Abstract
There are pronounced disparities among black compared to white Americans for risk of end-stage renal disease. This study examines whether similar relationships exist between poverty and racial disparities in chronic kidney disease (CKD) prevalence.
Methods
We studied 22,538 participants in the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study. We defined individual poverty as family income below USD 15,000 and a neighborhood as poor if 25% or more of the households were below the federal poverty level.
Results
As the estimated glomerular filtration rate (GFR) declined from 50–59 to 10–19 ml/min/ 1.73 m2, the black:white odds ratio (OR) for impaired kidney function increased from 0.74 (95% CI 0.66, 0.84) to 2.96 (95% CI 1.96, 5.57). Controlling for individual income below poverty, community poverty, demographic and comorbid characteristics attenuated the black:white prevalence to an OR of 0.65 (95% CI 0.57, 0.74) among individuals with a GFR of 59–50 ml/min/1.73 m2 and an OR of 2.21 (95% CI 1.25, 3.93) among individuals with a GFR between 10 and 19 ml/min/ 1.73 m2.
Conclusion
Household, but not community poverty, was independently associated with CKD and attenuated but did not fully account for differences in CKD prevalence between whites and blacks.
Key Words: Chronic kidney disease, Poverty, Racial disparities
Introduction
Blacks experience a fourfold greater risk of end-stage renal disease (ESRD) compared to whites [1] for reasons that are poorly understood. Lower socioeconomic status (SES), measured either by personal education [2,3,4], annual household income [5,6] or both [7,8], is associated with the disparate risk for ESRD among blacks. In addition, an association between neighborhood poverty and increased risk of ESRD has been reported for blacks compared to whites in some [9,10] but not all studies [11].
Population-based studies on earlier stages of chronic kidney disease (CKD) have found that, in contrast to ESRD rates, rates of earlier stages of CKD are comparable [12,13] or increased [14] among whites compared to blacks. It is not clear why this reversal occurs and to what degree the association between SES and the racial disparities observed for incident ESRD patients can account for the decreased prevalence of less severe CKD among blacks compared to whites. For example, the Atherosclerosis Risk in Communities (ARIC) Study found that only white males had an increased risk of progressive CKD in lower SES neighborhoods [15] and that the estimated glomerular filtration rate (GFR) was higher among black ARIC participants.
These observations suggest that the association between SES, race and decreased GFR is complex. The purpose of this report is to describe the association between race, SES, and the level of GFR to determine if individual and neighborhood poverty independently contribute to racial disparities in CKD prevalence.
Methods
Study Design and Participants
Renal REGARDS is an ancillary study of the ongoing REasons for Geographic And Racial Differences in Stroke cohort study [16,17]. The REGARDS cohort is a national random sample of non-institutionalized individuals aged 45 years and older, 20% of whom reside in the coastal plain of North Carolina (NC), South Carolina (SC), and Georgia (GA), 30% in the remainder of NC, SC, and GA and the southeastern states of Tennessee, Mississippi, Alabama, Louisiana and Arkansas, and 50% in the remaining 42 contiguous states. Enrollment was targeted to be approximately half black, half white, and half female. Enrollment of the cohort was completed in October 2007.
Data
Data were obtained from each participant in a telephone interview followed by a subsequent in-home examination and included age, gender, race, previous history of stroke and coronary heart disease, and education. Hypertension was defined as either self-reported use of antihypertensive medications or a systolic blood pressure of >140 mm Hg or a diastolic blood pressure of >90 mm Hg measured during the home examination, where systolic blood pressure and diastolic blood pressure were the average of two measures taken in the seated position. Diabetes was defined as taking insulin or oral hypoglycemics, or either a fasting blood glucose of ≥126 mg/dl or a non-fasting blood glucose of ≥200 mg/dl. We defined hyperlipidemia as a ‘yes’ answer to the telephone interview question: ‘Have you ever been told by a doctor that you have high cholesterol or an abnormal level of fats in your blood?’
Serum creatinine is measured by colorimetric reflectance spectrophotometry using the Ortho Vitros Clinical Chemistry System 950IRC instrument (Johnson & Johnson Clinical Diagnostics, Rochester, N.Y., USA). In 2007, after completion of REGARDS recruitment, the REGARDS laboratory at the University of Vermont changed creatinine reagents to a method traceable to creatinine determined by isotope dilution mass spectrometry. Fifty samples were run in duplicate comparing the original method to the traceable method yielding the following calibration equation:
IDMS traceable creatinine = −0.06 + 0.953 ×creatinine.
