Abstract
Synaptic plasticity, the activity-dependent change in the strength of neuronal connections, is a proposed cellular mechanism of memory storage that is critically regulated by protein kinases such as cAMP-dependent protein kinase (PKA). Despite the fact that a neuron contains thousands of synapses, the expression of synaptic plasticity can be specific to subsets of synapses. This is surprising because signal transduction pathways underlying synaptic plasticity involve diffusible second messenger molecules such as cAMP and diffusible proteins such as the catalytic subunit of PKA. One way in which this specificity can be achieved is by the localization of signal transduction molecules to specific subcellular domains. Spatial compartmentalization of PKA signaling is achieved via binding to A kinase-anchoring proteins (AKAPs). We report here that pharmacological inhibition of PKA anchoring impairs synaptically activated late-phase long-term potentiation (L-LTP) in hippocampal slices. In contrast, potentiation that is induced by the pharmacological activation of the cAMP/PKA pathway, which can potentially affect all synapses within the neuron, is not impaired by inhibition of PKA anchoring. These results suggest that PKA anchoring may be particularly important for events that occur at the synapse during the induction of L-LTP, such as synaptic tagging and capture. Indeed, our results demonstrate that blocking PKA anchoring impairs synaptic tagging and capture. Thus our data highlight the idea that PKA anchoring may allow for specific populations of synapses to change in synaptic strength in the face of plasticity-related transcription that is cell-wide.
Keywords: cAMP-dependent protein kinase, A kinase-anchoring proteins, Hippocampus, Synaptic plasticity, Synaptic tagging and capture
Introduction
The cellular mechanisms underlying memory storage likely involve activity-dependent changes in the strength of neuronal connections (Martin et al., 2000). Long-lasting modifications in synaptic strength require products of transcription (Nguyen et al., 1994) and translation (Frey et al., 1988). Even though these newly generated molecules may be distributed throughout the cell, only a subset of these synapses remain changed over time (Nguyen et al., 1994). Out of thousands of synapses, how does a neuron identify those synapses that will selectively undergo long-term change? Furthermore, how is this degree of specificity possible when signal transduction pathways underlying synaptic plasticity involve diffusible molecules such as cAMP? One way in which specificity can be achieved is by localizing signal transduction molecules to specific subcellular domains. Indeed, PKA is concentrated in certain subcellular regions through interaction with a family of functionally distinct but structurally related proteins called A kinase-anchoring proteins (AKAPs), a protein family consisting of more than 50 members (reviewed in Wong and Scott, 2004). Spatial compartmentalization of PKA may contribute to the specificity of the cAMP/ PKA signaling pathway in affecting downstream proteins (for example, in studies by Fink et al., 2001, inhibition of PKA anchoring resulted in redistribution of RII and decreased compartmentalization of PKA).
A well known and widely studied form of synaptic plasticity is hippocampal long-term potentiation (LTP) (Bliss and Collingridge, 1993; Bliss and Lømo, 1973; Malenka and Nicoll, 1999). In slice preparations of the hippocampus, brief patterns of high-frequency stimulation increase the amplitude of subsequent synaptic potentials. At hippocampal Schaffer collateral-CA1 synapses, L-LTP requires NMDA receptor activation (Collingridge et al., 1983), protein synthesis (Frey et al., 1988), transcription (Nguyen et al., 1994), and PKA (Abel et al., 1997; Frey et al., 1993; Huang and Kandel, 1994; Matthies and Reymann, 1993; Woo et al., 2000, 2002, 2003). In area CA1, cAMP levels are increased 1 min after tetanic stimulation that induced L-LTP (Frey et al., 1993), with a corresponding increase in PKA activity briefly after stimulation (Roberson and Sweatt, 1996). Treatment of hippocampal slices with cAMP analogs induces a potentiation that resembles L-LTP, whereas treatment with PKA inhibitors blocks L-LTP (Frey et al., 1993). Transgenic mice expressing a dominant negative regulatory subunit of PKA have reduced L-LTP in area CA1 and exhibit impaired performance in hippocampal-dependent memory and place cell stability (Abel et al., 1997; Rotenberg et al., 2000; Woo et al., 2000, 2002, 2003). Therefore, PKA activity is crucial in L-LTP and long-term memory.
