Abstract
Change of CD20 expression was examined in cases of diffuse large B-cell lymphoma (DLBCL). CD20 expression after treatment with anti-CD20 antibody (rituximab, Rx) for DLBCL was examined in 23 cases who received serial biopsy by immunohistochemistry (IHC) and flow cytometry (FCM). CD20– by IHC and/or FCM was defined as CD20–. Four cases were CD20– at initial biopsy but became CD20+ after chemotherapy with Rx (CH-R) (group A). Recurrent tumors in three group A cases became resistant to CH-R. Initial and recurrent tumors were CD20+ before and after CH-R in 17 cases (group B). Tumors before CH-R were CD20– in two cases (group C) and continued to be CD20– in one and turned CD20+ in the other with survival time after the relapse of 8 and 23 months, respectively. Evaluation of CD20 expression with immunohistochemical and flow cytometric methods is used for the prediction of responsiveness of relapsed DLBCL for CH-R.
Key Words: Diffuse large B-cell lymphoma, CD20, Rituximab, Relapse, Clonality
Introduction
CD20, a hydrophobic transmembrane protein with a molecular weight of approximately 35 kD, is expressed on pre B and mature B lymphocytes [1] and B-cell lymphoma. Rituximab (Rx) is a chimeric anti-human CD20 antibody, and approved for the use in treatment of B-cell lymphomas [2] and immune-related diseases such as rheumatoid arthritis [3]. Action mechanisms of Rx for elimination of nonneoplastic and neoplastic B cells include complement-dependent cytotoxicity, antibody-dependent cellular cytotoxicity, and stimulation of apoptotic pathway [2]. Rx was originally employed for the treatment of low-grade or follicular lymphoma. Later combined use of Rx with conventional chemotherapy (cyclophosphamide, doxorubicin, vincristine and prednisone) (R-CHOP) was found to be effective for more aggressive diffuse large B-cell lymphoma (DLBCL) [4]. DLBCL, the most common category, is defined as a diffuse proliferation of large B-lymphoid cells.
R-CHOP is now employed as a standard therapy for DLBCL, but recurrence of disease is not infrequently encountered. In such cases, histologic examination of relapsed tumors is usually not performed because the DLBCL does not further transform to more aggressive lymphoma or become lower-grade one. To know whether relapsed DLBCL continues to express CD20 is essential for the decision of continuous administration of Rx, but information for this is quite limited at present.
In this study, change of CD20 expression in DLBCL after chemotherapy with Rx (CH-R) was studied. Histopathologic findings, expression of CD20 in tumor cells as revealed by immunohistochemistry (IHC) and flow cytometry (FCM), and identification of clone of cells before and after CH-R were evaluated and compared. Clinical relevance of loss of CD20 expression in DLBCL is discussed.
Patients
From November 1999 to October 2008, a total of 3,902 cases of lymphoproliferative diseases were registered with the Osaka Lymphoma Study Group, Osaka, Japan. Histologic specimens obtained by biopsy were fixed in 10% formalin and routinely processed for paraffin embedding. Histologic sections, cut at 4 μm, were stained with hematoxylin and eosin and immunoperoxidase procedure (ABC method). All of the histologic sections were classified according to the WHO classification by one of the authors (K.A.). Brief clinical findings were available in all cases. A diagnosis of malignant lymphoma was confirmed in 3,115 of 3,902 cases (79.8%). The number of DLBCL cases was 1,382, which comprised 44.4% of all lymphomas. Of these 1,382 DLBCL cases, histologic examinations for relapsed tumors after administration of chemotherapeutic regimens including Rx were performed in 23 (1.7%) cases; these cases constituted the basis of the present study. This study was approved by the institutional research board of Osaka University Graduate School of Medicine. Clinical information available in the 23 patients included age, sex, primary site, stage, international prognostic index, response to Rx treatment, and follow-up. The follow-up period for the survivors ranged from 8.6 to 93.6 (median 50.2) months.
