Abstract
Objective
To compare the effectiveness of a continence pessary to evidence-based behavioral therapy for stress incontinence and to assess whether combined pessary and behavioral therapy is superior to single-modality therapy.
Methods
Multi-site, randomized clinical trial (“Ambulatory Treatments for Leakage Associated with Stress Incontinence” (ATLAS)) randomized 446 women with stress incontinence to pessary, behavioral therapy, or combined treatment. Primary outcome measures, at 3months, were Patient Global Impression of Improvement (PGI-I) and the stress incontinence subscale of the Pelvic Floor Distress Inventory (PFDI). A priori, to be considered clinically superior, combination therapy had to be better than both single-modality therapies. Outcomes measures were repeated at 6 and 12 months. Primary analyses used intention-to-treat approach.
Results
At 3 months, 40% of the pessary group and 49% of the behavioral group were “much better” or “very much better” on PGI-I (p=0.09). Compared to the pessary group, more women in the behavioral group reported having no bothersome incontinence symptoms (49% vs. 33%, p=0.006) and treatment satisfaction (75% vs. 63%, p=0.02). Combination therapy was significantly better than pessary on PGI-I (53%, p=0.02) and PFDI (44%, p=0.05), but not better than behavioral therapy; it was therefore not superior to single-modality therapy. Group differences were not sustained to12 months on any measure, and patient satisfaction remained above 50% for all treatment groups.
Conclusions
Behavioral therapy resulted in greater patient satisfaction and fewer bothersome incontinence symptoms than pessary at 3 months, but differences did not persist to 12 months. Combination therapy was not superior to single-modality therapy.
Introduction
Stress urinary incontinence affects approximately 15% to 35% of women in population-based studies.1,2 While surgical treatments are generally safe and highly effective, women with stress incontinence symptoms may wish to avoid or defer surgery for medical or personal reasons. Further, expert consensus groups recommend that non-surgical options should be offered as first-line therapy for incontinence.3
Evidence-based non-surgical therapies for treatment of stress incontinence are limited to behavioral therapy, which combines pelvic floor muscle training and exercise together with instruction in skills and strategies for active use of muscles to prevent urine loss.3–10 However, behavioral therapy requires individual motivation, and not all women are willing to adhere to a daily regimen and continued practice. Additionally, expert practitioners are often required to guide this type of therapy.
Continence pessaries represent a promising alternative or complementary non-surgical approach to the treatment of stress incontinence. These devices are believed to work by augmenting urethral closure during increased intra-abdominal pressure and thus increasing urethral resistance.11, 12 There are few studies describing the effectiveness of pessaries for treatment of stress incontinence and most are based on small samples of participants with short-term follow-up.13,14,15 There are no randomized trials comparing continence pessaries to evidence-based alternatives.
This trial was designed to evaluate the effectiveness of a continence pessary compared to established behavioral therapy in women with stress incontinence. In addition, as the therapeutic mechanisms of the continence pessary and behavioral therapy are thought to be different, we wished to determine whether combined therapy was superior to single-modality treatment.
Materials and Methods
The methods used in the Ambulatory Treatments for Leakage Associated with Stress Incontinence (ATLAS) trial have been reported previously.16 Eligible women at least 18 years old with symptoms of stress only or stress predominant mixed incontinence symptoms were enrolled. Institutional review board approval was obtained at each site and the data coordinating center, and all participants provided written informed consent.
Participants were stratified by 7-day bladder diary results, including incontinence type (stress only versus mixed) and severity (<14 vs. ≥14 total incontinence episodes) and then randomized to one of three treatment arms: pessary, behavioral therapy, or a combination of the two treatments using a permuted block randomization schedule. The Data Coordinating Center performed the randomization and provided each site with sets of sealed envelopes; the next envelope in the correct stratum was opened by the Interventionist only after the woman satisfied all the inclusion/exclusion criteria. Behavioral therapy was considered an active treatment control, as level I evidence exists for behavioral therapy’s efficacy.3,4,5
Interventionists (registered nurses, nurse practitioners and physical therapists) at each of the nine participating clinical sites administered all behavioral treatments after centralized training and standardization of procedures. Behavioral therapy was implemented in 4 visits at approximately 2-week intervals. Visits included instructions for pelvic floor muscle training and exercise, with additional skills and strategies for active use of muscles to prevent stress and urge incontinence. Participants were given individualized prescriptions for daily pelvic floor muscle exercise and practice. Pessary treatment included a physician or nurse fitting the participant with a continence ring or dish. While most were fitted successfully in one visit, up to 3 clinic visits at 1–2 week intervals were permitted to achieve optimal fitting. At the end of the 8-week treatment period, participants in the behavioral and combined treatment groups were provided with an individualized home maintenance program to sustain their skills and muscle strength. Women in the pessary and combined treatment groups were encouraged to continue routine pessary use. Furthermore, women in the combination group could continue in the trial while using only one of the therapies. For example, if they could not be successfully fit with a pessary, they could continue behavioral therapy alone.