An interesting property of hippocampal L-LTP is that of pathway specificity. A two-pathway experimental setup, where two independent sets of presynaptic inputs converge on a common set of postsynaptic neurons, can be used to monitor synaptic activity in two separate populations of synapses on the same synaptic neurons. In this two-pathway setup, only synapses that received L-LTP stimulation remain persistently potentiated; synapses that did not receive L-LTP stimulation do not undergo long-term functional change (Nguyen et al., 1994). This pathway specificity with which subsets of synapses become stably potentiated over time suggests that plasticity-related gene products are selectively used by those synapses that have received L-LTP stimulation to increase synaptic strength. Frey and Morris tested the idea that plasticity-related proteins, widely distributed throughout the cell, can be captured at specific “tagged” synapses (Frey and Morris, 1997). They demonstrated using two-pathway experiments that transient synaptic potentiation induced by weak stimulation in one pathway can be converted to stable synaptic potentiation if it is paired with strong stimulation in the other pathway. Thus synaptic activity seems to “tag” active synapses. It has been proposed that strong stimulation of synapses not only tags the synapses but also induces transcriptional and translational activity; weak stimulation of synapses only tags the synapses, but these tags have the ability to “capture” the products of gene expression produced by the neuron in response to strong stimulation in other synapses (Barco et al., 2002, 2005; Frey and Morris 1997, 1998; Sajikumar and Frey 2004; Sajikumar et al., 2005; Young and Nguyen, 2005).
Because selectively modifying subsets of synapses likely requires highly compartmentalized signal transduction pathways, we explore here the hypothesis that PKA anchoring is essential for hippocampal L-LTP. To inhibit PKA–AKAP interactions, we use a cell-permeable form of the Ht31 peptide, a truncated form of an AKAP that competitively binds the regulatory subunits of PKA, blocking the interaction of the regulatory subunit with most AKAPs (Carr et al., 1992). We demonstrate that synaptically activated L-LTP is impaired by this pharmacological inhibitor of PKA anchoring in a dose-dependent manner. We provide further evidence for the compartmentalized nature of PKA signaling in L-LTP by showing that synaptic potentiation caused by the global elevation of cAMP does not require PKA anchoring. Because tagging synapses for long term changes is specific to subsets of synapses, we investigate whether PKA anchoring is required to maintain the spatial specificity that is critical to synaptic tagging. Using a two-pathway paradigm, we demonstrate that the process of synaptic tagging and capture is impaired by inhibition of PKA anchoring. Thus the spatial specificity of the PKA signaling pathway, mediated by AKAPs, is critical to long-term synaptic changes in the hippocampus.
Materials and methods
Electrophysiology
Hippocampal slices were prepared as described previously (Abel et al., 1997). Briefly, 2- to 6-month-old male and female C57BL/6J (Jackson Labs) mice were sacrificed by cervical dislocation, brains were removed and hippocampi were rapidly dissected in the presence of chilled, oxygenated artificial cerebrospinal fluid (aCSF). Transverse slices (400 μm) were prepared using a tissue chopper and placed in an interface recording chamber (Fine Science Tools, Foster City, CA). ACSF (pH 7.4) containing 124 mM NaCl, 4.4 mM KCl, 1.3 mM MgSO4, 1 mM NaH2PO4, 26.2 mM NaHCO3, 2.5 mM CaCl2 and 10 mM D-glucose bubbled with 95% O2/5% CO2, was constantly perfused over slices at a rate of approximately 1 ml/min. Slices were allowed to recover for approximately 1.5 h before recording. To elicit field excitatory postsynaptic potentials (fEPSPs) from Schaffer collateral-CA1 synapses, bipolar nichrome wire (0.5 mm; AM Systems, Carlsborg, WA) extracellular stimulating electrodes were placed in stratum radiatum of CA1. Field EPSPs were recorded extracellularly using a glass micropipette (1.5 mm OD; AM Systems, Carlsborg, WA) electrode filled with aCSF with a resistance of 2–4 MΩ Data were acquired using ClampEx 9.2 and a Digidata1322 A/D converter (Axon Instruments, Union City, CA) at 20 kHz and low pass filtered at 2 kHz with a 4-pole Bessel filter. To examine basal synaptic transmission, input-output curves were