Immunohistochemical and Flow Cytometric Analyses
Monoclonal antibodies used for IHC were CD20, CD79a, and CD3 (DakoCytomation, Glostrup, Denmark, dilution at 1:400, 1:100 and 1:50, respectively). CD79a expression was essential for the diagnosis of B-cell lymphoma in cases not expressing CD20. IHC revealed that the cases expressing CD20 were clearly divided into two groups: almost all cells expressed CD20 in one group (CD20+ cases) and less than 10% cells did in the other (CD20- cases). Flow cytometric analysis was performed with Becton Dickinson laser flow cytometer (FACS Calibur) by three-color analysis technique. Gating was done using forward and side scatter criteria. Cases with labeling of more than 20% of the large lymphoid cells were judged as CD20+ or CD19+. When the percentage of CD20 labeling cells was less than 20%, positivity for CD19 was a requisite for CD20- DLBCL. According to the criteria used by Kennedy et al. [5], cases showing CD20 negativity by IHC and/or FCM were defined as CD20- in this study.
Clonality Analysis with Use of Immunoglobulin Gene Rearrangement (GeneScan Analysis)
DNA was extracted from the paraffin-embedded samples with phenol-chloroform extraction-based protocol, followed by ethanol precipitation and redissolved in TE buffer. Immunoglobulin (Ig) gene rearrangement was assessed by eight PCRs with 41 primers according to BIOMED-2 protocols [6]. For this, fluorescence-labeled (6-FAM, VIC, NED, PET) custom-made primers were purchased from Applied Biosystems (Tokyo, Japan). The amplified PCR products with internal size standard (GeneScan-600 LIZ, Applied Biosystems) were analyzed using ABI PRISM 310 genetic analyzer with DS-33 dye-set and software v.3.1. (Applied Biosystems). Samples for clonality analysis before and after CH-R were not available in 12 cases.
Results
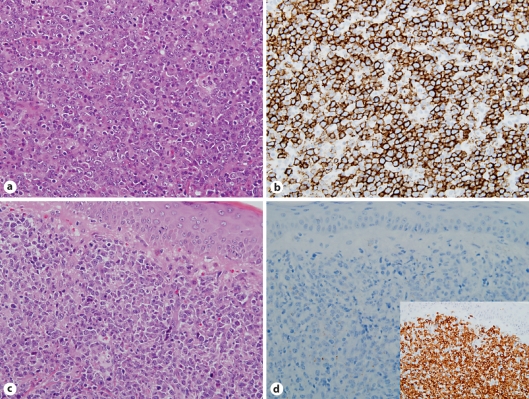
Change of CD20 expression pattern in the initial and recurrent tumors is summarized in table 1. Tumors in four (19%) of 21 cases which were CD20+ at initial biopsy became CD20- after CH-R (group A) (fig. 1). In the remaining 17 cases, tumor cells remained CD20+ in the recurrent lesions (group B). Tumors in another two cases were CD20- by FCM but CD20+ by IHC before CH-R, thus judged CD20- (group C): they continued to be CD20- by FCM but CD20+ by IHC in one and turned to be CD20+ by FCM in the other. Clinical findings in the four group A cases are summarized in table 2. The interval between the last administration of Rx and CD20- relapse in group A cases ranged from 1 to 12 (mean 4.5) months. The remaining 19 cases in groups B and C (group non-A) comprised 11 males and 8 females with a median age of 63 years. The primary site was lymph node in 3 cases, skin in 2, testis in 2, one each of breast, colon, popliteal region, prostate, stomach, thyroid gland, and tonsil, and unknown in 5. Stage was I in one case, II in 7, III in 2, and IV in 9.
Table 1.
