Abstract
Scent marking and over-marking are important forms of communication between the sexes for many terrestrial mammals. Over the course of three experiments, we determined whether the amount of time individuals investigate the scent marks of opposite-sex conspecifics is affected by four days of olfactory experience with those conspecifics. In experiment 1, female meadow voles, Microtus pennsylvanicus, spent more time investigating the scent mark of the novel male conspecific than that of the familiar male donor, whereas male voles spent similar amounts of time investigating the scent mark of the familiar female and a novel female conspecific. In experiment 2, voles were exposed to a mixed-sex over-mark in which subjects did not have four days of olfactory experience with either the top-scent donor or the bottom-scent donor. During the test phase, male and female voles spent more time investigating the scent mark of the opposite-sex conspecific that provided the top-scent mark than that of a novel, opposite-sex conspecific. Male and female voles spent similar amounts of time investigating the scent mark of the bottom-scent donor and that of a novel opposite-sex conspecific. In experiment 3, voles were exposed to a mixed-sex over-mark that contained the scent mark of an opposite-sex conspecific with which they had four days of olfactory experience. During the test phase, male voles spent more time investigating the mark of the familiar, top-scent female than the scent mark of a novel female donor but spent similar amounts of time investigating the mark of the familiar, bottom-scent female and that of a novel female donor. In contrast, female voles spent more time investigating the mark of a novel male donor than that of either the familiar, top-scent male or that of the familiar, bottom-scent male. The sex differences in the responses of voles to scent marks and mixed-sex over-marks are discussed in relation to the natural history and non-monogamous mating system of meadow voles.
Keywords: over-marks, voles, olfactory experience, odor communication
Introduction
Not all social interactions have to be directly witnessed by individuals in order for those individuals to learn information about the participants (Kappeler 1998; Valone 2007; Amy & Leboucher 2009). When animals encounter over-marks, overlapping scent marks of two different conspecifics, they can learn various identifying features about the top-scent donor and the bottom-scent donor (Johnston et al. 1994; Ferkin et al. 1999; Johnston 2003). Individuals will typically encounter over-marks of two same-sex conspecifics in areas that are visited or contested by rival conspecifics (Ferkin & Pierce 2007). Studies on golden hamsters, pygmy lorises, and voles have reported on the responses of individuals to same-sex over-marks. The studies on voles and hamsters shared similar methods to examine the response of conspecifics to over-marks involving an exposure phase followed by a test phase (Johnston et al. 1994, 1995, 1997a, b; Ferkin et al. 1999; Woodward et al. 1999; Fisher et al. 2003). For example, during the exposure phase, subject voles were presented with an over-mark containing the scent marks of two same-sex conspecifics. After exposure to the over-mark, the subjects were presented simultaneously with the scent mark of the donor that provided the top-scent mark and the scent mark of the donor that provided the bottom-scent mark during the exposure phase; the two scent marks were separate, distinct, and not overlapping. During the test phase, voles spent more time investigating the mark of the top-scent donor than the bottom-scent donor (Johnston et al. 1997a, b; Ferkin et al. 1999). Further experiments on voles and hamsters have shown that the top-scent mark did not mask the bottom-scent mark (Woodward et al. 1999, 2000; Johnston 2003; Pierce et al. 2007).
Terrestrial mammals may also encounter the overlapping scent marks of a same-sex conspecific and an opposite-sex conspecific (Hurst 1990; Ferkin et al. 2004; Ferkin 2010). However, little is known about how conspecifics respond to these mixed-sex over-marks, and whether individuals exposed to mixed-sex over-marks later spend more time investigating the mark of the top-scent donor than the mark of the bottom-scent donor. A study on meadow voles, Microtus pennsylvanicus, found that after exposure to a mixed-sex over-mark, individuals spent more time investigating the scent mark of the opposite-sex donor than that of the same-sex donor. The preference for the mark of the opposite-sex conspecific was independent of whether that donor was previously the top- or bottom-scent mark of the over-mark (Woodward et al. 2000). This finding led to the speculation that mixed-sex over-marks may be a signal associated with finding a mate (Ferkin & Pierce 2007).
For many terrestrial mammals, the home ranges of opposite-sex conspecifics overlap (Madison 1980a; Swihart & Slade 1989; Erlinge et al. 1990). Consequently, individuals may have olfactory experience with the scent marks of neighboring opposite-sex conspecifics and become familiar with them. It has been well established that familiarity affects interactions and preferences between same- and opposite-sex conspecifics (Bekoff 1981; Kareem & Barnard 1982; Blaustein et al. 1987; Ferkin 1992; Cheetham et al. 2008). Prairie voles, a socially monogamous species, displayed partner preferences for familiar opposite-sex conspecifics over novel opposite-sex conspecifics. In contrast, montane voles and meadow voles, two non-monogamous species, showed partner preferences for novel opposite-sex conspecifics over familiar opposite-sex conspecifics (Ferguson et al. 1986; Shapiro et al. 1986; Williams et al. 1992; Salo & Dewsbury 1995; Ricankova et al. 2007). It is not clear if these partner preferences predict the responses non-monogamous voles may display when they encounter scent marks and over-marks of familiar opposite-sex conspecifics.
The present study had three goals. The first goal was to test the hypothesis that olfactory experience with a scent donor affects how opposite-sex conspecifics respond to their scent marks. The second goal was to test the hypothesis that animals respond differently to the scent marks of opposite-sex conspecifics when these marks are encountered separately or when they are first encountered as over-marks and then separately. The third goal was to test the hypothesis that four days of olfactory experience with an opposite-sex conspecific affects how a meadow vole responds to its scent mark after it is first encountered as part of a mixed-sex over-mark. We tested these hypotheses by first exposing voles to the scent marks or over-marks of a familiar, opposite-sex donor, and later testing them for their responses to the simultaneous presentation of the scent mark of that donor and the scent mark of a novel, opposite-sex conspecific.