generated by measuring the initial slope of the fEPSP in response to systematic increases in the strength of the stimulus. Slices that had maximum amplitude responses of less than 4 mV were rejected. The stimulus strength was then set to elicit approximately 40% of the maximum initial fEPSP amplitude. Paired pulse facilitation was then examined at interpulse intervals between 25–300 ms. For LTP experiments, test pulses were delivered to Schaffer collaterals once every minute for 20 min. Slices that did not have stable baseline responses for 20 min were rejected. After 20 min, LTP was induced electrically by using one of two protocols. A tetraburst (four 1 s, 100 Hz tetanic stimulus trains delivered 5 min apart) was used to induce L-LTP. After induction of synaptic potentiation, test pulses were delivered once per minute for two hours. In E-LTP experiments, a single 1 s, 100 Hz tetanic stimulus was used to induce a transient potentiation that was followed for one hour. For synaptic tagging experiments, two stimulating electrodes were positioned in such a way as to activate two separate sets of inputs onto the same postsynaptic population of neurons. Pathway independence was assessed by the absence of paired-pulse facilitation (50 ms interval) between the two pathways. Strong stimulation (tetra-burst protocol) was used to induce L-LTP in S1. Weak stimulation (one 1 s, 100 Hz) in S2 was used to assess the efficacy of the synaptic tagging and capture process. All experiments were conducted according to National Institutes of Health guidelines for animal care and use and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Reagents
Custom peptide synthesis of stearated Ht31 (stHt31) and Ht31P (stHt31P) was conducted by Quality Controlled Biochemicals (Hopkinton, MA). The sequences of the peptides were: stHt31, St-N-DLIEEAASRIVDA-VIEQVKAAGAY-C; stH31P, St-N-DLIEEAASR-PVDAVPEQVKAAGAY-C (Carr et al., 1992; Vijayaraghavan et al., 1997). Peptides were delivered as lyophilized powder and resuspended at a stock concentration of 10 mM in 50 mM Tris–HCl (pH 7.0) and 0.05% DMSO to increase peptide solubility. Unless specified, peptides were used at 10 μM final concentration in aCSF in all experiments described.
Forskolin (50 μM; Sigma, St. Louis, MO; de Souza et al., 1983), is an adenylyl cyclase activator. 3-Isobutyl-1-methylxanthine (IBMX, 30 μM; Sigma; Kalderon et al., 1980; Wojcikiewicz et al., 1984) is a phosphodiesterase inhibitor. Forskolin and IBMX were independently dissolved in 50 mM Tris–HCl (pH 7.0) and 0.05% DMSO and used together to induce a form of chemical LTP that is PKA dependent (Woo et al., 2002).
Data analysis
Synaptic strength was measured by the initial slope of the fEPSP. A 20 min stable baseline was acquired prior to pharmacological manipulations or L-LTP stimulation. Field EPSP at each subsequent time point is normalized to the averaged baseline value and plotted to generate LTP graphs. Two way repeated measures ANOVA with post-hoc Student Newman-Keuls test (SYSTAT 7.0.1 software) was used to determine statistical differences between treatment groups. ANO-VA was performed on the last 20 min time points of each recording. In Fig. 2b, the normalized fEPSP values at 100–120 min posttetanus are averaged and plotted for each drug treatment group. For the analysis of the input–output properties at Schaffer collateral-CA1 synapses, presynaptic fiber volley amplitudes were binned at 0.1 mV intervals prior to ANOVA.
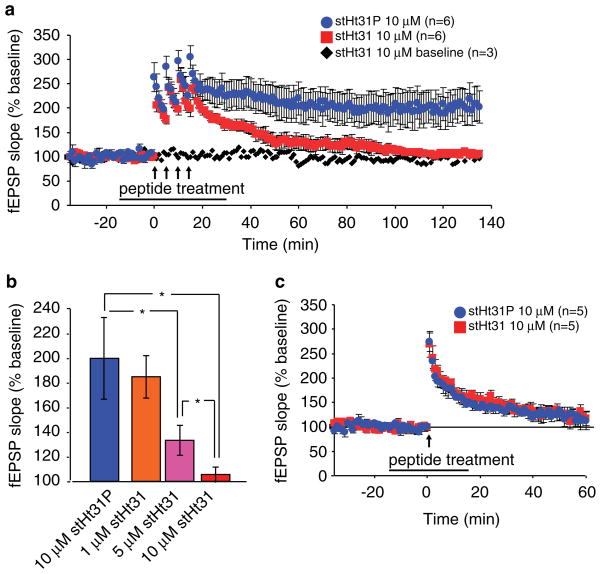
Fig. 2.
Pharmacological inhibition of PKA anchoring impairs 4-train L-LTP in a dose-dependent fashion. (a) Treatment with 10 μM stHt31 (duration indicated by black bar) impairs L-LTP induced by four 1 s, 100 Hz trains of electrical stimuli, while 10 μM stHt31P does not alter L-LTP. stHt31 (10 μM) in the absence of LTP induction does not alter baseline responses recorded for 2.5 h. fEPSP responses in the absence of L-LTP induction were not altered by stHt31 treatment. (b) Comparison of the average fEPSPs from the experiments in (a) at 100–120 min posttetanus. L-LTP impairment is dose dependent: compared to the stHt31P group, 1 μM stHt31 does not significantly impair L-LTP, 5 μM stHt31 significantly impaired L-LTP as compared to stHt31P treatment, but not to the same extent as compared to the 10 μM stHt31 group. (c) Treatment with stHt31 does not impair a PKA-independent form of E-LTP induced by one 1 s, 100 Hz train of electrical stimulus.
