Abstract
[carbonyl-11C]Benzyl acetate ([11C]1) has been proposed as a potential agent for imaging glial metabolism of acetate to glutamate and glutamine with positron emission tomography (PET). [11C]1 was synthesized from [11C]carbon monoxide, iodomethane and benzyl alcohol via palladium-mediated chemistry. The radiosynthesis was automated with a modified Synthia platform controlled with in-house developed Labview software. Under production conditions, [11C]1 was obtained in 10% (n = 6) decay–corrected radiochemical yield from [11C]carbon monoxide in > 96% radiochemical purity and with an average specific radioactivity of 2,415 mCi/µmol. The total radiosynthesis time was about 45 min. Peak uptake of radioactivity in brain (SUV = 3.1) was relatively high and may be amenable to measuring uptake and metabolism of acetate in glial cells of the brain.
Keywords: [11C]benzyl acetate, carbon-11, [11C]carbon monoxide, carbonylation, PET
Introduction
Radiolabeled acetate has been proposed as a potential in vivo marker of cerebral oxidative metabolism1 and also of glial cell metabolism2, specifically the incorporation of acetate into glutamate and glutamine. However, the brain uptake of radiolabeled acetate following intravenous administration is inadequate.3 [carbonyl-14C]Benzyl acetate, among several 14C-labeled aryl acetates, displays much higher brain-blood barrier permeability and brain retention than [carbonyl-14C]acetate in rat.4–6 Benzyl acetate (1) is readily hydrolyzed to acetate within brain. The labeling of 1 in its carbonyl position with the positron-emitter carbon-11 (t1/2 = 20.4 min) might therefore provide an agent for the delivery of [carbonyl-11C]acetate into brain for the purpose of imaging glial metabolism in vivo with positron emission tomography (PET).
Classically, 11C-labeled esters have been synthesized via the carboxylation of Grignard7–12 or organolithium reagents13,14 with cyclotron-produced [11C]carbon dioxide, followed by chlorination with a suitable reagent, such as thionyl chloride, oxalyl chloride or phthaloyl chloride, and finally reaction of the resulting 11C-labeled acid chloride with the appropriate alcohol. On a practical note, methods using Grignard reagents are demanding because freshly prepared reagent is usually required to avoid low specific radioactivity and low radiochemical yield. Any ingress of atmospheric carbon dioxide into the reagent will lower the specific radioactivity of the reacting [11C]carbon dioxide and therefore of the radioactive product. Grignard reagents, which are complex mixtures of species participating in the ‘Schlenk equilibrium’15, may also vary in reactivity with concentration and age. Furthermore, such reagents are sensitive to trace moisture and oxygen, so increasing the difficulty of their use in small-scale automated radiochemistry. Organolithium reagents show similar handling difficulties and sensitivity to moisture.
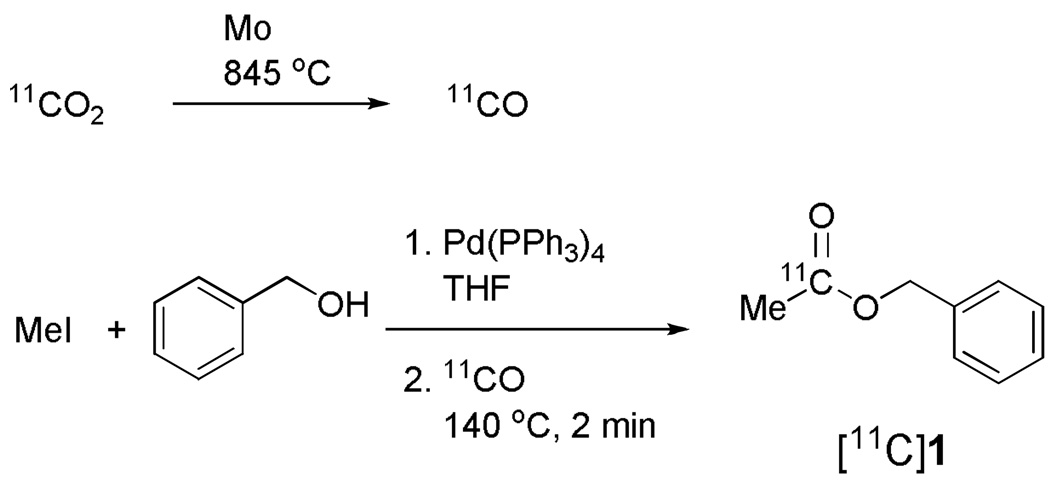