We used meadow voles as the model species for testing this hypothesis. In nature, male and female voles have overlapping home ranges, and male voles may enter the territories of and encounter the scent marks of multiple females (Madison 1980a). Male and female meadow voles mate with multiple individuals (Madison 1980b; Boonstra et al. 1993). However, male and female voles have limited interactions with one another (Dewsbury 1990) and individuals may or may not have olfactory experience with the scent marks of nearby opposite-sex conspecifics.
General Materials and Methods
Animals
We used meadow voles that were descendants of those captured in Pennsylvania, Kentucky, and Ohio, USA. Every 18–24 months, the voles in the colony were mated with captured free-living voles. In this study, meadow voles were born and raised under a long photoperiod (14:10 h, L: D, lights on at 0700h CST). All voles were weaned between 19–21 days of age, housed with littermates until 33–36 days of age, and thereafter housed singly in clear plastic cages (18 × 12.5 × 10 cm). Cages contained cotton nesting material, water, and food (Harlan Teklad Rodent Diet, #8640, Madison, WI, USA). Meadow voles were housed in the animal facility at the University of Memphis. Female meadow voles are induced ovulators and do not undergo regular estrus cycles (Milligan 1982; Keller 1985). Adult female voles born and reared in long photoperiod are sexually receptive (Meek & Lee 1993). Long-photoperiod meadow voles respond preferentially to the scent marks of opposite-sex conspecifics (Ferkin & Johnston 1995; Ferkin et al. 2004). Voles used in this study were 3–7 mo-old, sexually mature, but not sexually experienced. All testing was carried out between 0900 and 1200 h CST.
Experiment 1— Do meadow voles spend different amounts of time investigating the scent mark of a familiar opposite-sex conspecific compared to that of a novel opposite-sex conspecific?
Olfactory Experience
In this study, we used methods detailed by Ferkin (1988) to allow voles to gain olfactory experience with a particular opposite-sex conspecific. On day 1, all cotton nesting material was removed from the cages of the scent donors and replaced with fresh cotton. The cotton nesting material remained in each cage approximately 24 h. On day 2, this cotton nesting material was traded with the cotton belonging to an opposite-sex conspecific. On day 3, the traded cotton was discarded and replaced with fresh cotton. The procedure was repeated until the voles' cotton had been traded with their selected partners' cotton four times. On day 9, voles selected as subjects were tested.
Experimental Design
In this experiment, 30 male and 30 female voles (subjects) were allowed four days of olfactory experience with an opposite-sex conspecific. After they gained olfactory experience, these subjects were given an opportunity to investigate either: (a) the scent marks of an opposite-sex conspecific with which they had four days of olfactory experience and a novel, opposite-sex conspecific (n = 15 male subjects and n = 15 female subjects), or (b) the scent marks of two novel, opposite-sex conspecifics (n = 15 male subjects and n = 15 female subjects); these subjects also had four days of olfactory experience with an opposite-sex conspecific, but were tested with the scent marks of two novel, opposite-sex conspecifics. All subjects underwent a single preference test with a unique pair of scent donors. The novel scent donors were individuals with which the investigating voles had no prior olfactory experience.
We followed the methods for measuring the amount of time voles investigated the scent marks of conspecifics employed by Ferkin and Johnston (1995). Briefly, we presented voles with a glass slide (2.5 × 7.6 cm) that contained the feces scent marks of two opposite-sex conspecifics. Feces scent marks are sexually discriminable, deposited by voles when they scent mark and over-mark, and found in their nesting material (Ferkin & Johnston 1995; Ferkin et al. 2004). We divided the glass slide into three equal sections (each 2.5 cm in length); one end section contained the feces scent mark of a particular opposite-sex scent donor, and the other end section contained the feces scent mark of another opposite-sex scent donor. The middle section of the slide contained no scent marks. To deposit the scent marks on the slide, we dragged one or two fresh fecal boli from one scent donor across the left-end section of a clean glass microscope slide and one or two fecal boli from the other scent donor across the right-end section of the same slide. One min separated the deposition of the scent marks of the two donors on the slide. The placement of a particular donor's scent mark on the left or right side of the slide was random. The scent marks were roughly the same size, approximately 1.2 cm × 0.3 cm (l × w). After both scent marks were placed on the slide, we waited 5 min before we suspended the slide in the home cage of the subject. We recorded for 5 min the amount of time that male and female subjects licked or sniffed (the subject's nose came within 2 cm) each scent mark on the slide (Ferkin & Johnston 1995). The observer was blind to the position of the donors' scent marks on the slide. Each test slide was used once and then discarded.
To be included in the data analysis, subjects had to have investigated the scent marks of both donors and spent more time investigating the scent marks of the two donors than they did investigating the clean portion of the slide (Ferkin & Johnston 1995). No subjects were excluded from the data analysis in this and subsequent experiments.
Statistics
To analyze the data, we used a two-way analysis of variance (ANOVA) where the main factors were sex of the subject and sex of the donors. To do so, we created two continuous variables (Pierce et al. 2007). One variable was the quotient of the amount of time voles spent investigating the scent mark of the vole with which they had four days of olfactory experience (X) divided by the time they spent investigating the scent mark of the novel vole (Y1) plus the mark of the vole with which they had four days of olfactory experience (X). The resulting formula would be X/Y1+X. The other variable was the quotient of the amount of time voles spent investigating the scent mark of the novel vole 1 or novel vole 2 divided by the time they spent investigating the scent marks of the novel vole 1 plus the time they spent investigating the scent marks of novel vole 2. The resulting formula would be Y1/Y1+Y2. We performed post hoc multiple pairwise comparisons using the Holm-Sidák method to determine if differences existed between males and females in the amount of time they spent investigating the scent marks of the two scent donors. Statistically significant differences were accepted at p < 0.05. Kolmogorov-Smirnov tests indicated that the data were not normally distributed. Thus, the data were arcsine square root transformed for statistical analysis (SigmaPlot 11.0). The non-transformed data are presented in figure 1.