Results
Basal synaptic transmission is normal in stHt31 treated hippocampal slices
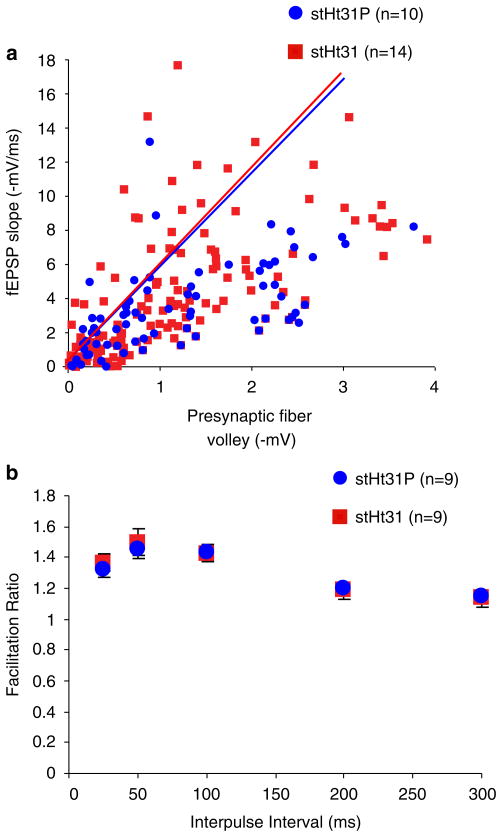
To examine the role of PKA anchoring in hippocampal synaptic plasticity, we bath-applied a membrane-permeable (stearated) form of the Ht31 peptide (stHt31) to hippocampal slices. Stearated Ht31P (stHt31P), a peptide identical to stHt31 except for proline substitutions to prevent binding to PKA, was used as negative control (Carr et al., 1992; Vijayaraghavan et al., 1997). At the Schaffer collateral-CA1 synapses, input-output properties, as assessed by the scatter plots of fEPSPs and their corresponding presynaptic fiber volley amplitudes, were not statistically different between stHt31- (n = 15 slices) and stHt31P- (n = 10) treated slices (F1, 229 = 1.37, p>0.05) (Fig. 1a). Paired-pulse facilitation (PPF), the short-term enhancement of synaptic efficacy following delivery of two closely spaced stimuli that is sensitive to alterations in presynaptic function (Manabe et al., 1992), was not significantly different between the two peptide treatments (n = 9 for each) at interpulse intervals from 25 to 300 ms (F1, 89 = 0.027, p>0.05) (Fig. 1b), suggesting that stHt31 treatment does not alter presynaptic function.
Fig. 1.
Measures of basal synaptic transmission are normal in slices treated with stHt31. (a) Input–output curves illustrating each presynaptic fiber volley amplitude with its corresponding initial fEPSP at various stimulus intensities are not different between hippocampal slices from C56BL/6J mice treated with stHt31 (10 μM) and stHt31P (10 μM). (b) Paired-pulse facilitation, the short-term enhancement of synaptic efficacy following delivery of two closely spaced stimuli, is not significantly different in slices treated with stHt31 and stHt31P.
Inhibition of PKA anchoring impairs synaptically activated L-LTP
Because changing the strength of neuronal connections in subsets of synapses likely requires PKA signals that are restricted to these synapses, we specifically examined whether PKA anchoring is required for a PKA-dependent form of L-LTP induced synaptically at Schaffer collateral-CA1 synapses by four 1 s, 100 Hz trains of electrical stimuli. We observed that L-LTP was impaired by 10 μM stHt31 peptide treatment relative to 10 μM stHt31P control peptide treatment (n = 6 slices for each, p<0.05 in the post-hoc test) (Fig. 2a).