Outcomes were assessed at 3, 6, and 12 months after randomization, with primary outcomes assessed at 3 months. All research personnel who conducted physical examinations for efficacy and safety and telephone interviewers who collected patient-oriented outcome data were blinded to treatment group assignment. Two primary outcomes were assessed using validated measures. First, the Patient Global Impression of Improvement (PGI-I),17 where success was defined as a response of “much better” or “very much better.” In addition, using the Urogenital Distress Inventory-stress incontinence subscale of the Pelvic Floor Distress Inventory (PFDI),18 success was defined as the absence of bothersome stress incontinence symptoms, as indicated by an answer of “no” to all six of the stress incontinence subscale items or a response of “yes,” but with a bother of “not at all” or “somewhat.” Women responding otherwise were considered treatment failures, as were those who received other treatment for incontinence.
Secondary outcomes included the proportion of participants with at least 75% reduction in frequency of incontinence episodes on 7-day bladder diary and patient satisfaction with treatment, assessed using the validated Patient Satisfaction Question (PSQ).19 A self-administered treatment adherence questionnaire was completed at each follow-up time-point. Adverse events (AEs) were collected during treatment visits.
Power calculations determined that 150 women per group would provide 80% power to detect a 15% difference in the success rate between pessary and behavioral therapy defined by the PGI-I at 3 months, as well as 80% power to detect whether combined therapy was superior to both treatments alone (assuming a 75% success rate for the combined group and 60% for the individual treatment groups).
Baseline characteristics of the three groups were compared using Mantel-Haenszel tests or ANOVA. An intention-to-treat analysis was performed, in which participants who received any other treatment for incontinence or withdrawals were considered failures. A secondary per protocol analysis included participants who reported that they had adhered to the assigned treatment at each follow-up visit. In both intention-to-treat and per protocol analyses, missing values were imputed using the “Next Value Carried Backward” method: if an outcome measure was missing, the next available follow-up value was used. All observations that could not be filled using the above method were set to “Failure.” All analyses were repeated by resetting these observations to “Success.” Logistic regression, adjusted for the two stratification factors, was used to determine whether the two individual treatment arms had different treatment success rates. Similarly, each of the two individual treatment groups was compared to the combination group in separate analyses. A priori, the combination treatment was considered superior to single-modality therapy only when both statistical tests were significant at the 5% level. Statistical significance was defined at 5%, except for the PGI-I endpoint at 3 months, where a 4.92% level was used, because a planned interim analysis was performed, and thus the nominal significance level was adjusted for the final analysis.
Results
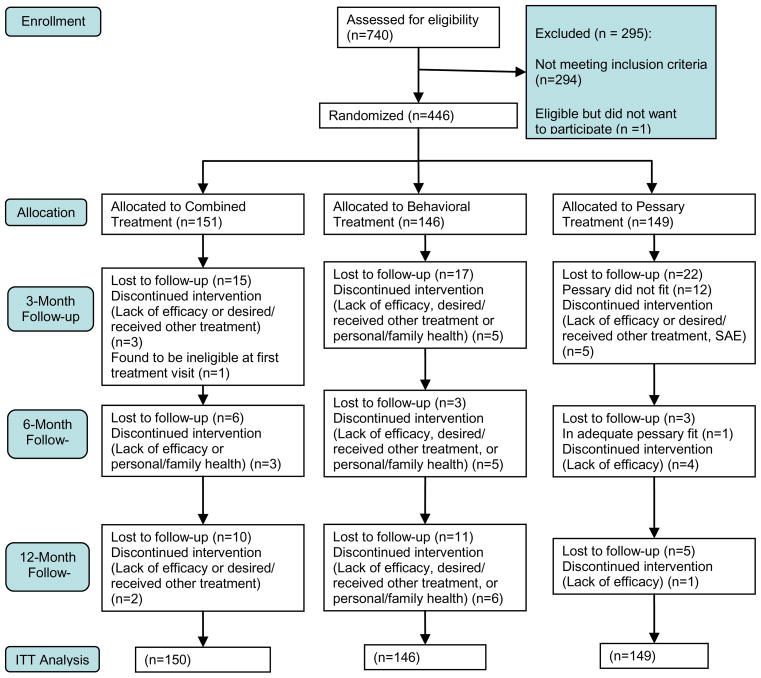
Between May 2005 and October 2007, 741 women were assessed for eligibility (Figure 1), and 446 participants were randomly assigned to pessary only (N=149), behavioral therapy only (N=146), or combination therapy (N=151). One randomized participant was subsequently found to be ineligible and not included in the analysis. Participants in the three treatment groups were similar in demographic and medical characteristics (Table 1).