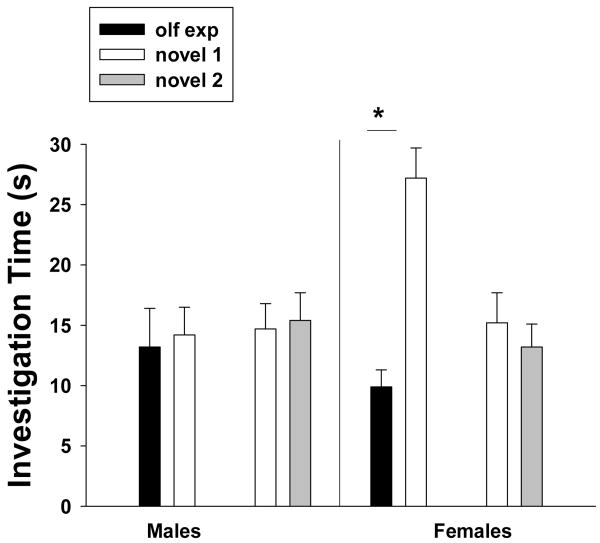
Figure 1.
The amount of time (mean ± sem) that male and female meadow voles spent investigating the scent mark of an opposite-sex conspecific with which they had four days of olfactory experience (olf exp) and that of a novel, opposite-sex conspecific as well as the time they spent investigating the scent marks of two different novel, opposite-sex conspecifics. The scent marks used in the pairings were encountered separately and were not part of a mixed-sex over-mark. * indicates a statistical difference in investigation time between scent-mark pairings (p < 0.05; multiple paired comparisons, Holm-Sidák Method).
Experiment 2— After exposure to a mixed-sex over-mark, do meadow voles differ in the amount of time that they spend investigating the scent mark of the opposite-sex conspecific that provided the top- or bottom-scent mark compared to that of a novel opposite-sex conspecific?
Experimental Design
Our experimental design involved two phases, the exposure phase and the test phase, both of which took place in the subjects' home cages. This exposure-testing procedure matches the one used by Woodward et al. (2000). Specifically, we determined the amount of time that voles spent investigating the opposite-sex donor that provided top- or bottom-scent mark compared to the time voles spent investigating the scent mark of a novel, opposite-sex donor.
Exposure phase
During the exposure phase of this experiment, 30 male and 30 female subjects were presented with a mixed-sex over-mark; subject voles had no prior olfactory experience with the donors of the mixed-sex over-mark. Fifteen male and 15 female voles were exposed to a mixed sex over-mark in which the top-scent donor was an opposite-sex conspecific and the bottom-scent donor was a same-sex conspecific. The remaining 15 male and 15 female subjects were exposed to an over-mark in which the top-scent donor was a same-sex conspecific and the bottom-scent donor was an opposite-sex conspecific.
We used the feces scent mark of an opposite-sex conspecific and that of a same-sex conspecific to create the mixed-sex over-mark. To do so, we collected fresh fecal boli from each scent donor before each exposure. One or two fecal boli from a scent donor were dragged across the center of a glass microscope slide (2.5 × 7.6 cm). Five min later, a similar amount of the feces from another donor was dragged over the top of the previously deposited scent mark, such that the two marks overlapped, and the resulting configuration was a “+” shape. Each feces scent mark was approximately 0.4–0.5 cm in length and 0.1–0.2 cm in width. Thus, we were able to control for the size of the scent marks (Ferkin et al. 1999; Woodward et al. 2000).
Five min after the second scent mark was placed on the slide, the slide was placed into the subject's home cage against the wall opposite the subject's nest. The slide was suspended 2 cm above the substrate by a clean metal clip and hook. Subjects were exposed to this slide for 5 min. This slide was placed in the cage of only one subject and then discarded after the exposure phase was completed; the exposure slide was not used during the test phase. In all observations, the observer was blind to the identity of the top- and bottom-scent mark donors.
Test Phase
The test phase began 5 min after completion of the 5-min exposure phase and followed the methods used in previous over-marking studies (Ferkin et al. 1999; Woodward et al. 2000). We presented the 30 male and 30 female subjects used in the exposure phase of this experiment with a glass slide (2.5 × 7.6 cm) that contained the feces scent marks of the opposite-sex conspecific that provided the top- or bottom-scent mark during the exposure phase on one edge of the slide and on the other edge the scent mark of a novel, opposite-sex donor. The middle section of the slide contained no scent marks. Thus, 15 male and 15 female subjects were given a choice between the scent mark of a novel opposite-sex conspecific and the scent mark of the opposite-sex conspecific that provided the top-scent mark. The remaining 15 male and 15 female subjects were given a choice between the scent mark of a novel opposite-sex donor and the scent mark of the opposite-sex conspecific that provided the bottom-scent mark.
To deposit the scent marks on the slide, we dragged one or two fresh fecal boli from one scent donor across the left side of a clean glass microscope slide and one or two fecal boli from the other scent donor across the right side of the same slide (Ferkin et al. 1999). One min separated the deposition of the scent marks of the two donors on the slide. The placement of a particular donor's scent mark on the left or right side of the slide was random. The scent marks were roughly the same size, approximately 1.2 cm × 0.3 cm (l × w). After both scent marks were placed on the slide, we waited 5 min before we suspended the slide in the home cage of the subject. For 5 min, we recorded the amount of time that male and female subjects licked or sniffed (the subject's nose came within 2 cm) each scent mark on the slide (Ferkin et al. 1999; Woodward et al. 1999, 2000). The observer was blind to the left-side or right-side position of the donors' scent marks on the slide. Each test slide was used once and then discarded. Subjects investigated the exposure slide for an average of 58.6 ± 15.2 s.