Furthermore, impairment in this form of L-LTP was dependent on the dosages of stHt31 (F3, 539 = 8.18, p<0.05; main effect of dose). During the period between 100 and 120 min post-tetanus, the average fEPSPs were 203 ± 34% for the stHt31P control group, 183 ± 19% for the 1 μM stHt31 group, 130 ± 11% for the 5 μM stHt31 group, and 106 ± 6% for the 10 μM stHt31 group (Fig. 2b). Compared to the stHt31P treatment group, 1 μM stHt31 did not significantly impair L-LTP (n = 6, p>0.05; post-hoc Student-Neumann-Keuls test on each drug concentration). stHt31 (5 μM) significantly impaired L-LTP (n = 6, p<0.05) as compared to stHt31P treatment. According to the post-hoc test, 5 and 10 μM stHt31 treatment groups are also different (p<0.05), suggesting that 5 μM stHt31 does not impair hippocampal L-LTP as severely as with 10 μM stHt31. Baseline fEPSP responses in the absence of L-LTP induction were not altered by stHt31 treatment (n = 3) (Fig. 2a).
In contrast to the observed impairment of L-LTP, a PKA-independent form of synaptic plasticity, induced by one 1 s, 100 Hz train of electrical stimuli that elicited a short lasting form of LTP called E-LTP (Huang and Kandel, 1994), was not impaired by stHt31 inhibition of PKA anchoring (F1, 199 = 0.02, p>0.05) (Fig. 2c).
Inhibition of PKA anchoring does not impair synaptic potentiation that is induced by global activation of cAMP
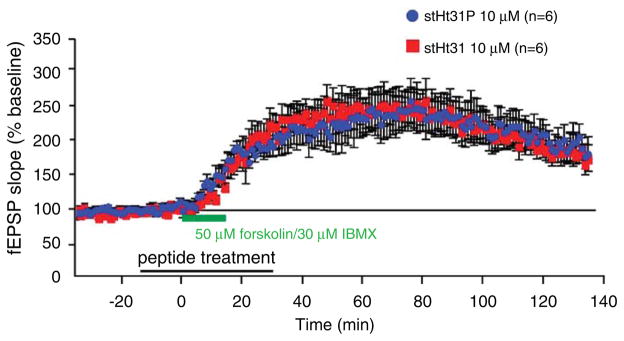
Our observations suggest that hippocampal L-LTP requires highly compartmentalized PKA signaling that is sensitive to inhibition of PKA anchoring. Synaptic potentiation caused by the global elevation of cAMP may not require restricted microdomains of PKA signals at synapses and may not need the spatial signaling specificity conferred by PKA anchoring to AKAPs. Therefore, we examined whether PKA anchoring is essential for a PKA-dependent form of LTP (Woo et al., 2002) that is induced by the cell-wide activation of cAMP. We observed that long lasting potentiation induced by forskolin (50 μM) and IBMX (30 μM) treatment was not statistically different between the stHt31 treatment group and the stHt31P control group (n = 6 slices for each, F1, 239 = 0.19, p>0.05) (Fig. 3a).
Fig. 3.
Pharmacological inhibition of PKA anchoring does not impair potentiation induced by forskolin/IBMX treatment. Acute application of 50 μM forskolin and 30 μM IBMX for 15 min (indicated by green bar) elicited potentiation that is unaffected by stHt31 treatment as compared to stHt31P treatment (indicated by black bar).
Inhibition of PKA anchoring impairs synaptic tagging
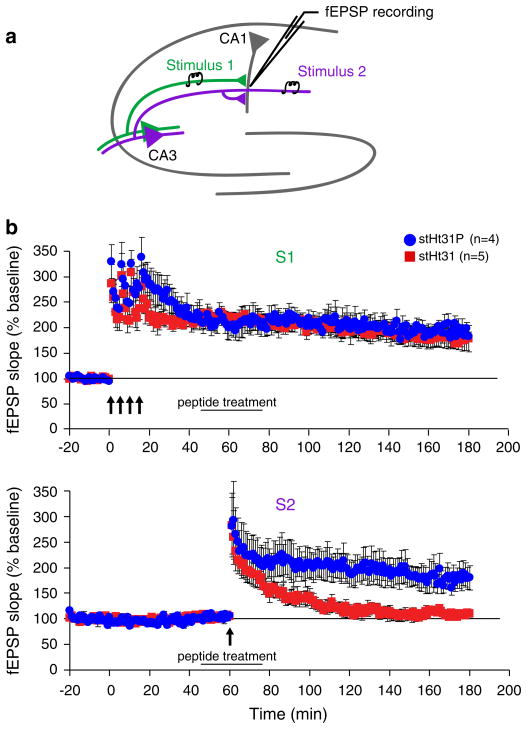
Because tagging synapses for long-term changes involves PKA activity (Barco et al., 2002; Young et al., 2006) and is specific to subsets of synapses, we next examined whether PKA anchoring is required for synaptic tagging and capture processes. Two stimulating electrodes were placed on either side of the recording electrode, to elicit two independent input pathways (S1 and S2) to the same population of postsynaptic neurons (Fig. 4a). The independence of these two sets of inputs was confirmed by the absence of paired pulse facilitation when the two pathways were stimulated in succession at a 50ms interval. A “strong” stimulation protocol consisting of four 1 s, 100 Hz stimulus trains (4-train) spaced five minutes apart was used to induce L-LTP in pathway S1 (Fig. 4b, top panel). One hour after the induction of L-LTP, pathway S2 was tetanized by a “weak,” single-burst stimulation protocol consisting of a single 1 s, 100 Hz train that normally induces a short-lasting form of E-LTP (Huang and Kandel, 1994) (also see Fig. 2c). However, when this weak stimulus was delivered 1 h after L-LTP induction in the other pathway, stable potentiation was elicited that persisted for several hours, a process referred to as synaptic tagging and capture (Frey and Morris, 1997). In the presence of the control stHt31P peptide, pairing weak stimulation in S2 1 h after strong stimulation in S1 induced long-lasting strengthening in pathway S2 (Fig. 4b, bottom panel). In contrast, stHt31 treatment during weak stimulation to S2 impaired long-lasting changes in fEPSP in S2 (Fig. 4b, bottom panel) and is significantly different from stHt31P treatment (F1, 179 = 20.21, p<0.01). It is interesting to note that application of stHt31 1 h after strong stimulation in S1 does not impair L-LTP in S1 (F1, 179 = 0.61, p>0.05), suggesting that the requirement for anchored PKA signaling falls during the induction of L-LTP.
