Abstract
We report on serial MRI findings of cerebral lesions in a 55-year-old man with severe Marchiafava-Bignami disease (MBD). The first MRI change on fluid-attenuated inversion recovery images was hyperintensity in the genu of the corpus callosum and in the frontoparietal cortex. Following this change, a splenial lesion appeared. The first MRI change in the genu of the corpus callosum was not associated with a change in diffusion on diffusion-weighted MRI imaging, suggesting a pathological change involving vasogenic edema. Development of cortical lesions in the initial stage confirms that cortical lesions result from the primary pathogenetic process induced by alcoholic intoxication and malnutrition in MBD.
Key Words: Marchiafava-Bignami disease, Corpus callosum, MRI, Diffusion-weighted imaging
Introduction
Marchiafava-Bignami disease (MBD) is a rare neurological complication of chronic alcoholism, pathologically characterized by callosal lesions consisting of demyelination and necrosis [1,2,3]. Recently, MRI findings of the callosal and cortical lesions in the acute and chronic stages have been investigated [4,5,6,7]; however, the evolution of these lesions is still unknown. We present serial changes of callosal and cortical lesions on MRI in an acute state in a case with severe MBD.
Case Report
A 55-year-old man with a long history of alcohol abuse (Japanese liquor, shochu distilled from barley) was found by his neighbor lying on the floor, losing consciousness after groaning loudly. He was admitted to our hospital. Physical examination on admission revealed a body temperature of 36.7°C, a blood pressure of 198/117 mm Hg and a heart rate of 134 beats/min. Chest and abdomen were unremarkable. Neurological examination revealed a stuporous state, coarse horizontal nystagmus and positive plantar reflexes on both sides. Laboratory tests showed increased total bilirubin (2.0 mg/dl; normal < 1.2 mg/dl) and gamma-glutamyl transpeptidase (143 IU/l; normal < 40 IU/l). Blood sugar and blood ammonia levels were normal, and the level of vitamin B1 was 20 ng/ml (normal > 19 ng/ml). Blood gas analysis revealed mild metabolic acidosis. Cerebrospinal fluid was normal.
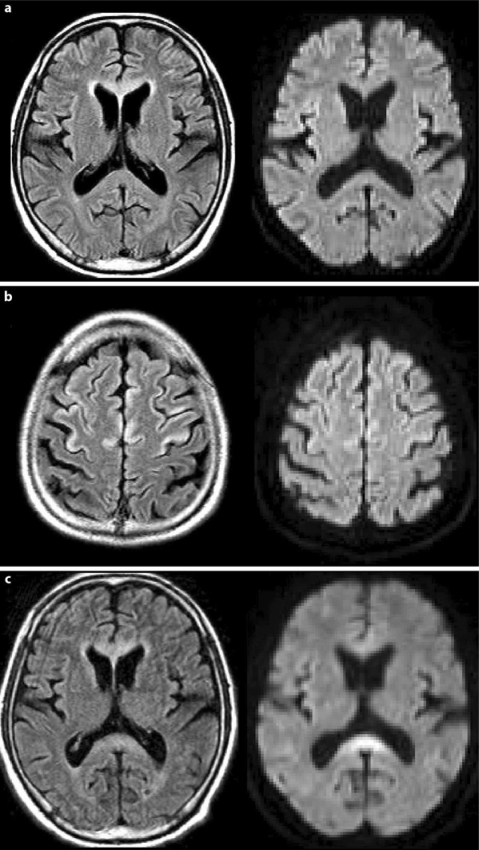
MRI taken on admission revealed a high intensity area on fluid-attenuated inversion recovery (FLAIR) images in the genu of the corpus callosum and in the frontoparietal cortex (fig. 1a, b). Diffusion-weighted MRI imaging (DWI) showed an abnormal signal only in the cortex. A follow-up MRI taken 30 h after admission newly revealed hyperintensity in the splenium on a FLAIR image (fig. 1c). DWI demonstrated hyperintensity in the genu and splenium (fig. 1c).
Fig. 1.
Initial MRI taken on admission. a The FLAIR image shows a hyperintense lesion in the genu of the corpus callosum and frontoparietal cortex but DWI shows no alteration. b The FLAIR image shows hyperintense lesions in the frontoparietal cortex with slight hyperintensity on DWI. c Second MRI taken 30 h after admission. The FLAIR image shows a new hyperintense lesion in the splenium. DWI demonstrates hyperintensity in the genu and splenium.
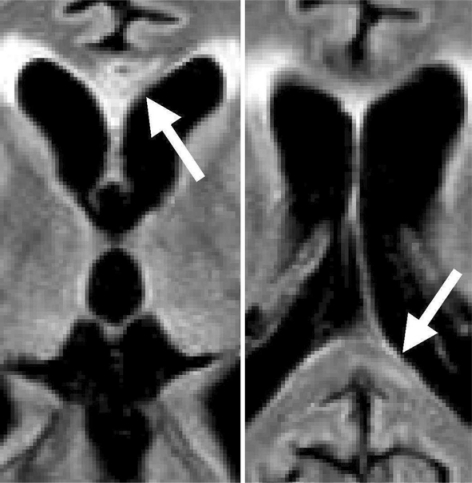
On the basis of the clinical history and characteristic MRI findings, we diagnosed the patient as having MBD and started administration of thiamine and other vitamins of the B complex before the second MRI. The patient was given 1,000 mg/day thiamine intravenously for 2 weeks and 200 mg/day thiamine for the next 5 weeks, but he did not show recovery and was discharged 7 weeks after onset. As he was in a persistent stuporous state with severe cognitive decline, neuropsychological signs, including disconnection syndromes, could not be evaluated. MRI taken 45 days after admission showed cystic necrosis in the central layer of the genu and linear necrosis in the splenium on FLAIR images (fig. 2).