Figure 1.
Enrollment and Disposition.
Table 1.
Selected Participant Characteristics at Baseline
| Variable | All N=445 | Combined N=150 | Behavioral N=146 | Pessary N=149 | P-value1 |
|---|---|---|---|---|---|
| Age-Mean SD2 | 49.8 (11.9) | 49.5 (11.8) | 49.6 (13.0) | 50.2 (11.0) | 0.86 |
| Race | |||||
| White/Caucasian | 379 (85.4%) | 122 (81.3%) | 132 (90.4%) | 125 (84.5%) | 0.26 |
| Black/Afr American | 45 (10.1%) | 20 (13.3%) | 10 (6.8%) | 15 (10.1%) | |
| Other | 20 (4.5%) | 8 (5.3%) | 4 (2.7%) | 8 (5.4%) | |
| Vaginal Deliveries Median (Q1, Q3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.31 |
| Menstrual Status | 0.91 | ||||
| Pre-menopausal | 214 (48.1%) | 68 (45.3%) | 71 (48.6%) | 75 (50.3%) | |
| Post-menopausal | 188 (42.2%) | 66 (44.0%) | 62 (42.5%) | 60 (40.3%) | |
| Not sure | 43 (9.7%) | 16 (10.7%) | 13 (8.9%) | 14 (9.4%) | |
| Currently Receiving Estrogen Therapy | 67 (15.1%) | 16 (10.7%) | 24 (16.4%) | 27 (18.1%) | 0.17 |
| Prior non-surgical UI treatment | 92 (20.7%) | 27 (18.0%) | 35 (24.0%) | 30 (20.1%) | 0.46 |
| Prior UI surgery | 27 (6.1%) | 10 (6.7%) | 7 (4.8%) | 10 (6.7%) | 0.71 |
| Hysterectomy | 110 (24.8%) | 32 (21.3%) | 39 (26.9%) | 39 (26.4%) | 0.50 |
| Incontinence Type | |||||
| Stress Only | 204 (45.8%) | 70 (46.7%) | 65 (44.5%) | 69 (46.3%) | 0.92 |
| Mixed | 241 (54.2%) | 80 (53.3%) | 81 (55.5%) | 80 (53.7%) | |
| Incontinence Freq | |||||
| ≥14 episodes/week | 202 (45.4%) | 67 (44.7%) | 67 (45.9%) | 68 (45.6%) | 0.98 |
| <14 episodes/week | 243 (54.6%) | 83 (55.3%) | 79 (54.1%) | 81 (54.4%) | |
Mantel-Haenszel test or ANOVA controlling for stratification based on baseline incontinence episodes and incontinence type.
Ages ranged from 18 to 89 years.
After randomization, dropout patterns differed among the three treatment groups (p=0.015) with the pessary only group having the highest attrition rate of 26% (39/149) at 3 months, followed by 15% (22/146) in the behavioral therapy group and 12% (18/150) in the combination therapy group. Reasons for withdrawal by group are shown in Table 2.
Table 2.
Reasons for Withdrawal from Study
| Reasons for withdrawal | Combined N=150 | Behavioral N=146 | Pessary N=149 | All N=445 |
|---|---|---|---|---|
| 3 MONTH | ||||
| Inadequate continence pessary fit1 | 0(0%) | 0(0%) | 12(8.1%) | 12(2.7%) |
| Lack of Efficacy | 1(0.7%) | 2(1.4%) | 1(0.7%) | 4(0.9%) |
| Personal/family health | 0(0%) | 1(0.7%) | 1(0.7%) | 2(0.5%) |
| Received/desired alternate treatment after receiving the assigned therapy. | 2(1.3%) | 2(1.4%) | 2(1.3%) | 6(1.4%) |
| Too busy | 3(2.0%) | 4(2.7%) | 0(0%) | 7(1.6%) |
| Unwilling to (continue to) participate | 12(8.0%) | 10(6.9%) | 11(7.4%) | 33(7.4%) |
| Wanted other treatment arm when informed of the randomly assigned treatment. | 0(0%) | 3(2.1%) | 11(7.4%) | 14(3.2%) |
| Withdrew due to SAE2 | 0(0%) | 0(0%) | 1(0.7%) | 1(0.2%) |
| TOTAL | 18 | 22 | 39 | 79 |
| 12 MONTH (based on cumulative counts) | ||||
| Inadequate continence pessary fit1 | 0(0%) | 0(0%) | 13(8.7%) | 13(2.9%) |
| Lack of Efficacy | 4(2.7%) | 6(4.1%) | 6(4.0%) | 16(3.6%) |
| Personal/family health | 1(0.7%) | 6(4.1%) | 1(0.7%) | 8(1.8%) |
| Received/desired alternate treatment after receiving the assigned therapy. | 3(2.0%) | 4(2.7%) | 2(1.3%) | 9(2.0%) |
| Too busy | 4(2.7%) | 4(2.7%) | 1(0.7%) | 9(2.0%) |
| Unwilling to (continue to) participate | 27(18.0%) | 22(15.1%) | 17(11.4%) | 66(14.8%) |
| Wanted other treatment arm when informed of the randomly assigned treatment. | 0(0%) | 5(3.4%) | 12(8.1%) | 17(3.8%) |
| Withdrew due to SAE2 | 0(0%) | 0(0%) | 1(0.7%) | 1(0.2%) |
| TOTAL | 39 | 47 | 53 | 139 |
Patients in the combined therapy group could continue despite failed continence pessary fitting.