Fig. 4.
Pharmacological inhibition of PKA anchoring impairs synaptic tagging and capture. (a) For the synaptic tagging and capture experiments, two stimulating electrodes were positioned along the Schaffer collateral fiber pathways in such a way as to activate two separate sets of stimulus inputs (S1 and S2) onto the same postsynaptic population of neurons in CA1. (b) In S1, L-LTP induced by four 1 s, 100 Hz trains of electrical stimuli is normal in stHt31-treated slices (duration indicated by black bar), when the peptide is applied at a later time point (30 min) after L-LTP induction. In S2, weak stimulation (one 1 s, 100 Hz train) in the presence of the stHt31P control peptide resulted in long-lasting potentiation. Stearated Ht31 treatment during weak stimulation in S2 impairs this long-lasting potentiation.
Discussion
The cellular mechanisms facilitating pathway specificity, where only synapses that receive tetanizing stimuli remain potentiated over time (Nguyen et al., 1994), likely requires spatial compartmentalization of signal transduction pathways that are involved in L-LTP. We present evidence for this compartmentalization by demonstrating that pharmacological inhibition of PKA anchoring impairs a synaptically activated form of synaptic plasticity but spares another PKA-dependent form of plasticity induced by global elevation of cAMP. The co-application of forskolin and IBMX will activate adenylyl cyclases and remove barriers to cAMP diffusion formed by phosphodiesterases, resulting in cell-wide activation of cAMP. This global cAMP signal is able to induce a long-lasting form of LTP that is not dependent on microdomains of anchored PKA. In contrast, L-LTP induced in subsets of synapses by four trains of high-frequency stimulation is impaired by stHt31, suggesting that PKA anchoring allows micro-domains of PKA signals to be discretely activated following synaptic L-LTP stimulation. Further, application of stHt31 1 h after strong stimulation in S1 does not impair L-LTP in S1 (Fig. 4b, top panel), indicating that there is a critical time window during the induction of L-LTP in which PKA anchoring is essential for the expression of L-LTP. These results highlight the critical importance of PKA anchoring in the local activation of PKA required to restrict synaptic changes to specific subsets of synapses.
The process of tagging specific synapses to capture products of plasticity-related genes has been shown to require PKA activity (Barco et al., 2002; Young et al., 2006). We extend this finding and demonstrate that inhibition of PKA anchoring impairs the process of synaptic tagging and capture. The idea of synaptic tagging and capture reconciles two fundamental properties of L-LTP: that it requires the products of transcription and translation, and that it exhibits pathway specificity. Only tagged synapses have the ability to capture plasticity-related molecules that are generated by transcription and translation. Thus the spatial specificity of these signaling events is critical to the expression of long-term synaptic changes. We demonstrate that pharmacological inhibition of PKA anchoring prevents synaptic tagging and capture. Therefore, the impairment in L-LTP caused by stHt31 (Fig. 2b) can be attributed, at least in part, to this failure in synaptic tagging and capture when PKA anchoring is disrupted. By varying the order of strong and weak stimulation in S1 and S2 and applying the stHt31 peptide during different time windows, future experiments will delineate whether PKA anchoring is selectively required for the synaptic tagging or the synaptic capture process.