Statistics
The data were analyzed by using a two-way ANOVA with the main factors as sex of the subject and type of over-mark. Post hoc multiple pairwise comparisons used the Holm-Sidák method. We also created a continuous variable, which was the quotient of the amount of time voles spent investigating the scent mark of the opposite-sex conspecific that was part of the over-mark (the top- or bottom-scent donor) divided (/) by the time they spent investigating the scent mark of the novel vole plus (+) the time voles spent investigating the scent mark of the top- or bottom-scent donor. Kolmogorov-Smirnov tests indicated that the data were not normally distributed. Thus, the data were arcsine square root transformed for statistical analysis (SigmaPlot 11.0). The non-transformed data are presented in figure 2.
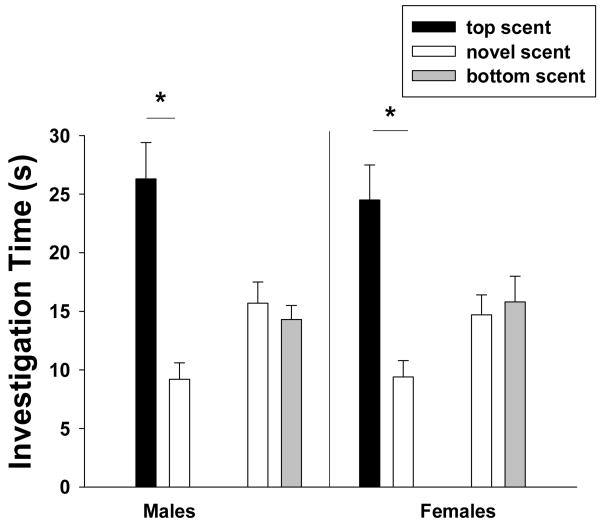
Figure 2.
The amount of time (mean ± sem) that male and female meadow voles spent investigating the scent mark of the top-scent, opposite-sex donor of the mixed-sex over-mark and that of a novel, opposite-sex conspecific as well as the time they spent investigating the bottom-scent, opposite-sex donor of a mixed-sex over-mark and that of a novel, opposite-sex conspecific. * indicates a statistical difference in investigation time between scent-mark pairings (p < 0.05; multiple paired comparisons, Holm-Sidák Method).
Experiment 3— After exposure to a mixed-sex over-mark, do meadow voles differ in the amount of time that they spend investigating the scent mark of a familiar opposite-sex conspecific that provided the top- or bottom-scent mark compared to that of a novel opposite-sex conspecific?
Exposure Phase
The experimental design of experiment 3 is identical to the exposure-testing procedure used in experiment 2 with one notable exception. During the exposure phase, 30 male and 30 female subjects were presented with a mixed-sex over-mark with which they had four days of olfactory experience with the opposite-sex donor of that over-mark; the subjects had no prior olfactory experience with the same-sex conspecific donor of the over-mark. Thus, 15 of these male subjects and 15 of these female subjects were presented with a slide containing a mixed sex over-mark in which the familiar opposite-sex conspecific was the top-scent donor. The remaining 15 male and 15 female subjects were exposed to a mixed-sex over-mark in which the familiar opposite-sex conspecific was the bottom-scent donor. Subjects investigated the exposure slide for an average of 105.1 ± 9.8 s.
Test Phase
The test phase began 5 min after completion of the exposure phase and was nearly identical to the test phase detailed in experiment 2 with these notable exceptions. We presented the 30 male and 30 female subjects used in the exposure phase of this experiment with a glass slide that contained the feces scent marks of the familiar opposite-sex conspecific that provided the top-scent mark or the bottom-scent mark during the exposure phase on one edge of the slide and on the other edge the scent mark of a novel opposite-sex donor. The middle section of the slide contained no scent marks. During the 5-min test, we recorded the amount of time that 15 of these male subjects and 15 of these female subjects investigated the scent mark of a novel, opposite-sex conspecific and the scent mark of the familiar, top-scent donor. We also recorded the amount of time that the remaining 15 male and 15 female subjects spent investigating the scent mark of a novel donor and the scent mark of the familiar, bottom-scent donor.
Statistics
The data were analyzed with a 2 × 2 factorial design ANOVA and post hoc multiple pairwise comparisons using the Holm-Sidák method. The main effects were sex of subject and scent mark pairing during the test phase (opposite-sex, top-scent donor vs. novel, opposite-sex donor and opposite-sex, bottom-scent donor vs. novel, opposite-sex donor). We also created a continuous variable, which was the quotient of the amount of time voles spent investigating the scent mark of the opposite-sex conspecific that was part of the over-mark (the familiar top- or bottom-scent donor) divided (/) by the time they spent investigating the scent mark of the novel vole plus (+) the time voles spent investigating the scent mark of the top- or bottom-scent donor. Kolmogorov-Smirnov tests indicated that the data were not normally distributed. Thus, the data were arcsine square root transformed for statistical analysis (SigmaPlot 11.0). The non-transformed data are presented in the figure 3.