Fractured right leg and ankle requiring surgical repair.
Intention-to-treat analysis revealed that, overall, 46% of participants reported that they were “much better” or “very much better” at 3 months (pessary 40%, behavioral 49%, combination therapy 53%) on the PGI-I, with no statistically significant differences between the pessary and behavioral therapy groups. The combined therapy group was not better than single therapy: The PGI-I success rate in the combined therapy group was significantly higher than that in the pessary alone group (p=0.02), but not different from the behavioral therapy group (Table 3). Twenty-three percent (104/445) reported that they were “a little bit better,” 7% (32/445) reported “no change,” and 1% (6/445) reported that they were worse; the others were dropouts (n=79) or missing the PGI-I measure at 3 months (n=31).
Table 3.
Summary of outcomes by treatment group and time period.
| Intention-to-Treat | Per Protocol | |||||
|---|---|---|---|---|---|---|
| Outcome | Combined N=150 | Behavioral N=149 | Pessary N=146 | Combined | Behavioral | Pessary |
| “Much better” or “very much better” on PGI-I1 | ||||||
| 3 month | 80 (53.3%)2 | 72 (49.3%) | 59 (39.6%)2 | 47/69 (68.1%) | 69/112 (61.6%) | 53/99 (53.5%) |
| 12 month | 49 (32.7%) | 48 (32.9%) | 47 (31.5%) | 12/27 (44.4%) | 44/83 (53.0%) | 39/67 (58.2%) |
| No bothersome stress incontinence symptoms3 | ||||||
| 3 month | 66 (44.0%)4 | 71 (48.6%)5 | 49 (32.9%)4,5 | 38/69 (55.1%) | 66/112 (58.9%) | 45/99 (45.5%) |
| 12 month | 49 (32.7%) | 59 (40.4%) | 52 (34.9%) | 11/27 (40.7%) | 52/83 (62.7%) | 41/67 (61.2%) |
| > 75% reduction in weekly urinary Incontinence episodes6 | ||||||
| 3 month | 80 (53.3%) | 68 (46.7%) | 69 (46.3%) | 42/69 (60.9%) | 61/112 (54.5%) | 64/99 (64.7%) |
| 12 month | 52 (34.7%) | 54 (37.0%) | 51 (34.2%) | 14/27 (51.9%) | 47/82 (56.6%) | 39/67 (58.2%) |
| Satisfaction with treatment | ||||||
| 3 month | 118 (78.7%)7 | 110 (75.3%)8 | 94 (63.1%)7,8 | 63/69 (91.3%) | 104/112(92.9%) | 87/99 (87.9%) |
| 12 month | 81 (54.0%) | 79 (54.1%) | 75 (50.3%) | 23/27 (85.2%) | 72/83 (86.7%) | 61/67 (91.0%) |
PGI-I, Patient Global Impression ofI mprovement.
Combined group is different from pessary group (p=0.02), but not different from behavioral group (p=0.49).
Defined as an answer of either “no” or “yes” with a bother component of “not at all” or “somewhat” to all of the seven Urogenital Distress Inventory-Stress Incontinence Subscale items of the Pelvic Floor Distress Inventory;
Combined group is different from pessary group (p=0.05), but not different from behavioral group (p=0.42).
Pessary group is different from behavioral group (p=0.006).
On the 7-day bladder diary
Combined group is different from pessary group (p=0.003), but not different from behavioral group (p=0.50).
Pessarygroup is different from behavioral therapy group (p=0.03).
All other results in tabledo not differ (p> 0.05).