Antigen expression pattern and peak pattern of GeneScan analysis before and after Rx
| Case | Before Rx |
After Rx |
Peak pattern of GeneScan analysis before and after Rx | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| first biopsy |
second biopsy |
first biopsy |
second biopsy |
third biopsy |
||||||||
| IHC | FCM | IHC | FCM | IHC | FCM | IHC | FCM | IHC | FCM | |||
| Group A (4 cases) | ||||||||||||
| 1 | CD20+ | NA | nd | nd | CD20+ | NA | CD20– | NA | nd | nd | change | |
| CD79a+ | ||||||||||||
| 2 | CD20+ | NA | CD20+ | CD20+ | CD20− | NA | nd | nd | nd | nd | change | |
| CD79a+ | ||||||||||||
| 3 | CD20+ | CD20+ | nd | nd | CD20− | CD20− | nd | nd | nd | nd | NA | |
| CD79a+ | CD19+ | |||||||||||
| 4 | CD20+ | CD20+ | nd | nd | CD20+ | CD20− | nd | nd | nd | nd | NA | |
| CD19+ | ||||||||||||
| Group B (17 cases) | ||||||||||||
| 5–21 | CD20+ | CD20+* | CD20+ | CD20+* | CD20+ | CD20+* | CD20+ | NA | CD20+ | NA | change | 6 cases |
| identical | 1 case | |||||||||||
| Group C (2 cases) | ||||||||||||
| 22 | CD20+ | CD20− | nd | nd | CD20+ | CD20− | nd | nd | nd | nd | identical | |
| CD19+ | CD19+ | |||||||||||
| 23 | CD20+ | NA | CD20+ | CD20− | CD20+ | CD20+ | CD20+ | CD20+ | nd | nd | change | |
| CD19+ | ||||||||||||
Rx = Rituximab; IHC = immunohistochemistry; FCM = flow cytometry; NA = data not available; nd = not done.
Data were available in part of the cases.
Fig. 1.
a Initial DLBCL (case 3). H&E. b Tumor cells were CD20+ at IHC. c Recurrent DLBCL after Rx treatment showing similar features to the initial DLBCL. H&E. d Recurrent tumor cells were CD20-. Inset Tumor cells were CD79a+. All ×400.
Table 2.
Summary of clinical findings in group A patients
| Case | Age at first diagnosis, years | Sex | Primary site | Stage | IPI | Response to Rx | Interval between last Rx and CD20− relapse | Treatment for CD20− relapse | Follow-up, months |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 71 | F | stomach | II | LI | PD following relapse | 4 months | nd | 26.0 DT |
| 2 | 48 | M | LN | III | LI | relapse following PR | 12 months | MECP | 93.6 A (PR) |
| 3 | 72 | M | LN | IV | H | PD following relapse | 1 month | ESHAP + Bleo VNCOP-B | 12.4 DT |
| 4 | 67 | M | LN | II | L | PD following relapse | 1 month | Rx only CHOP-R high-dose MTX | 8.6 A (PD) |
LN = Lymph node; IPI = international prognostic index; L = low; LI = low-intermediate; H = high; PR = partial remission; PD = progressive disease; nd = not done; Bleo = bleomycin; Rx = rituximab; MTX = methotrexate; A = alive; DT = death due to tumor; MECP = mitoxantrone, etoposide, carboplatin and prednisolone; ESHAP = etoposide, cytarabine, cisplatinum and methylprednisolone; VNCOP-B = etoposide, mitoxantrone, cyclophosphamide, vincristine, prednisolone and bleomycin; CHOP-R = cyclophosphamide, doxorubicin, vincristine, prednisolone and rituximab.
There were no prominent differences in histologic features of DLBCL between group A and non-A cases before and after CH-R. Mean mitotic counts before CH-R at 10 high-power fields ranged from 2.0 to 4.5 (median 2.6) and from 1.0 to 7.5 (median 2.1) in group A and non-A cases, respectively, and those after CH-R ranged from 2.3 to 5.3 (median 4.4) and from 1.0 to 8.5 (median 3.3) in group A and non-A cases, respectively. Mitotic counts in the recurrent tumors were higher than those in the initial tumors in both group A and non-A cases.
The mode of Rx administration was as follows: all patients received Rx with 375 mg/m2 per one dose, and total doses until last relapse ranged from 3 to 14 (mean 9) and from 2 to 20 (mean 6.3) doses in group A and non-A cases, respectively. The interval between the first Rx medication and last relapse ranged from 2.3 to 25.7 (mean 14.1) months and from 2.6 to 63.1 (mean 22.0) in group A and non-A cases, respectively.