Fig. 2.
MRI taken 45 days after admission. FLAIR images show cystic necrosis in the central layer of the genu and linear necrosis in the splenium.
Discussion
Chronic alcoholism and acute consciousness disturbance following a suspected seizure, along with characteristic lesions of the corpus callosum, confirmed the diagnosis of MBD in our patient. The serial MRI findings in the acute state in our patient disclosed progression of the callosal lesions in MBD: the first MRI revealed a hyperintense lesion in the genu on FLAIR images, and the follow-up MRI revealed a new hyperintense lesion in the splenium of the corpus callosum. It has been reported that the first change on MRI is diffuse swelling of the corpus callosum, followed by a genu lesion, and finally by a splenium lesion [4, 5]. The mode of progression of the callosal lesion in our patient was in accordance with the reports, except for a lack of diffuse swelling of the corpus callosum.
The first MRI change in the genu of the corpus callosum was not associated with an apparent change in diffusion on DWI, and hyperintense signals on DWI appeared in the lesion 30 h after the first MRI. These findings suggest that the initial change in the corpus callosum was partly vasogenic edema and that the lesion was then converted into cytotoxic edema [8, 9]. The necrotic lesions in the corpus callosum in the chronic stage in our patient confirm the cytotoxic process.
Our patient had cortical lesions in addition to callosal lesions in the acute stage. It has been suggested that cortical lesions on MRI are occasionally associated with MBD, especially in cases with poor prognosis and severe cognitive decline [6]; our patient also had a poor prognosis. Cortical lesions have frequently been found in pathological studies in MBD [10] and have been speculated to have Morel's laminar sclerosis as their pathology [7]. The cortical lesions already existed when the initial vasogenic changes appeared in the genu of the callosum in our patient, which suggests that they resulted from the primary pathological process induced by alcoholic intoxication and malnutrition in MBD.
In conclusion, the serial MRI findings in the acute stage of MBD demonstrate that the callosal lesions progress from the genu to the splenium, and that the cortical lesions appear at the initial stage of the pathological process in MBD.
References
- 1.Marchiafava E, Bignami A. Sopra un'alterazione del corpo calloso osservata in soggetti alcoolisti. Riv Patol Nerv Ment. 1903;8:544–549. [Google Scholar]
- 2.Koeppen AH, Barron KD. Marchiafava-Bignami disease. Neurology. 1978;28:290–294. doi: 10.1212/wnl.28.3.290. [DOI] [PubMed] [Google Scholar]
- 3.Harper C, Butterworth R. Nutritional and metabolic disorders. In: Graham DI, Lantos PL, editors. Greenfield's Neuropathology. 6th ed. London: Arnold; 1997. pp. 616–617. [Google Scholar]
- 4.Chang KH, Cha SH, Han MH, Park SH, Nah DL, Hong JH. Marchiafava-Bignami disease: serial changes in corpus callosum. Neuroradiol. 1992;34:480–482. doi: 10.1007/BF00598954. [DOI] [PubMed] [Google Scholar]
- 5.Arbelaez A, Pajon A, Castillo M. Acute Marchiafava-Bignami Disease: MR findings in two patients. Am J Neuroradiol. 2003;24:1955–1957. [PMC free article] [PubMed] [Google Scholar]
- 6.Ménégon P, Sibon I, Pachai C, Orgogozo JM, Dousset V. Marchiafava-Bignami disease: Diffusion-weighted MRI in corpus callosum and cortical lesions. Neurology. 2005;65:475–477. doi: 10.1212/01.wnl.0000171348.55820.89. [DOI] [PubMed] [Google Scholar]
- 7.Johkura K, Naito M, Naka T. Cortical involvement in Marchiafava-Bignami disease. Am J Neuroradiol. 2005;26:670–673. [PMC free article] [PubMed] [Google Scholar]
- 8.Schaefer PW, Buonanno FS, Gonzalez RG, Schwamm LH. Diffusion-weighted imaging discriminates between cytotoxic and vasogenic edema in a patient with eclampsia. Stroke. 1997;28:1082–1085. doi: 10.1161/01.str.28.5.1082. [DOI] [PubMed] [Google Scholar]
- 9.Doelken M, Lanz S, Alibek S, Richter G, Doerfler A. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI. Diagn Interv Radiol. 2007;13:125–128. [PubMed] [Google Scholar]
- 10.Castaigne P, Buge A, Cambier J, Escourolle R, Rancurel G. Clinical and neuropathological studies of 10 cases of Marchiafava-Bignami disease. Neurol Neurochir Pol. 1973;7:183–184. [PubMed] [Google Scholar]