Also at 3 months, the proportion of women without bothersome stress incontinence symptoms, as assessed by the PFDI, was 33% in the pessary group and 49% in the behavioral group (p=0.006). The combined group had a 44% success rate, which was significantly different from the pessary group, but not different from the behavioral group and therefore not better than single therapy. Approximately 50% of subjects in each group showed at least 75% reduction in incontinence episodes on bladder diary at 3 months, with no significant differences among groups. More women in the behavioral group than the pessary group reported being satisfied with therapy at 3 months (75% vs 63%, p=0.03). Patient satisfaction was 79% in the combined therapy group, which was not different from behavioral therapy (Table 3).
The per protocol analysis of the women who reported adherence to assigned therapies is also shown in Table 3. Among these participants, success rates at 3 months ranged from 54–68% as measured with the PGI-I, from 46–59% as measured by PFDI, and from 88–93% satisfied as measured by the satisfaction questionnaire, with no statistically significant treatment differences among groups.
Between 3 and 12 months, treatment success declined regardless of definition, with no statistically significant differences between groups at 12 months (Table 3). In the entire group of randomized participants at 12 months, 32% reported that they were “much better” or “very much better” on the PGI-I, 36% denied bothersome stress incontinence symptoms on the PFDI, and 35% had at least a 75% reduction of incontinence episodes on bladder diary. In the per protocol analysis, depending on group assignment and outcome used, 41–61% of women were effectively treated at 12 months. Patient satisfaction at 12 months was 50–54% in the intention-to-treat analysis and 85–91% in the per protocol analysis with no significant between-group differences.
The most commonly reported AE at 3 months was vaginal discharge (16% in pessary, 6% in behavioral, 9% in combination therapy), and 7% of women reported a vaginal yeast infection, but there were no vaginal erosions. The most common unexpected AEs were urologic (N=10, 3 in pessary, 2 in behavioral, 5 in combination therapy) consisting of 7 urinary tract infections (UTIs), 2 possible UTIs and 1 possible case of urinary retention.
Discussion
The ATLAS findings support the conclusion that behavioral therapy was more effective than pessary at three months and that both therapies resulted in reasonable patient satisfaction. Nonsurgical therapies may be less effective than surgical therapy. For example, women undergoing surgery have been shown to have a significantly greater improvement in the Pelvic Floor Distress Inventory and urinary and prolapse subsacles of the Pelvic Floor Impact Questionnaire than women using pessaries. 20 However, women who choose nonsurgical therapy may have different goals than those who choose surgical therapy.
While pessaries have been used for years in the conservative management of stress incontinence in women,21 this study is the first randomized clinical trial to compare the effectiveness of the continence pessary to evidence-based behavioral therapy for stress incontinence symptoms. This claim is based on a PubMed search, the 2009 World Health Organization sponsored International Consultation on Incontinece review of the world's literature and a review of a Cochran literature. PubMed search terms utilized included English language reports for "pessary,", "stress incontinence," "nonsurgical treatment stress incontinence," "behavioral therapy," "pelvic floor muscle therapy," and "randomized trials" over the past 20 years. The search was conducted in March 2009. The International Consultation on Incontinence reviews are cited in references 3, 4, and 21 and the pertinent Cochrane reivew is reference 4. We did not study a sham treatment group because of level I evidence, summarized by a Cochrane review4, demonstrating that behavioral therapy is more effective than no treatment or sham treatment. While we did not detect a significant difference between pessary and behavioral therapy with respect to participants’ subjective report of improvement (“much better” or “very much better” on the PGI-I), more women in the behavioral group reported treatment satisfaction and a lack of bothersome urinary symptoms and than women in the pessary group at 3 months. However, by one year after randomization, in both the intent-to-treat and in the per-protocol analyses, there were no statistically significant differences in outcomes between these two groups. By intention to treat, one-third of all women, and over one-half of women still using the assigned treatment, had successful non-surgical treatment.
Our rate of pessary discontinuation is similar to that seen in a previous pessary study in which approximately a third of intention-to-treat participants withdrew early.13 Clinically, this suggests that women can quickly determine whether or not a pessary is a viable therapy for them, and move on to other treatments if not.
The pessary and behavioral therapy most likely treat stress incontinence through different mechanisms. Therefore, we hypothesized that combining the two therapies would produce better outcomes than either therapy alone. The criterion for judging superiority was that combination therapy must be more effective than both continence pessary alone and behavioral therapy alone. Our data indicated that combination therapy was not superior to single-modality therapy.