Rx administration was stopped when CD20 turned to be negative by IHC in the recurrent tumors in cases 1, 2 and 3. CD20 became negative by FCM but persistently positive by IHC in case 4. Rx administration was continued in case 4, but the patient showed progressive disease four months after CD20 negativity by FCM. The remaining two group A cases (cases 1 and 3) became resistant to CH-R at relapse. In case 2, Rx was not employed for the relapsed lesion, thus response of the relapsed tumor to Rx was unknown. 5 of 13 group B and 2 of 2 group C patients became resistant to CH-R at relapse. Information for response for Rx administration at relapse was not available in four group B cases.
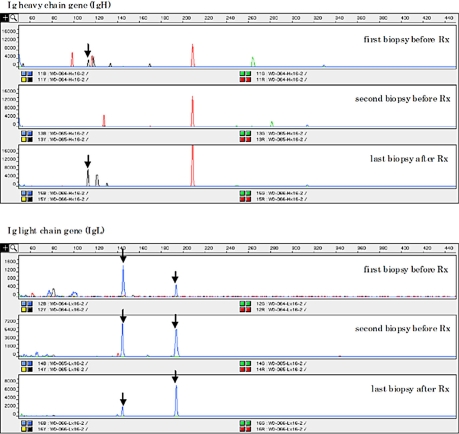
Peak patterns in the GeneScan analysis before and after CH-R were compared in the 2 of 4 group A, 7 of 17 group B, and 2 of 2 group C cases (table 1). All of the group A, B and C cases showed peaks before and after CH-R, indicating monoclonality for Ig genes with at least one primer (fig. 2). All of these cases showed partial or complete persistence of the same sized peaks before and after CH-R, indicating the same origin of tumor cells.
Fig. 2.
Polymerase chain reaction-based clonality analysis for Ig gene rearrangement (GeneScan analysis) in case 2 revealed the different peak pattern before and after Rx treatment with partial persistence of same sized peaks (↓).
Discussion
Information for CD20 expression in DLBCL after CH-R is quite limited at present. When the criteria for CD20 negativity employed in the previous study was applied in this study [5], 4 (19%) of the present 21 cases changed from CD20+ before CH-R to CD20- after CH-R. This frequency was significantly lower than that reported by Kennedy et al. from Australia [5], i.e., 6 of 10 cases (p < 0.05). The mean administrated dose of Rx until CD20- relapse in Kennedy et al.'s cases (5 doses) was lower than that of our cases (9 doses), but the difference was not significant. The mean interval between the last administration of Rx and CD20- relapse in the Kennedy et al.'s cases (5.3 months) was relatively close to that in our cases (4.5 months). At present, factors influencing the CD20- relapse of DLBCL are unknown.
The recurrent DLBCL in one of the group A cases showed CD20 negativity by FCM but positivity by IHC. A similar phenomenon was observed in one of Kennedy et al.'s cases [5]. Those two cases showed progressive disease under Rx treatment. Therefore CD20 negativity by FCM also might be a sign for resistance to Rx treatment. FCM and IHC analyses detect the different epitopes of CD20: extracellular surface epitopes by FCM and intracytoplasmic ones by IHC. Mutation or deletion of genes encoding the surface epitopes with preserved intracytoplasmic ones or mask of the surface epitopes by binding of CD20 molecules might occur in these cases. There seem to be no prominent differences in clinical findings including age, sex, primary site of tumors, and mode of chemotherapy between group A and non-A cases.
GeneScan analysis revealed partial or complete persistence of the same sized peaks in all cases, indicating the same origin of tumor cells before and after CH-R. However change a in peak pattern was found in 2 of 2 group A, 6 of 7 group B and 1 of 2 group C cases, respectively, suggesting the presence of genetic instability. These findings might partly explain the occurrence of CD20- DLBCL after CH-R.