Alternatively, [carbonyl-11C]esters have also been prepared via transition metal-16,17 or radical- mediated18,19 carbonylation reactions of organo-halides with [11C]carbon monoxide,20 derived from cyclotron-produced [11C]carbon dioxide. The radiochemical yields are quite low because alcohols are weak nucleophiles.16 Nevertheless, useful radiochemical yields can still be obtained through careful choice of appropriate alkyl halides and alcohols. Transition metal-mediated [11C]carbon monoxide chemistry, does not require freshly prepared reagents and has low sensitivity to moisture.19 Moreover, the normally very low level of atmospheric carbon monoxide poses negligible risk for contaminating [11C]carbon monoxide reagent and thereby for diluting the specific radioactivity of labeled product. For these reasons along with our interest in exploring the carbonylation chemistry, we chose to use palladium-mediated [11C]carbon monoxide chemistry to produce [11C]1 in adequate activity for evaluation in monkey as a potential PET radiotracer. A Synthia apparatus,21 equipped with a high pressure micro-autoclave22 and controlled with in-house developed software, was used to prepare [11C]1 automatically from [11C]carbon monoxide within a lead-shielded hot-cell (Figure 1). The brain uptake of [11C]1 in a rhesus monkey was measured with PET.
Figure 1.
Synthesis of [carbonyl-11C]benzyl acetate via Pd-mediated [11C]carbon monoxide chemistry
Experimental
General Procedures
LC-MS analysis was performed on a Surveyor LC system equipped with a Quest LC QDECA ESI probe (Thermo Electron Corp.; San Jose, CA). GC-MS was performed on a Trace gas chromatograph (Thermo Finnigan; San Jose, CA) equipped with Polaris Q mass spectrometer. Acetonitrile residue was determined with gas chromatography on a 6850 Network GC instrument (Agilent Technologies, Foster City, CA) equipped with a DB-WAX capillary column (30 m length, 0.25 mm i.d.). Radioactivity was measured with a calibrated Atomlab™ 300 dose calibrator (Biodex Medical Systems; Shirley, NY), and corrected for background and physical decay of carbon-11.
No-carrier-added (NCA) [11C]carbon dioxide was prepared by the 14N(p,α)11C nuclear reaction by bombarding a nitrogen gas target (1% oxygen, pressure 300 p.s.i.) with a beam of protons (16 MeV, 25 µA) from a cyclotron (PETrace; GE) for 40 min. Semi-preparative scale and analytical HPLC were performed on two different systems comprising a System Gold 126 solvent module (Beckman Coulter; Fullerton, CA) coupled with a 166 UV absorbance detector (plus a Flow-count radioactivity detector (diode for semi-preparative or PMT for QC analysis; Bioscan, Washington DC).
Animal procedures
Animal procedures were performed in strict accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals-23 and were approved by the National Institute of Mental Health (NIMH) Animal Care and Use Committee.
Radiochemistry
The procedure was fully automated using a modified Synthia21 module under control with Labview-based software developed in-house. Cyclotron-produced NCA [11C]carbon dioxide was first collected in a 5-loop stainless steel tube trap filled with molecular sieves (13×) at room temperature. The trap was purged with helium at 90 mL/min for 2 min to remove oxygen. [11C]Carbon dioxide was released in a helium stream (16 mL/min) at 360 °C into a cryogenic trap filled with silica. [11C]Carbon monoxide was obtained by single pass conversion of the [11C]carbon dioxide over a mixture of molybdenum wire24 (99.97%, 0.05 mm diameter, Strem Chemicals, Newburyport, MA) and powder (99.95%, 100 mesh, Strem Chemicals) in a quartz tube (22 cm length, 0.7 cm I.D.) heated at 845 °C. [11C]Carbon monoxide was first collected on silica gel in a stainless steel tube cooled with liquid nitrogen and then released into the autoclave22 for reaction by warming the trap with a lamp.