We chose a global impression of improvement (PGI-I) and the lack of bothersome stress incontinence symptoms as our primary outcome measures, because we felt they were most relevant to the patient’s experience. In one trial that used a similar validated global perception of improvement (GPI) in women 40–78 years of age who completed a program of biofeedback-assisted behavioral training for stress-predominant incontinence, 57% of participants reported that they were “much better.” 10 Those findings are similar to the per protocol results for adherent patients in the current trial. Global ratings have been used infrequently as a primary outcome measure in stress incontinence intervention trials.4–6 We therefore used other more traditional outcome measures such as symptoms as assessed by the PFDI and bladder diary as secondary endpoints to enhance comparability of our results with those of previous studies.
The minimum important difference (MID) for the stress incontinence subscale of the PFDI has been estimated previously using blinded, pooled baseline data from this trial to define a clinically significant change in score.22 At the 3- and 12-month outcomes, all three groups exceeded this MID, confirming the clinical relevance of the improvements demonstrated in this trial. Fifty percent of women in our trial experienced at least 75% reduction of incontinence episodes on bladder diary. This treatment effect is comparable to that found in a previous behavioral trial in which 58% of women achieved similar reductions.6 Our results in the per protocol analysis fall at the lower end of the 60%–85% range for reduction in incontinence episodes reported in other behavioral intervention trials.4–10
This lower range of reduction of incontinence may be explained by several limitations to our study. Our evidence-based behavioral therapy groups had only 4 visits with the interventionist over approximately 8 weeks. Two studies have suggested that increased intensity of pelvic floor muscle training may yield improved results for stress incontinence.8,23 Although we considered the merits of comparing the pessary to a higher intensity pelvic floor muscle training program, we chose to study an evidence-based behavioral intervention program that more closely reflected the number of provider visits typically approved by US insurance companies, some of which authorize as few as two visits for incontinence treatment. The relationship of the cost of such visits to the cost of fitting and maintaining a pessary, particularly relative to patient satisfaction, woudl be interesting analyses to pursue in future studies.
Declines in adherence over time likely contributed to the attenuation of successful outcomes for all 3 groups by 12 months. Only 45% of women reported that they were still using the pessary at one year and only 57% of women reported that they were continuing to practice their pelvic floor muscle exercises. These results are similar to prior uncontrolled studies of pessary use for stress incontinence, in which 50–60% of women continued use of continence pessary after 1 year.14 Barriers to long-term use of pessary deserve examination, and further studies will be required to determine whether adherence to pelvic floor muscle exercises and other behavioral strategies is modifiable.
In conclusion, three months after randomization, more women assigned to behavioral therapy had no bothersome stress incontinence symptoms and more were satisfied with treatment outcomes than those assigned to pessary. Differences did not persist to 12 months. One year after initiating therapy, one-third of all women, and over one-half of women still using the assigned treatment, were improved based on patient-reported outcomes and even more were satisfied. Thus, the one-year data support the consideration of pessary as a reasonable alternative for women wishing to avoid or defer stress incontinence surgery and not interested in or able to adhere to behavioral therapy. Individualization of care should continue to be the cornerstone of our approach to patients. Because combined therapy was not superior to single-modality therapy, we recommend offering a single therapy as the initial approach to non-surgical treatment of SUI.
Acknowledgments
Source of funding: Supported by grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (U10 HD41261, U10 HD 41250, U10 HD54136, U10 HD41249, U10 HD41267, U10 HD41248, U10 HD41268, U10 HD41263, U10 HD54214, U10 HD54241, and U10 HD54215); National Institute of Diabetes and Digestive and Kidney Diseases and NIH Office of Research on Women’s Health
Pelvic Floor Disorders Network:
Cleveland Clinic
Mathew D. Barber, MD, MHS, Principal Investigator
Marie Fidela R. Paraiso, MD, Co-Investigator
Mark D. Walters, MD, Co-Investigator
J. Eric Jelovsek, MD, Co-Investigator
Linda McElrath, RN, Research Nurse Coordinator
Donel Murphy, RN, MSN, Research Nurse
Cheryl Williams, Research Assistant
Duke University
Anthony G. Visco, MD, Principal Investigator
Jennifer Wu, MD, Co-Investigator
Alison Weidner, MD, Co-Investigator
Cindy Amundsen, MD, Co-Investigator
Mary J. Loomis, RN, BSN, Research Coordinator
Loyola University
Linda Brubaker, MD, MS, Principal Investigator
Kimberly Kenton, MD, MS, Investigator
MaryPat FitzGerald, MD, MS, Investigator
Elizabeth Mueller, MD, MSME, Investigator
Mary Tulke, RN, Research Nurse Coordinator
Kathy Jesse, RN, Research Nurse Coordinator
Kathy Marchese, RN, Research Nurse Coordinator
University of Alabama at Birmingham
Holly E. Richter, PhD, MD, Principal Investigator
Kathryn L. Burgio, PhD, Co-Principal Investigator
R. Edward Varner, MD, Co-Investigator
Robert L. Holley, MD, Co-Investigator
Patricia S. Goode, MD, Co-Investigator
L. Keith Lloyd, MD, Co-Investigator
Alayne D. Markland, DO, Co-Investigator
Tracey Wilson, MD, Co-Investigator
Velria Willis, RN, BSN, Research Coordinator
Nancy Saxon, BSN, Research Nurse Clinician
LaChele Ward, LPN, Research Specialist
Lisa S. Pair, CRNP
University of California, San Diego and Kaiser Permanente, San Diego
Charles W. Nager, MD Principal Investigator
Shawn A. Menefee, MD, Co- Investigator
Emily Lukacz, MD, Co-Investigator
Margie Kahn, MD, Co-Investigator
Karl M. Luber, MD, Co-Investigator
Leah Merrin, Research Coordinator
Giselle Zazueta-Damian, Research Coordinator
Patsy Riley, R.N.