Complete remission and overall response rate of relapsed DLBCL for Rx treatment were reported to be 8 and 33%, respectively, and the response rate of recurrent tumors for Rx treatment was reported to be significantly lower in DLBCL formerly treated with Rx than that not treated with Rx [7]. In group B cases in whom tumors continued to be CD20+, the response rate of the relapsed tumors for CH-R was 62% (8 of 13 cases). Generally relapsed tumors after CH-R occasionally become resistant to the same regimens, but negativity of CD20 is a robust sign for resistance to CH-R. Therefore CD20 negativity in recurrent DLBCL might partly explain the low response rate for CH-R and poor prognosis. Indeed, 3 of 3 group A patients became resistant to CH-R at relapse, and 2 of them showed disease progression and died.
Before CH-R, tumors in two group C cases were CD20- by FCM. One of the two cases remained CD20- by FCM at relapse, but the other became CD20+. The former case first showed complete remission, but finally died of disease 8 months after relapse. The latter case repeated the remission and relapse and was treated by CH-R at every relapse. This patient finally died of disease 23 months after the second relapse (the first relapse after CH-R). Taken together, CD20 negativity could be a sign for poor response to CH-R, although much more information is necessary to confirm this point.
In conclusion, evaluation of CD20 expression with immunohistochemical and flow cytometric methods is a reliable guide for employment of CH-R for DLBCL. Loss of CD20 expression after CH-R was less frequently observed in our cases from Japan than in those from Australia.
Acknowledgement
Supported in part by grants (20014012, 20590364 and 20014010) from the Ministry of Education, Science, Culture, Sports and Technology, Japan.
References
- 1.Nadler LM, Ritz J, Hardy R, Pesando JM, Schlossman SF, Stashenko P. A unique cell surface antigen identifying lymphoid malignancies of B cell origin. J Clin Invest. 1981;67:134–140. doi: 10.1172/JCI110005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, Newman RA, Hanna N, Anderson DR. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. 1994;83:435–445. [PubMed] [Google Scholar]
- 3.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 4.Feugier P, Van Hoof A, Sebban C, Solal-Celigny P, Bouabdallah R, Fermé C, Christian B, Lepage E, Tilly H, Morschhauser F, Gaulard P, Salles G, Bosly A, Gisselbrecht C, Reyes F, Coiffier B. Long-term results of the R-CHOP study in the treatment of elderly patients with diffuse large B-cell lymphoma: a study by the Groupe d'Etude des Lymphomes de l'Adulte. J Clin Oncol. 2005;23:4117–4126. doi: 10.1200/JCO.2005.09.131. [DOI] [PubMed] [Google Scholar]
- 5.Kennedy GA, Tey SK, Cobcroft R, Marlton P, Cull G, Grimmett K, Thomson D, Gill D. Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin's lymphoma: a retrospective review. Br J Haematol. 2002;119:412–416. doi: 10.1046/j.1365-2141.2002.03843.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Dongen JJ, Langerak AW, Brüggemann M, Evans PA, Hummel M, Lavender FL, Delabesse E, Davi F, Schuuring E, García-Sanz R, van Krieken JH, Droese J, González D, Bastard C, White HE, Spaargaren M, González M, Parreira A, Smith JL, Morgan GJ, Kneba M, Macintyre EA. Design and standardization of PCR primers and protocols for detection of clonal immunoglobulin and T-cell receptor gene recombinations in suspect lymphoproliferations: report of the BIOMED-2 Concerted Action BMH4-CT98-3936. Leukemia. 2003;17:2257–2317. doi: 10.1038/sj.leu.2403202. [DOI] [PubMed] [Google Scholar]
- 7.Martín A, Conde E, Arnan M, Canales MA, Deben G, Sancho JM, Andreu R, Salar A, García-Sanchez P, Vázquez L, Nistal S, Requena MJ, Donato EM, González JA, León A, Ruiz C, Grande C, González-Barca E, Caballero MD, Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea (GEL/TAMO Cooperative Group) R-ESHAP as salvage therapy for patients with relapsed or refractory diffuse large B-cell lymphoma: the influence of prior exposure to rituximab on outcome. A GEL/TAMO study. Haematologica. 2008;93:1829–1836. doi: 10.3324/haematol.13440. [DOI] [PubMed] [Google Scholar]