Abstract
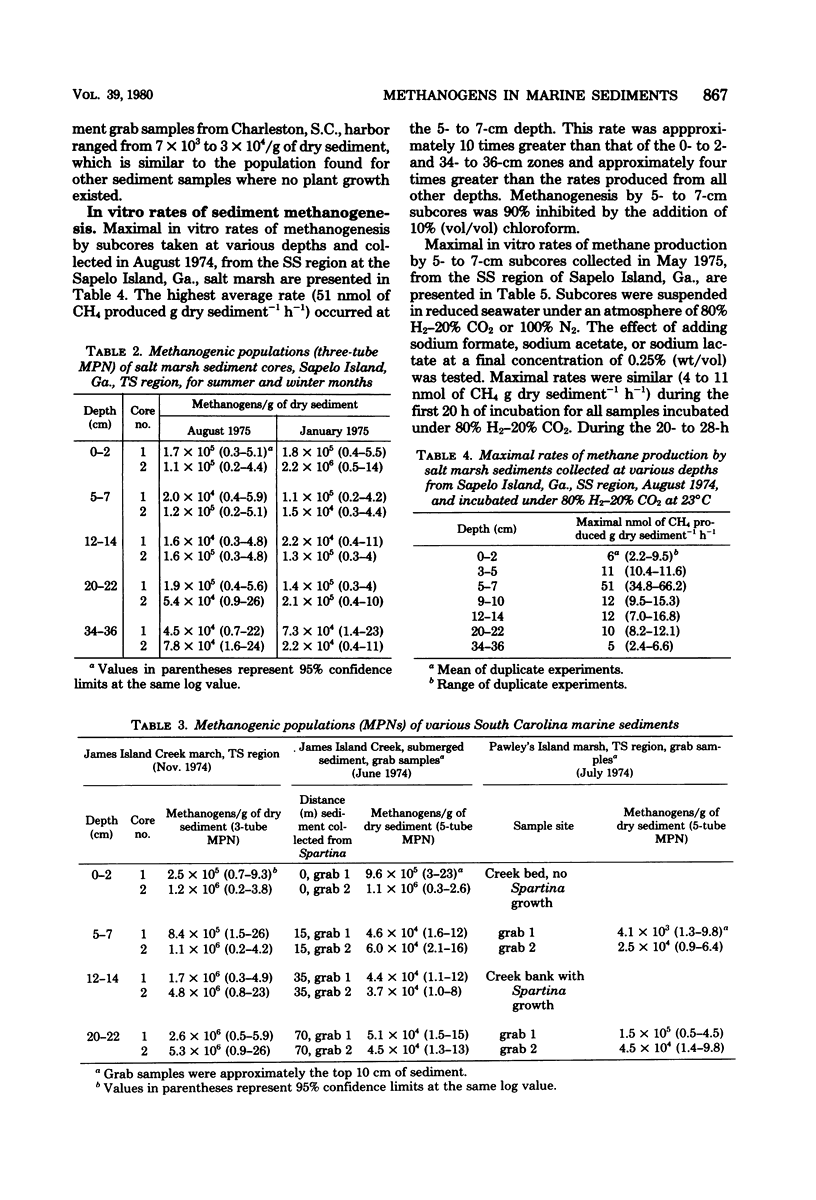
Most probable numbers (MPNs) of methanogens in various salt marsh and estuarine sediments were determined with an anaerobic, habitat-simulating culture medium with 80% H2 plus 20% CO2 as substrate. Average MPNs for the short Spartina (SS) marsh sediments of Sapelo Island, Ga., were maximal at the 5- to 7-cm depth (1.2 × 107/g of dry sediment). Populations decreased to approximately 880/g of dry sediment at the 34- to 36-cm depth. There was no significant difference between summer and winter populations. In tall Spartina (TS) marsh sediments, average populations were maximal (1.2 × 106/g of dry sediment) in the upper 0- to 2-cm zone; populations from the 5- to 36-cm zones were similar (average of 9 × 104/g of dry sediment). Methanogenic populations for TS sediments of James Island Creek marsh, Charleston, S.C., were similar (average of 3 × 106/g of dry sediment) for all depths tested (0 to 22 cm), which was comparable to the trend observed for TS sediments at Sapelo Island, Ga. Sediment grab samples collected along a transect of James Island Creek and its adjacent Spartina marsh had MPNs that were approximately 20 times greater for the region of Spartina growth (average of 106/g of dry sediment) compared with the channel (approximately 5 × 104 methanogens per g of dry sediment). A similar trend was found at Pawley's Island marsh, S.C., but populations were approximately one order of magnitude lower. In vitro rates of methanogenesis with SS sediments incubated under 80% H2-20% CO2 showed that the 5- to 7-cm region exhibited maximal activity (51 nmol of CH4 g−1 h−1), which was greater than rates for sediments above and below this depth. SS sediment samples (5 to 7 cm) incubated under 100% N2 and supplemented with formate exhibited rates of methanogenesis similar to those generated by samples under 80% H2-20% CO2. Replacing the N2 atmosphere with H2 resulted in an eightfold decrease in the rate of methanogenesis. In vitro methanogenic activity by TS salt marsh sediments, incubated under 80% H2-20% CO2, was similar for all depths tested (0 to 22 cm). TS sediment samples (0 to 7 cm) supplemented with formate and incubated under 100% N2 had greater rates of methanogenesis compared with unsupplemented samples.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. I. Field observations. Antonie Van Leeuwenhoek. 1974;40(2):285–295. doi: 10.1007/BF00394387. [DOI] [PubMed] [Google Scholar]
- Martens C. S., Berner R. A. Methane production in the interstitial waters of sulfate-depleted marine sediments. Science. 1974 Sep 27;185(4157):1167–1169. doi: 10.1126/science.185.4157.1167. [DOI] [PubMed] [Google Scholar]
- Oremland R. S. Methane production in shallow-water, tropical marine sediments. Appl Microbiol. 1975 Oct;30(4):602–608. doi: 10.1128/am.30.4.602-608.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter M. J., Hungate R. E. Characterization of Methanobacterium mobilis, sp. n., isolated from the bovine rumen. J Bacteriol. 1968 May;95(5):1943–1951. doi: 10.1128/jb.95.5.1943-1951.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Nelson D. R., Klevickis S. C., Zeikus J. G. Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl Environ Microbiol. 1977 Feb;33(2):312–318. doi: 10.1128/aem.33.2.312-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Winfrey M. R. Temperature limitation of methanogenesis in aquatic sediments. Appl Environ Microbiol. 1976 Jan;31(1):99–107. doi: 10.1128/aem.31.1.99-107.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