Lynn Hall, R.N.
Judy M. Condino, RN
University of Michigan
Cathie Spino, DSc, Principal Investigator
John T. Wei, MD, MS, Co-Principal Investigator
Morton B. Brown, PhD, Co-Investigator
Donna DiFranco, BS, Clinical Monitor
John O.L. DeLancey, MD, Co-Investigator
Dee Fenner, MD, Co-Investigator
Nancy K. Janz, PhD, Co-Investigator
Wen Ye, PhD, Statistician
Zhen Chen, MS, Statistician
Yang Wang Casher, MS, Database Programmer
University of Texas, Southwestern
Joseph Schaffer MD, Principal Investigator
David Rahn, MD, Co-Investigator
Clifford Wai, MD, Co-Investigator
Marlene Corton, MD, Co-Investigator
Gary Lemack, MD, Co-Investigator
Philippe Zimmern, MD Co-Investigator
Kelly Moore - Research Coordinator
Shanna Atnip, NP
Margaret Hull, NP
Pam Martinez, NP
Deborah Lawson, NP
University of Utah
Ingrid Nygaard, MD, Principal Investigator
Peggy Norton, MD, Co-Investigator
Yvonne Hsu, MD, Co-Investigator
Jan Baker, Interventionist
Linda Freeman, RN, Research Coordinator
Steering Committee Chair
Katherine E. Hartmann, MD, PhD
NIH Project Scientist
Susan Meikle, MD, MSPH
Other Participating Sites:
Magee Women’s Hospital, Pittsburgh, PA
Halina Zyczynski, MD, Principal Investigator
Wendy Leng, MD, Co-Investigator
Pamela Moalli, Ph.D, MD, Co-Investigator
Chaira Ghetti, MD, Co-Investigator
Jerry Lowder, MD, Co- Investigator
Judy Gruss and Karen Mislanovich, Nurse Coordinators
Interventionist: Karen Debes
Johns Hopkins Medical Institutes
Geoffrey Cundiff, M.D., Principal Investigator
Victoria Handa, M.D., Co-Investigator
Jamie Wright, M.D., Co-Investigator
Mary Elizabeth Sauter, N.P., Research Coordinator
Laura Scheufele, PT, Interventionist
References
- 1.Abrams P, Cardozo L, Fall M, et al. The Standardization of terminology of lower urinary tract function: Report from the Standardization Sub-committee of the International Continence Society. Neurourol Urodynam. 2002;21:167–78. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 2.Milsom I, Altman D, Lapitan MC, Nelson R, Sillen U, Thom D. Epidemiology of urinary (UI) and Faecal (FI) Incontinence and Pelvic Organ Prolapse. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence; 4th International Consultation on Incontinence; Paris: Health Publication Ltd; 2009. pp. 35–111. [Google Scholar]
- 3.Hay-Smith J, Berghmans B, Burgio K, et al. Adult Conservative Management. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence; 4th International Consultation on Incontinence; Paris: Health Publication Ltd; 2009. pp. 1025–1120. [Google Scholar]
- 4.Hay-Smith EJC, Bo K, Berghmans LCM, et al. Pelvic floor muscle training for urinary incontinence in women. Cochrane Database Syst. 2002:CD001407. doi: 10.1002/14651858.CD001407.pub2. Rev 1. [DOI] [PubMed] [Google Scholar]
- 5.Shamliyan TA, Kane RL, Wyman J, Wilt TJ. Systematic review: Randomized, controlled trials of nonsurgical treatments for urinary incontinence in women. Ann Intern Med. 2008;148:459–73. doi: 10.7326/0003-4819-148-6-200803180-00211. [DOI] [PubMed] [Google Scholar]
- 6.Berghmans LCM, Hendriks HJM, Bo K, et al. Conservative treatment of stress urinary incontinence in women: a systematic review of controlled clinical trials. Br J Urol. 1998;82:181–91. doi: 10.1046/j.1464-410x.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 7.Burns PA, Pranikoff K, Nochajski TH, Hadley EC, Levy KJ, Ory MG. A comparison of effectiveness of biofeedback and pelvic muscle exercise treatment of stress incontinence in older community-dwelling women. J Gerontol. 1993;48:M167–74. doi: 10.1093/geronj/48.4.m167. [DOI] [PubMed] [Google Scholar]