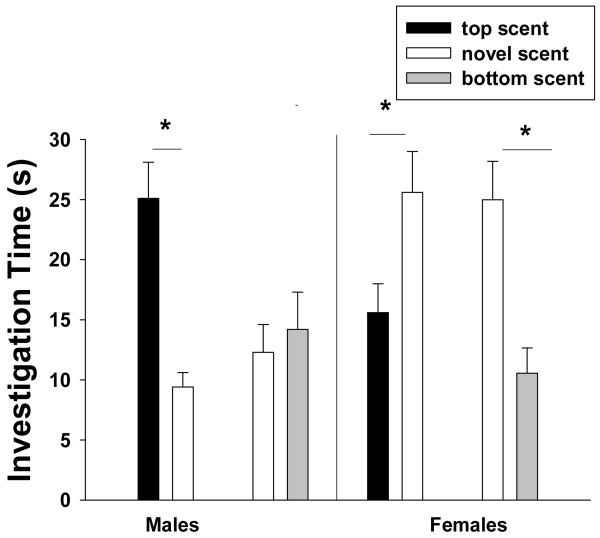
Figure 3.
The amount of time (mean ± sem) that male and female meadow voles spent investigating the scent mark of the opposite-sex, top-scent donor of a mixed-sex over-mark with which they had four days of olfactory experience and that of a novel, opposite-sex conspecific as well as the time they spent investigating the scent mark of the opposite-sex, bottom-scent donor of a mixed-sex over-mark and that of a novel, opposite-sex conspecific. * indicates a statistical difference in investigation time between scent mark pairings (p < 0.05; multiple paired comparisons, Holm-Sidák Method).
Results
Experiment 1
The amount of time that subjects spent investigating the scent marks of two opposite-sex conspecifics was affected by the sex of the subject (F1, 59 = 6.23, p = 0.0154) and their familiarity with the scent donors (F1, 59 = 8.55, p = 0.005). There was a significant interaction between sex of the subject and the familiarity of the scent donor (F1, 59 = 4.07, p = 0. 0482). Post hoc comparisons revealed that female voles spent more time investigating the scent mark of a novel male than the scent mark of a familiar male (Holm-Sidák method, p < 0.05; Fig. 1). In contrast, male voles spent similar amounts of time investigating the scent mark of a familiar female and the scent mark of a novel female (p > 0.05; Fig. 1). Male and female voles spent similar amounts of time investigating the scent marks of two novel, opposite-sex conspecifics (both comparisons, p > 0.05; Fig. 1). Subjects spent 1.3 ± 0.8 s investigating the clean, middle section of the slide.
Experiment 2
The amount of time that subjects spent investigating the scent mark of an opposite-sex donor from the exposure phase and the scent mark of a novel opposite-sex conspecific was not affected by the sex of the subject (F1, 59 = 0.874, p > 0.354), but was affected by the type of mixed-sex over-mark that voles had encountered during the exposure phase (F1, 59 = 12.18, p = 0.001). There was no significant interaction between the main effects (F1, 59 = 0.211, p = 0.647). During the test phase, male and female subjects spent more time investigating the mark of the opposite-sex conspecific that provided the top-scent mark during the exposure phase than the mark of the novel opposite-sex conspecific (Holm-Sidák method, both comparisons p < 0.05; Fig. 2). However, male and female voles spent similar amounts of time investigating the mark of a novel opposite-sex conspecific and the mark of the opposite-sex conspecific that provided the bottom-scent mark during the exposure phase (both comparisons, p > 0.05; Fig. 2). Subjects spent 1.7 ± 0.5 s investigating the clean, middle section of the test slide.
Experiment 3
The amount of time that voles spent investigating the scent mark of the opposite-sex donor from the exposure phase (the vole with which they had four days of olfactory experience) and the scent mark of a novel opposite-sex conspecific was affected by the sex of the subject (F1, 59 = 7.41, p = 0.008) and the type of over-mark they were exposed to during the exposure phase (F1, 59 = 8.33, p = 0.005). There was a significant interaction between the main effects (F1, 59 = 6.84, p = 0.011). During the test phase, male voles spent more time investigating the mark of the familiar, top-scent female than the scent mark of a novel female (Holm-Sidák method, p < 0.05; Fig. 3). However, male voles spent similar amounts of time investigating the mark of the familiar, bottom-scent female and the scent mark of a novel female (p > 0.05; Fig. 3). In contrast, during the test phase, female voles spent more time investigating the mark of a novel male than that of either the familiar, top-scent male or that of the familiar, bottom-scent male (both comparisons, p < 0.05; Fig. 3). Subjects spent 1.4 ± 0.6 s investigating the clean, middle section of the test slide.
Discussion
One goal of this study was to test the hypothesis that olfactory experience with a scent donor affects how opposite-sex conspecifics respond to that donor's scent marks. In experiment 1, meadow voles were exposed simultaneously to the scent marks of an opposite-sex conspecific with which they had olfactory experience and scent marks of a novel opposite-sex conspecific. Female voles preferred the scent marks of the novel male donor to those of the male donor with which they had olfactory experience, the familiar male. Female meadow and montane voles have also expressed a partner preference for a novel male over a familiar male conspecific (Ferguson et al. 1986; Shapiro et al. 1986). Male meadow voles, however, showed no preference between the scent marks of a novel female and those of a familiar female donor. Male meadow and montane voles, however, displayed a partner preference for a novel female conspecific over a familiar female conspecific (Ferguson et al. 1986; Shapiro et al. 1986). Olfactory experience with a scent donor was shown to have mixed effects on scent marking in previously marked areas by mice and river otters (Gilder & Slater 1978; Rostain et al. 2004; Arakawa et al. 2008), but its affects on scent marking in voles are not known. The number of agonistic and amicable behaviors displayed during encounters between male and female meadow voles was also not affected by familiarity between opposite-sex conspecifics (Ferkin 1988). Collectively, these observations suggest that familiarity with an opposite-sex conspecific has different effects on the behavior of male and female meadow voles.