We have demonstrated that anchoring of PKA to AKAPs is a critical component of the signal transduction pathways underlying hippocampal L-LTP. AKAPs such as AKAP79/150 and Yotiao have been implicated in anchoring PKA, along with other kinases and phosphatases, to synaptic proteins such as NMDA-and AMPA-receptors that play crucial roles in synaptic plasticity (Colledge et al., 2000; Westphal et al., 1999). However, in order to identify the specific AKAPs involved in providing compartmentalized PKA signaling during hippocampal L-LTP, one must selectively disrupt the interaction of PKA with each specific AKAP by introducing site-specific mutations in the PKA-binding domains of that particular AKAP. The challenge is to accomplish this disruption for each potential AKAP in a cell-type and region specific manner. Regulation of channel currents, receptor trafficking, gene transcription, protein synthesis, and neuronal morphology are just some of the potential mechanisms and sites of action for PKA during synaptic plasticity (for reviews see Nguyen and Woo, 2003; Bauman et al., 2004). These diverse pathways involve PKA targets that are located in different subcellular compartments and in pre- and post-synaptic neurons, highlighting the importance of PKA anchoring in mediating synaptic plasticity. Thus, future experiments will take advantage of proteomic, biochemical and electrophysiological techniques to identify the cellular and molecular components by which PKA anchoring regulates synaptic tagging and capture.
Acknowledgments
This research was supported by National Institute of Health Grant MH60244 (to T. Abel), predoctoral Training Program fellowship in Neuropsychopharmacology T32MH014654 (to C.B. McDonough, Dr. Irwin Lucki, PI), a Ruth L. Kirschstein NRSA Research Training Grant 5F31MH069136-02 (to C.B. McDonough) and the Human Frontier Science Program Research Grant RGP0001/2005-C (to T. Abel). T. Abel is a David and Lucile Packard Fellow.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Barco A, Alarcon JM, Kandel ER. Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell. 2002;108:689–703. doi: 10.1016/s0092-8674(02)00657-8. [DOI] [PubMed] [Google Scholar]
- Barco A, Patterson S, Alarcon JM, Gromova P, Mata-Roig M, Morozov A, Kandel ER. Gene expression profiling of facilitated L-LTP in VP16-CREB mice reveals that BDNF is critical for the maintenance of LTP and its synaptic capture. Neuron. 2005;48:123–137. doi: 10.1016/j.neuron.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Bauman AL, Goehring AS, Scott JD. Orchestration of synaptic plasticity through AKAP signaling complexes. Neuropharmacology. 2004;46:299–310. doi: 10.1016/j.neuropharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Lømo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DW, Stofko-Hahn RE, Fraser ID, Cone RD, Scott JD. Localization of the cAMP-dependent protein kinase to the postsynaptic densities by A-kinase anchoring proteins. Characterization of AKAP 79. J Biol Chem. 1992;267:16816–16823. [PubMed] [Google Scholar]
- Colledge M, Dean RA, Scott GK, Langeberg LK, Huganir RL, Scott JD. Targeting of PKA to glutamate receptors through a MAGUK-AKAP complex. Neuron. 2000;27:107–119. doi: 10.1016/s0896-6273(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol. 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza NJ, Dohadwalla AN, Reden J. Forskolin: a labdane diterpenoid with antihypertensive, positive inotropic, platelet aggregation inhibitory, and adenylate cyclase activating properties. Med Res Rev. 1983;3:201–219. doi: 10.1002/med.2610030205. [DOI] [PubMed] [Google Scholar]
- Fink MA, Zakhary DR, Mackey JA, Desnoyer RW, Apperson-Hansen C, Damron DS, Bond M. AKAP-mediated targeting of protein kinase A regulates contractility in cardiac myocytes. Circ Res. 2001;88:291–297. doi: 10.1161/01.res.88.3.291. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- Frey U, Morris RG. Weak before strong: dissociating synaptic tagging and plasticity-factor accounts of late-LTP. Neuropharmacology. 1998;37:545–552. doi: 10.1016/s0028-3908(98)00040-9. [DOI] [PubMed] [Google Scholar]