Iodomethane (80 µmol), benzyl alcohol (80 µmol) and Pd(PPh3)4 (1.4 µmol) were premixed in THF (150 µL) and loaded into the autoclave. [11C]Carbon monoxide was released from the cryogenic trap and directed into the autoclave at room temperature. The autoclave was sealed and heated at 140 °C for 2 min in a heating well. The reaction mixture was then flushed out of the autoclave with THF (0.7 mL), diluted with water (3 mL) and then injected onto a semi-preparative size reverse phase column (Luna C18, 5 μ̣ 100 Å, 250 × 10 mm i.d.; Phenomenex, Torrance, CA) eluted at 4 mL/min with a linear gradient of water (A)-MeCN (B), starting with 30% B for 4 min and increasing to 60% B in 2 min. Eluate was monitored for radioactivity and absorbance at 210 nm. The product fraction eluting between 14.3 and 15.0 min was collected. Acetonitrile was removed at 35 °C under reduced pressure (32 Pa). [11C]1 was formulated in saline containing ethanol (10% w/v) and filtered through a Millex-MP sterile filter (0.22 µm; Millipore, Carrigtwohill, Ireland). RCY was calculated from trapped [11C]carbon monoxide. The starting radioactivity of [11C]carbon monoxide was measured separately in six identical production runs for the computation of average RCY.
[11C]1 (tR = 5.4 min) was shown to co-elute with reference benzyl acetate from a Luna C18 column (5 μ̣ 100 Å, 250 × 4.6 mm i.d.; Phenomenex, Torrance, CA) eluted with acetonitrile-25 mM ammonium formate solution (60: 40, v/v) at 1.2 mL/min and monitored for absorbance at 210 nm and radioactivity. GC-MS analysis of [11C]1 showed identical retention time (tR = 10.69 min) and mass spectrum (for M+ m/z = 150) compared with non-radioactive standard.
PET imaging
A rhesus monkey (11.28 kg) was anesthetized with 1.5% isoflurane and injected intravenously with a bolus of NCA [11C]1 (5.99 mCi; 0.43 µg of carrier). Serial dynamic images were recorded on a High Resolution Research Tomography (HRRT; Siemens/CPS, Knoxville, TN, USA) PET camera over 120 min. Decay-corrected time-activity curves were obtained for irregular volumes of interest, selected from cerebral cortex (10.9 cm3) and cerebellum (3.2 cm3). Radioactivity was normalized for injected dose and monkey weight by expression as standardized uptake value (SUV):
Results and Discussion
[11C]1 was successfully produced for intravenous injection into monkey from the Pd-mediated 11C-carbonylation of methyl iodide in the presence of benzyl alcohol (Table 1). No [11C]1 was obtained from the use of methyl triflate in place of methyl iodide (Table 1, entry 1), possibly because of the greater direct reactivity of methyl triflate towards benzyl alcohol. The Pd ligand was generally used at 1–4% molar equivalent to the methyl iodide. (4-Dimethylamino)pyridine (DMAP) has previously been found useful for the syntheses of 11C-labeled esters from [11C]carbon monoxide16, but here its use as an acylation catalyst did not improve the radiochemical yield of [11C]1 (Table 1 entry 3). Use of a larger quantity of any of the reagents or longer reaction time, increased the yield only marginally. However, excess reagents, especially benzyl alcohol, complicated the subsequent HPLC purification of [11C]1 because benzyl alcohol (tR = 13.2–14.2 min) eluted very near [11C]1 (tR = 14.3–15.0 min). The conditions listed in entry 12 were selected as optimal for the production of [11C]1 for intravenous injection. The weak nucleophilicity of benzyl alcohol accounts for the overall low radiochemical yields.18
Table 1.
Synthesis of [11C]1 with Pd-mediated [11C]carbon monoxide chemistry under various conditions.
| Entry | Pd catalyst (µmol) |
MeI (µmol) |
C6H5CH2OH (µmol) |
Temp. (°C) |
Time (min) |
RCY of [11C]1a (%) |
|---|---|---|---|---|---|---|
| 1 | 2.4 | 62 (MeOTf) | 193 | 130 | 4 | 0 |
| 2 | 2.8 | 112 | 193 | 130 | 4 | 12.1 |
| 3 | 2.9 | 112 | 193 | 130 | 4 | 9.6b |
| 4 | 1.7 | 161 | 193 | 130 | 4 | 14.2 |
| 5 | 1.6 | 161 | 193 | 140 | 2 | 6.7 |
| 6 | 1.8 | 161 | 193 | 140 | 6 | 13.5 |
| 7 | 0.8 | 80 | 48 | 140 | 2 | 5.7 |
| 8 | 0.9–1.3 | 80 | 96 | 140 | 2 | 9.6 (n = 4) |
| 9 | 1.3 | 32 | 48 | 140 | 2 | 2.6 |
| 10 | 1.3 | 48 | 48 | 140 | 2 | 4.3 |
| 11 | 1.2 | 48 | 77 | 140 | 2 | 12.1 |
| 12 | 1.4 | 80 | 77 | 140 | 2 | 10.1 (n = 6) |
Decay-corrected radiochemical yield from trapped [11C]carbon monoxide
with DMAP (22 µmol).