Another goal of the present experiments was to test the hypothesis that animals respond differently to the scent marks of two opposite-sex conspecifics when their marks were encountered separately than when their marks were first encountered as over-marks and then separately. In experiment 2, we exposed voles to mixed-sex over-marks in which they had no prior olfactory experience with either the top- or bottom-scent donors. After exposure to the over-mark, voles spent more time investigating the scent mark of the opposite-sex conspecific that provided the top-scent mark than the scent mark of a novel opposite-sex conspecific that did not provide a scent mark during the exposure phase. Our results augment those obtained by Woodward et al. (2000), who found that after encountering a mixed-sex over-mark, prairie voles and meadow voles later preferred the scent mark of the opposite-sex donor to that of the same-sex donor, independent of whether the opposite-sex donor was encountered first as the top-scent donor or the bottom-scent mark in the over-mark. Interestingly, we found that male and female voles showed no preference between the mark of the bottom-scent donor and the mark of an opposite-sex conspecific donor that was not part of the over-mark. Thus, the position of the opposite-sex donor's mark in the mixed-sex over-mark determines how conspecifics will respond to its scent mark relative to the scent mark of a novel, opposite-sex conspecific. The results of experiments 1 and 2 demonstrated that voles respond differently to scent marks encountered first as part of an over-mark and scent marks not previously encountered as part of an over-mark. It is likely that over-marks provide information about the scent donors that scent marks encountered separately do not provide (Hurst & Beynon 2004). Such additional information may allow individuals to assess simultaneously two scent donors (Johnston et al. 1994; Rich & Hurst 1998) and identify possible associations between the top-scent donors and bottom-scent donors (Woodward et al. 2000; Ferkin & Pierce 2007).
The final goal of our study was to test the hypothesis that four days of olfactory experience with an opposite-sex conspecific affects how meadow voles respond to a mixed-sex over-mark. We tested this hypothesis in experiment 3. Briefly, voles were first exposed to the scent mark of a familiar, opposite-sex conspecific as part of a mixed-sex over-mark. Later, during the test phase, we measured the amount of time voles investigated the scent mark of the familiar, opposite-sex donor and that of a novel opposite-sex conspecific. Sex differences existed in how male and female voles responded to over-marks containing the scent marks of familiar, opposite-sex donors. During the test phase, male voles spent more time investigating the scent mark of the familiar female donor than the scent mark of a novel female donor. It is possible that males may be spending more time with the familiar female donor because she was the top-scent donor in the mixed-sex over-mark and not because of any value that she may have gained by being familiar to that male. Being the top-scent female of a mixed-sex over-mark may provide a signal to males that she is seeking mates (Woodward 2000). Thus, she may somehow be more attractive to investigating males because of her prior association with the bottom-scent male (Dugatkin 1992). However, after exposure to a mixed-sex over-mark in which the familiar female was the bottom-scent donor, males spent similar amounts of time investigating the mark of the novel female and that of the familiar female.
Likewise, after exposure to a mixed-sex over-mark, males that were not familiar with the bottom-scent female spent similar amounts of time investigating her scent mark and that of a novel female donor (Woodward et al. 2000; this study). Males behave as if the scent marks of females that are bottom-scent donors are similar to those of novel female donors in their attractivity. Thus, familiarity with the bottom-scent female did not alter a male meadow vole's response to her scent mark relative to that of a novel female. It is interesting that males do not prefer the scent mark of a novel female donor to that of the bottom-scent donor as the novel scent donor was not associated with another male. The bottom position in the over-mark may signal to males that she has already found a mate because of her association with the top-scent male (Gibson et al. 1991; Woodward et al. 2000). Alternatively, by not being part of a mixed-sex over-mark, and as a result not being associated with the scent mark of a male, the novel female donor's scent mark could indicate that she may or may not have mated with another male. In any case, male voles may not respond favorably to the scent marks of the bottom-scent female because he is attempting to reduce competition with the top-scent male, who may have mated the bottom-scent female (Woodward et al. 1999; delBarco-Trillo & Ferkin 2004).
Male and female meadow voles appear to have a memory for the scent marks of familiar, opposite-sex conspecifics. Yet, female meadow voles differed from male voles in their response to mixed-sex over-marks containing the scent marks of a familiar, opposite-sex conspecific. During experiment 3, female voles spent more time during the test phase investigating the mark of the novel male donor than the scent mark of the familiar male conspecific, independent of whether he was the top- or bottom-scent donor. Female voles may not respond preferentially to a familiar male if his scent mark was part of an over-mark with the scent mark of another female (Woodward et al. 2000; Ferkin & Pierce 2007), which may make that male less attractive to a female seeking a mate (Gibson et al. 1991). The preference for the novel male over the familiar male found in experiment 3 also matched that of female voles in experiment 1, in which subjects were not exposed first to a mixed-sex over-mark. The preference for a novel male is similar to the partner preference found among females in other non-monogamous species (Ferguson et al. 1986; Shapiro et al. 1986; Cheetham et al. 2008). It is also possible that by preferring novel males to familiar males, female voles may be encouraging sperm competition between males (delBarco-Trillo & Ferkin 2004), increasing paternity confusion (Waser & DeWoody 2006), or avoiding repeated copulations with the same partners (Tregenza & Hosken 2005; Ivy et al. 2005; LaDage & Ferkin 2006). It is interesting, however, that in experiment 2, we found that if female voles were exposed to a mixed-sex over-mark in which they were not familiar with the opposite-sex donor, they later spent more time investigating the scent mark of the male if he was the top-scent donor compared to that of a novel male donor. This finding suggests that the brief 5-min encounter with a male's scent mark during the exposure phase was not sufficient for the female to become familiar with that male. Our results suggest that female voles are likely to show differences in investigation times depending on their familiarity with the male's scent mark. If, as in experiment 2, females are not familiar with a male's scent mark, they respond preferentially to the top-scent male.
Acknowledgments
We thank Nicholas Hobbs, Barbara Haertl, and Lara LaDage for reading earlier drafts of this manuscript and Louis Ebensperger and two anonymous reviewers for providing helpful suggestions. This research was supported by NIH grants HD 049525 and an ARRA supplement, and NSF grant IOB 0444553 to MHF.