- 8.Bo K, Talseth T, Holm I. Single blind, randomised controlled trial of pelvic floor exercises, electrical stimulation, vaginal cones, and no treatment in management of genuine stress incontinence in women. BMJ. 1999;318:487–93. doi: 10.1136/bmj.318.7182.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henalla SM, Hutchins CJ, Robinson P, MacVicar J. Non-operative methods in the treatment of female genuine stress incontinence of urine. J Obstet Gynacol. 1989;9:222–25. [Google Scholar]
- 10.Goode PS, Burgio KL, Locher JL, et al. Effect of behavioral training with or without pelvic floor electrical stimulation on stress incontinence in women: A randomized control trial. JAMA. 2003;290:345–52. doi: 10.1001/jama.290.3.345. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia NN, Bergman A. Pessary test in women with urinary incontinence. Obstet Gynecol. 1985;65:220–26. [PubMed] [Google Scholar]
- 12.Komesu YM, Ketai LH, Rogers RG, et al. Restoration of continence by pessaries: magnetic resonance imaging assessment of mechanism of action. Am J Obstet Gynecol. 2008;198:563.e1. doi: 10.1016/j.ajog.2008.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farrell SA, Baydock S, Amir B, et al. Effectiveness of a new self-positioning pessary for the management of urinary incontinence in women. Am J Obstet Gynecol. 2007;196:474.e1–474.e8. doi: 10.1016/j.ajog.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed urinary incontinence. Intl Urogynecol J Pelvic Floor Dysfunct. 2004;15:302–7. doi: 10.1007/s00192-004-1163-7. [DOI] [PubMed] [Google Scholar]
- 15.Nygaard I. Prevention of exercise incontinence with mechanical devices. J Reprod Med. 1995;40:89–94. [PubMed] [Google Scholar]
- 16.Richter HE, Burgio KL, Goode PS, et al. Non surgical management of stress urinary incontinence: ambulatory treatments for leakage associated with stress (ATLAS) trial. Clinical Trials. 2007;4:92–101. doi: 10.1177/1740774506075237. [DOI] [PubMed] [Google Scholar]
- 17.Yalcin I, Bump RC. Validation of two global impression questionnaires for incontinence. Am J Obstet Gynecol. 2003;189:98–101. doi: 10.1067/mob.2003.379. [DOI] [PubMed] [Google Scholar]
- 18.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185:1388–95. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 19.Burgio KL, Goode PS, Richter HE, Locher JL, Roth DE. Global ratings of patient satisfaction and perception of improvement with treatment for urinary incontinence: Validation of three global patient ratings. Neurourol Urodynamics. 2006;25:411–17. doi: 10.1002/nau.20243. [DOI] [PubMed] [Google Scholar]
- 20.Barber MD, Walters MD, Cundiff GW, PESSRI Trial Group Responsiveness of the Pelvic Floor Distress Inventory (PFDI) and Pelvic Floor Impact Questionnaire (PFIQ) in women undergoing vaginal surgery and pessary treatment for pelvic organ prolapse. Am J Obstet Gynecol. 2006;194:1492–8. doi: 10.1016/j.ajog.2006.01.076. [DOI] [PubMed] [Google Scholar]
- 21.Cottenden A, Bliss DZ, Buckley B, et al. Management Using Continence Products. In: Abrams P, Cardozo L, Khoury S, Wein A, editors. Incontinence; 4th International Consultation on Incontinence; Paris: Health Publication Ltd; 2009. pp. 1519–1642. [Google Scholar]
- 22.Barber MD, Spino C, Janz NK, et al. The minimum important difference for the urinary scales of the Pelvic Floor Distress Inventory and Pelvic Floor Impact Questionnaire. Am J Obstet Gynecol. 2009;200:580.e1–580.e7. doi: 10.1016/j.ajog.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bo K, Hagen RH, Kvarstein B, et al. Pelvic floor muscle exercise for the treatment of female stress urinary incontinence: III. Effects of two different degrees of pelvic floor muscle exercises. Neurol Urodyn. 1990;9:489–502. [Google Scholar]