Although the boiling point of benzyl acetate is 206 °C, we observed heavy loss of NCA [11C]1 during its formulation for intravenous injection, where acetonitrile must be removed from the HPLC fraction under reduced pressure. The pressure, temperature and time had to be carefully controlled to conserve [11C]1 (Table 2). Removal of acetonitrile at 35 °C and at reduced pressure (32 Pa) for 30 s achieved a good balance between retaining most (~ 70%) of the radioactive product and removing solvent to an acceptably safe level (20 mg/kg)25,26 for intravenous injection.
Table 2.
Formulation efficiency for four production runs.
| Entry | [11C]1 HPLC fraction |
Rotary evaporation conditions |
Final dose | Recovery efficiency |
MeCN |
|---|---|---|---|---|---|
| (mCi) | (° C) × (s) | (mCi) | (%) | (mg/mL)* | |
| 1 | 3.60 | 80 × 30 | 1.33 | 45 | 0.395 |
| 2 | 13.8 | 70 × 52 | 1.36 | 12 | 0.034 |
| 3 | 0.80 | 35 × 30 | 0.42 | 70 | 9.32 |
| 4 | 12.2 | 35 × 35 | 5.70 | 59 | 5.50 |
Measured by GC using PrCN as external standard.
For production runs, formulated NCA [11C]1 was obtained in 10% (n = 6) decay-corrected radiochemical yield from [11C]carbon monoxide in > 96% radiochemical purity and with an average specific radioactivity of 2,415 mCi/µmol (n = 6). The total radiosynthesis time was about 45 min from the end of radionuclide production. The specific radioactivity value for [11C]1 is at the lower end of the range achieved in our laboratory for the production of other 11C-labeled radiotracers from [11C]carbon dioxide under similar cyclotron irradiation conditions, and indicates that contamination of the radiosynthetic mixture by extraneous carbon monoxide is not greatly problematic.
After administration of NCA [11C]1 to monkey, radioactivity entered brain quickly and reached quite high levels of 3.1 and 2.1 SUV in cerebral cortices and cerebellum, respectively, at 1.75 min (Figure 2). Thereafter, radioactivity inside brain declined slowly.
Figure 2.
Brain region time-activity curves after intravenous injection of [11C]1 into a rhesus monkey.
Conclusions
Palladium-mediated [11C]carbonylation chemistry provided a practically convenient route to [11C]1, avoiding air-sensitive reagents. It is envisaged that the automated process could be used to produce other 11C-labeled esters, as may be required. [11C]1 has acceptably high brain uptake and radioactivity is well-retained. Further study is needed to assess the suitability [11C]1 as a PET radiotracer of brain glial metabolism.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health (National Institute of Mental Health; project #s, Z01-MH-002793 and Z01-MH-002795). We thank Mr. D. Smith of Mink Hollow Systems Inc. (Gaithersburg, MD, USA) for assistance with software code-writing.