Literature Cited
- Amy M, Leboucher G. Effects of eavesdropping on subsequent signaling behaviours in male canaries. Ethology. 2009;115:239–246. [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. A new test paradigm for social recognition evidenced by urinary scent marking behavior in C57BL/6J. Behav Brain Res. 2008;190:97–104. doi: 10.1016/j.bbr.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa H, Arakawa K, Blanchard DC, Blanchard RJ. Social features of scent-donor mice modulate scent marking of C57BL/6J recipient males. Behav Brain Res. 2009;205:138–145. doi: 10.1016/j.bbr.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein AR, Bekoff M, Daniels TJ. Kin recognition in vertebrates (excluding primates): empirical evidence. In: Fletcher DJC, Michener CD, editors. Kin Recognition in Animals. John Wiley and Sons; Chichester: 1987. pp. 287–332. [Google Scholar]
- Bekoff M. Mammalian sibling interactions: Genes, facilitative environments, and the coefficient of familiarity. In: Gubernick DJ, Klopfer PH, editors. Parental Care in Mammals. Plenum; New York: 1981. pp. 307–346. [Google Scholar]
- Brown RE, Macdonald DW, editors. Social odours in mammals. Oxford University Press; Oxford, UK: 1985. [Google Scholar]
- Cheetham SA, Thom MD, Beynon RJ, Hurst JL. The effect of familiarity on mate choice. In: Hurst JL, Beynon RJ, Roberts SC, Wyatt TD, editors. Chemical Signals in Vertebrates 11. Springer Publ.; New York: 2008. pp. 271–280. [Google Scholar]
- delBarco-Trillo J, Ferkin MH. Male mammals respond to a risk of sperm competition conveyed by odours of conspecific males. Nature. 2004;431:446–449. doi: 10.1038/nature02845. [DOI] [PubMed] [Google Scholar]
- Dewsbury DA. Individual attributes generate contrasting degrees of sociality in voles. In: Tamarin RH, Ostfeld RS, Pugh SR, Bujalska G, editors. Social Systems and Population Cycles in Voles. Birkhäuser Verlag; Basel, Switzerland: 1990. pp. 1–9. [Google Scholar]
- Erlinge S, Hoogenboom I, Agrell J, Nelson J, Sandell M. Density-related home-range size and overlap in adult field voles (Microtus agrestis) in southern Sweden. J Mammal. 1990;71:597–603. [Google Scholar]
- Dugatkin LA. Sexual selection and imitation: females copy the mate choice of others. Amer Nat. 1992;139:1384–1389. [Google Scholar]
- Ferkin MH. The effect of familiarity on social interactions in meadow voles, Microtus pennsylvanicus: a laboratory and field study. Anim Behav. 1988;36:1816–1822. [Google Scholar]
- Ferkin MH. Age affects over-marking of opposite-sex scent marks in meadow voles. Ethology. 2010;116:24–31. doi: 10.1111/j.1439-0310.2009.01711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferkin MH, Pierce AA. Perspectives on over-marking: is it good to be on top? J Ethology. 2007;25:107–116. [Google Scholar]
- Ferkin MH, Dunsavage J, Johnston RE. What kind of information do meadow voles (Microtus pennsylvanicus) use to distinguish between the top and bottom scent of an over-mark? J Comp Psychol. 1999;113:43–51. [Google Scholar]
- Ferkin MH, Johnston RE. Meadow voles, Microtus pennsylvanicus, use multiple sources of scent for sex recognition. Anim Behav. 1995;49:37–44. [Google Scholar]
- Ferkin MH, Lee DN, Leonard ST. The reproductive state of female voles affects their scent marking behavior and the responses of male conspecifics to such marks. Ethology. 2004;110:257–272. [Google Scholar]
- Ferkin MH, Tamarin RH, Pugh SR. Cryptic relatedness and the opportunity for kin recognition in microtine rodents. Oikos. 1992;63:328–332. [Google Scholar]
- Ferguson B, Fuentes SM, Sawrey DK, Dewsbury DA. Male preferences for unmated versus mated females in two species of voles (Microtus ochrogaster and M. montanus) J Comp Psychol. 1986;100:243–247. [Google Scholar]
- Fisher HS, Swaisgood RR, Fitch-Snyder H. Countermarking by male pygmy lorises (Nycticebus pygmaeus): do females use odor cues to select mates with high competitive ability? Behav Ecol Sociobiol. 2003;53:123–130. [Google Scholar]
- Gibson RM, Bradbury JW, Vehrencamp SL. Mate choice in lekking sage grouse: The roles of vocal display, female site fidelity, and copying. Behav Ecol. 1991;2:165–180. [Google Scholar]
- Gilder PM, Slater PJB. Interest of mice in conspecific male odours is influenced by degree of kinship. Nature. 1978;274:364–365. doi: 10.1038/274364a0. [DOI] [PubMed] [Google Scholar]
- Hurst JL. Urine marking in populations of wild house mice, Mus domesticus Rutty. III. Communication between the sexes. Anim Behav. 1990;40:233–243. [Google Scholar]
- Hurst JL, Beynon RJ. Scent wars: the chemobiology of competitive signalling in mice. Bioessays. 2004;26:1288–1298. doi: 10.1002/bies.20147. [DOI] [PubMed] [Google Scholar]
- Ivy TM, Weddle CB, Sakaluk SK. Females use self-referent cues to avoid mating with previous mates. Proc Biol Sci. 2005;272:2475–2478. doi: 10.1098/rspb.2005.3222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RE. Chemical signals and reproductive behavior. In: Vandenbergh JG, editor. Pheromones and Reproduction in Mammals. Academic Press; New York: 1983. pp. 3–37. [Google Scholar]