- Frey U, Krug M, Reymann KG, Matthies H. Anisomycin, an inhibitor of protein synthesis, blocks late phases of LTP phenomena in the hippocampal CA1 region in vitro. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Recruitment of long-lasting and protein kinase A-dependent long-term potentiation in the CA1 region of hippocampus requires repeated tetanization. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- Kalderon AE, Dobbs JW, Greenberg ML. Localization of 3′,5′-cyclic adenosine monophosphate phosphodiesterase (cAMP-PDEase) activity in isolated bovine thyroid plasma membranes. Histochemistry. 1980;65:277–289. doi: 10.1007/BF00493177. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Manabe T, Renner P, Nicoll RA. Postsynaptic contribution to long-term potentiation revealed by the analysis of miniature synaptic currents. Nature. 1992;355:50–55. doi: 10.1038/355050a0. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Matthies H, Reymann KG. Protein kinase A inhibitors prevent the maintenance of hippocampal long-term potentiation. Neuroreport. 1993;4:712–714. doi: 10.1097/00001756-199306000-00028. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Woo NH. Regulation of hippocampal synaptic plasticity by cyclic AMP-dependent protein kinases. Prog Neurobiol. 2003;71:401–437. doi: 10.1016/j.pneurobio.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER. Requirement of a critical period of transcription for induction of a late phase of LTP. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. Transient activation of cyclic AMP-dependent protein kinase during hippocampal long-term potentiation. J Biol Chem. 1996;271:30436–30441. doi: 10.1074/jbc.271.48.30436. [DOI] [PubMed] [Google Scholar]
- Rotenberg A, Abel T, Hawkins RD, Kandel ER, Muller RU. Parallel instabilities of long-term potentiation, place cells, and learning caused by decreased protein kinase A activity. J Neurosci. 2000;20:8096–8102. doi: 10.1523/JNEUROSCI.20-21-08096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajikumar S, Frey JU. Late-associativity, synaptic tagging, and the role of dopamine during LTP and LTD. Neurobiol Learn Mem. 2004;82:12–25. doi: 10.1016/j.nlm.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Sajikumar S, Navakkode S, Sacktor TC, Frey JU. Synaptic tagging and cross-tagging: the role of protein kinase Mzeta in maintaining long-term potentiation but not long-term depression. J Neurosci. 2005;25:5750–5756. doi: 10.1523/JNEUROSCI.1104-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan S, Goueli SA, Davey MP, Carr DW. Protein kinase A-anchoring inhibitor peptides arrest mammalian sperm motility. J Biol Chem. 1997;272:4747–4752. doi: 10.1074/jbc.272.8.4747. [DOI] [PubMed] [Google Scholar]
- Westphal RS, Tavalin SJ, Lin JW, Alto NM, Fraser ID, Langeberg LK, Sheng M, Scott JD. Regulation of NMDA receptors by an associated phosphatase-kinase signaling complex. Science. 1999;285:93–96. doi: 10.1126/science.285.5424.93. [DOI] [PubMed] [Google Scholar]
- Wojcikiewicz RJ, Dobson PR, Brown BL. Muscarinic acetylcholine receptor activation causes inhibition of cyclic AMP accumulation, prolactin and growth hormone secretion in GH3 rat anterior pituitary tumour cells. Biochim Biophys Acta. 1984;805:25–29. doi: 10.1016/0167-4889(84)90032-6. [DOI] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space and time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, Nguyen PV. Genetic and pharmacological demonstration of differential recruitment of cAMP-dependent protein kinases by synaptic activity. J Neurophysiol. 2000;84:2739–2745. doi: 10.1152/jn.2000.84.6.2739. [DOI] [PubMed] [Google Scholar]
- Woo NH, Abel T, Nguyen PV. Genetic and pharmacological demonstration of a role for cyclic AMP-dependent protein kinase-mediated suppression of protein phosphatases in gating the expression of late LTP. Eur J Neurosci. 2002;16:1871–1876. doi: 10.1046/j.1460-9568.2002.02260.x. [DOI] [PubMed] [Google Scholar]
- Woo NH, Duffy SN, Abel T, Nguyen PV. Temporal spacing of synaptic stimulation critically modulates the dependence of LTP on cyclic AMP-dependent protein kinase. Hippocampus. 2003;13:293–300. doi: 10.1002/hipo.10086. [DOI] [PubMed] [Google Scholar]
- Young JZ, Nguyen PV. Homosynaptic and hetero-synaptic inhibition of synaptic tagging and capture of long-term potentiation by previous synaptic activity. J Neurosci. 2005;25:7221–7231. doi: 10.1523/JNEUROSCI.0909-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JZ, Isiegas C, Abel T, Nguyen PV. Metaplasticity of late-LTP: A critical role for PKA in synaptic tagging. Eur J Neurosci. 2006 doi: 10.1111/j.1460-9568.2006.04707.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]