References
- 1.Lear JL, Ackerman RF. Metab. Brain Disease. 1990;5:45–56. doi: 10.1007/BF00996977. [DOI] [PubMed] [Google Scholar]
- 2.Muir D, Berl S, Clarke DD. Brain Res. 1986;380:336–340. doi: 10.1016/0006-8993(86)90231-3. [DOI] [PubMed] [Google Scholar]
- 3.Hosoi R, Okada M, Hatazawa J, Gee A, Inoue O. J. Cerebr. Blood Flow & Metabolism. 2004;24:188–190. doi: 10.1097/01.WCB.0000098606.42140.02. [DOI] [PubMed] [Google Scholar]
- 4.Momosaki S. Fukuoka Daigaku Yakugaku Shuho. 2009;9:131–142. [Google Scholar]
- 5.Momosaki S, Hosoi R, Sanuki T, Todoroki K, Yamaguchi M, Gee A, Inoue O. Nucl. Med. Biol. 2007;34:939–944. doi: 10.1016/j.nucmedbio.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Inoue O, Hosoi R, Momosaki S, Yamamoto K, Amitani M, Yamaguchi M, Gee A. Nucl. Med. Biol. 2006;33:985–989. doi: 10.1016/j.nucmedbio.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Pike VW, Eakins MN, Allan RM, Selwyn AP. Int. J. Appl. Radiat. Isot. 1982;33:505–512. doi: 10.1016/0020-708x(82)90003-5. [DOI] [PubMed] [Google Scholar]
- 8.Pike VW, Horlock PL, Brown C, Clark JC. Int. J. Appl. Radiat. Isot. 1984;35:623–627. [Google Scholar]
- 9.Le Bars D, Malleval M, Bonnefoi F, Tourvieille C. J. Label. Compd. Radiopharm. 2006;49:263–267. [Google Scholar]
- 10.Pike VW, McCarron JA, Hume SP, Ashworth S, Opacka-Juffry J, Osman S, Lammertsma AA, Poole KG, Fletcher A, White AC, Cliffe IA. Med. Chem. Res. 1995;5:208–227. [Google Scholar]
- 11.Hwang D-R, Simpson NR, Montoya J, Mann JJ, Laruelle M. Nucl. Med. Biol. 1999;26:815–819. doi: 10.1016/s0969-8051(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 12.Pike VW, Halldin C, McCarron JA, Lundkvist C, Hirani E, Olsson H, Hume SP, Karlsson P, Osman S, Swahn C-G, Hall H, Wikström H, Mensonidas M, Poole KG, Farde L. Eur. J. Nucl. Med. 1998;25:338–346. doi: 10.1007/s002590050230. [DOI] [PubMed] [Google Scholar]
- 13.Van der Mey M, Janssen CGM, Janssens FE, Jurzak M, Langlois X, Sommen FM, Verreet B, Windhorst AD, Leysen JE, Herscheid JDM. Bioorg. Med. Chem. 2005;13:1579–1586. doi: 10.1016/j.bmc.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Reiffers S, Vaalburg W, Wiegman T, Wynberg H, Woldring MG. Intl. J. Appl. Radiat. Isot. 1980;31:535–539. [Google Scholar]
- 15.Seyferth D. Organometallics. 2009;28:1598–1605. [Google Scholar]
- 16.Kihlberg T, Långström B. J. Label. Compd. Radiopharm. 2001;44 Suppl. 1:S990–S992. [Google Scholar]
- 17.Eriksson J, Antoni G, Långström B. J. Label. Compd. Radiopharm. 2004;47:723–731. [Google Scholar]
- 18.Itsenko O, Kihlberg T, Långström B. Eur. J. Org. Chem. 2005:3830–3834. [Google Scholar]
- 19.Itsenko O, Norberg D, Rasmussen T, Långström B, Chatgilialoglu C. J. Am. Chem. Soc. 2007;129:9020–9031. doi: 10.1021/ja0707714. [DOI] [PubMed] [Google Scholar]
- 20.Långström B, Itsenko O, Rahman O. J. Label. Compd. Radiopharm. 2007;50:794–810. [Google Scholar]
- 21.Bjurling P, Reineck R, Westerburg G, Gee AD, Sutcliffe J, Långström B. Proc. VIth Workshop on Targetry and Target Chemistry; TRIUMF; 1995. pp. 282–284. [Google Scholar]
- 22.Kihlberg T, Långström B. WO2002/102711 A1. PCT Intl Appl. 2002
- 23.Clark JD, Baldwin RL, Bayne KA, Brown MJ, Gebhart GF, Gonder JC, Gwathmey JK, Keeling ME, Kohn DF, Robb JW, Smith OA, Steggerda J-AD, Vandenbergh JG, White WJ, Williams-Blangero S, VandeBerg JL. Guide for the Care and Use of Laboratory Animals. Washington DC: National Academy Press; 1996. [Google Scholar]
- 24.Zeisler SK, Nader M, Theobald A, Oberdorfer F. Appl. Rad. Isot. 1997;48:1091–1095. [Google Scholar]
- 25.Pozzani UC, Carpenter CP, Palm PE, Weil CS, Nair JH., III J. Occup. Envir Med. 1959;1:634–642. doi: 10.1097/00043764-195912000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Itoh T. unpublished results. [Google Scholar]