- Johnston RE. Chemical communication in rodent: from pheromones to individual recognition. J Mammal. 2003;84:1141–1162. [Google Scholar]
- Johnston RE, Chiang G, Tung C. The information in scent over-marks of golden hamsters. Anim Behav. 1994;48:323–330. [Google Scholar]
- Johnston RE, Munver R, Tung C. Scent counter marks: selective memory for the top scent by golden hamsters. Anim Behav. 1995;49:1435–1442. [Google Scholar]
- Johnston RE, Sorokin ES, Ferkin MH. Scent counter-marking by male meadow voles: Females prefer the top-scent male. Ethology. 1997a;103:443–453. [Google Scholar]
- Johnston RE, Sorokin ES, Ferkin MH. Female voles discriminate males' over-marks and prefer top-scent males. Anim Behav. 1997b;54:679–690. doi: 10.1006/anbe.1997.0471. [DOI] [PubMed] [Google Scholar]
- Kappeler PM. To whom it may concern: the transmission and function of chemical signals in Lemur catta. Behav Ecol Sociobiol. 1998;42:411–421. [Google Scholar]
- Kareem AM, Barnard CJ. The importance of kinship and familiarity in social interactions between mice. Anim Behav. 1982;30:594–601. [Google Scholar]
- Keller BL. Reproductive patterns. In: Tamarin RH, editor. Biology of New World Microtus. Vol. 8. Amer. Soc. Mammal. Sp. Publ.; Lawrence, Kansas: 1985. pp. 725–778. [Google Scholar]
- LaDage L, Ferkin MH. Male leopard geckos (Eublepharis macularius) can discriminate between two familiar females. Behaviour. 2006;143:133–149. [Google Scholar]
- LaDage L, Ferkin MH. Multiple mating increases fecundity, fertility and relative clutch mass in the female leopard gecko (Eublepharis macularius) Ethology. 2008;114:512–520. [Google Scholar]
- Madison DM. Space use and social structure in meadow voles, Microtus pennsylvanicus. Behav Ecol Sociobiol. 1980a;7:65–71. [Google Scholar]
- Madison DM. An integrated view of the social biology of Microtus pennsylvanicus. The Biologist. 1980b;62:20–33. [Google Scholar]
- Meek LR, Lee TM. Prediction of fertility by mating latency and photoperiod in nulliparous and primiparous meadow voles (Microtus pennsylvanicus) J Reprod Fert. 1993;97:353–357. doi: 10.1530/jrf.0.0970353. [DOI] [PubMed] [Google Scholar]
- Milligan SR. Induced ovulation in mammals. Oxford Reviews of Reproduction. 1982;4:1–46. [Google Scholar]
- Pierce AA, Vaughn A, Ferkin MH. Food deprivation affects the preference of female meadow voles for the mark of the top-scent donor of an over-mark. Ethology. 2007;113:480–486. [Google Scholar]
- Ricankova V, Sumbera B, Sedlacek F. Familiarity and partner preferences in female common voles, Microtus arvalis. J Ethology. 2007;25:95–98. [Google Scholar]
- Rich TJ, Hurst JL. Scent marks as reliable signals of the competitive abilities of mates. Anim Behav. 1998;56:727–735. doi: 10.1006/anbe.1998.0803. [DOI] [PubMed] [Google Scholar]
- Roberts SC. Scent marking. In: Wolff JO, Sherman PW, editors. Rodent Societies: an ecological and evolutionary perspective. Chicago University Press; Chicago: 2007. pp. 255–267. [Google Scholar]
- Rostain RR, Ben-David M, Groves P, Randall JA. Why do river otters scent mark? An experimental test of several hypotheses. Anim Behav. 2004;68:703–711. [Google Scholar]
- Salo AI, Dewsbury DA. Three experiments on mate choice in meadow voles, Microtus pennsylvanicus. J Comp Psych. 1995;118:37–47. doi: 10.1037/0735-7036.109.1.42. [DOI] [PubMed] [Google Scholar]
- Shapiro LE, Austin D, Ward SE, Dewsbury DA. Familiarity and female choice in two species of voles (Microtus ochrogaster and Microtus montanus) Anim Behav. 1986;34:90–97. [Google Scholar]
- Swihart RK, Slade NA. Differences in home-range size between sexes of Microtus ochrogaster. J Mammal. 1989;70:816–820. [Google Scholar]
- Tregenza T, Hosken D. Mate choice: Been there, done that. Current Biol. 2005;15:R959–R961. doi: 10.1016/j.cub.2005.11.019. [DOI] [PubMed] [Google Scholar]
- Valone TJ. From eavesdropping on performance to copying the behavior of others: a review of public information use. Behav Ecol Sociobiol. 2007;62:1–14. [Google Scholar]
- Waser PM, DeWoody JA. Multiple paternity in a philopatric rodent: the interaction of competition and choice. Behav Ecol. 2006;17:971–978. [Google Scholar]
- Williams JR, Catania KC, Carter CS. Development of partner preference in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Horm Behav. 1992;26:339–349. doi: 10.1016/0018-506x(92)90004-f. [DOI] [PubMed] [Google Scholar]
- Woodward RL, Jr, Schmick MK, Ferkin MH. Response of prairie voles, Microtus ochrogaster (Rodentia, Arvicolidae), to scent over-marks of two same-sex conspecifics: a test of the scent masking hypothesis. Ethology. 1999;105:1009–1017. [Google Scholar]
- Woodward RL, Bartos K, Ferkin MH. Meadow voles (Microtus pennsylvanicus) and prairie voles (M. ochrogaster) differ in their responses to over-marks from opposite- and same-sex conspecifics. Ethology. 2000;106:979–992. [Google Scholar]