In addition, in 2007, 200 samples were sent from the REGARDS laboratory to the Cleveland Clinic for calibration resulting in the following calibration equation:
Calibrated creatinine = −0.06 + 0.98 ×REGARDS creatinine.
As the two equations were nearly identical, the Vermont equation was used to convert original REGARDS creatinine values to IDMS-traceable values for determination of estimated GFR (eGFR), using the formula:
eGFR = 175 × standardized creatinine−1.154 × age−0.203 × 1.212 (if black) × 0.742 (if female).
This approach was used to obtain the eGFR values for the current article, and will be used in future publications concerning the REGARDS cohort.
A single serum creatinine measurement was available for each participant, and these values were used to define the eGFR. Estimated GFR was categorized as ≥60, 59–50, 49–40, 39–30, 29–20, and 19–10 ml/min/1.73 m2. Individuals with a GFR of >59 ml/min/1.73 m2 were collapsed into a single category, first to account for the imprecision of an eGFR above this level and second to conform with current diagnostic conventions that define individuals with a GFR of <60 ml/min/1.73 m2 as having CKD [18].
Individual poverty was estimated by reported family income. We ascertained annual family income by a series of 9 questions during the telephone interview that began with the phrase ‘Is your annual household income from all sources less than…?’, and then by specifying income levels from USD <5,000 to those USD >150,000. We then categorized the person's household income as low if the reported income was USD <15,000. This value in our data lies closest to the midrange of the federal poverty guidelines with an income threshold of USD 9,750 for a single individual and USD 19,350 for a family of 4 in 2005 [Fed Regist 2008;73:3971–3972].
Neighborhood poverty was estimated by the proportion of individuals residing below the federal poverty level in the census tract where the in-home examination was conducted. We geocoded the address used for the in-home interview to a specific census tract using US Census Bureau data to estimate the 1999 census tract poverty rate. Census tracts are small, stable subdivisions within a single county that include 2,500–8,000 persons who are similar with respect to demographic and socioeconomic characteristics [19]. The Census Bureau assigns each census tract household to being above or below the Federal poverty level based on income, family size and ages of family members [http://www.census.gov/hhes/www/poverty/povdef.html]. We defined a census tract as high poverty if 25% or more of the households were assigned to below the federal poverty level. We defined 5 neighborhood poverty categories: neighborhoods with 0–4.9, 5–9.9, 10–14.9, 15–24.9, and 25% or more of the households living below federal poverty level (high poverty).
Statistical Analysis
We described the baseline characteristics, prevalence of coronary heart disease and impaired kidney function, among REGARDS participants and compared them using t tests, ANOVA, and χ2 tests. Independent associations were assessed using multivariable logistic models [20].
We used multivariable logistic regression models to examine the association between the level of kidney function, race and poverty. Our dependent variable was race, with white race as the referent category. Our main exposure was the level of kidney function defined either as a GFR of ≥60 ml/min/1.73 m2, the non-CKD referent population, or by GFR decreasing by 10-ml/min/1.73 m2 decrements (increasing severity of CKD) between 59 and 10 ml/min/1.73 m2.
We assessed the potential confounding of the association between race and level of GFR by poverty measures in the following manner. First, we determined the association between GFR level and race (model 1). Next we successively added measures of household poverty (model 2), community poverty (model 3) and individual characteristics (model 4) to assess whether the association between race and level of GFR persisted after controlling for these covariates. Finally, we included an interaction term between race and poverty measures in the final model. All analyses included a covariate indicating the subject's region of residence. Analyses were conducted using SAS statistical software [21].
Results
There were 30,193 REGARDS subjects with a completed in-home examination by November 1, 2007. We excluded 24 individuals with missing race or geographic region, and 2,900 without a geocoded home address, 1,075 individuals with a missing serum creatinine and 75 individuals with an eGFR of <10 ml/min/1.73 m2. Finally, we excluded 3,581 individuals with missing information for at least one of the covariates included in our final multivariate analysis. Among those with missing covariates most (n = 3,208, 89.5%) were missing income data.
The mean (SD) age of the remaining 22,538 participants (74.6%) was 65.1 (9.4) years; 58.3% were white; 47.0% were male; 58.2% had hypertension; 20.9% had diabetes mellitus, and 14.8% were current smokers. Coronary heart disease was reported by 14.0% and a previous stroke was reported by 6.1%. An education less than high school was reported by 11.5%, and a family income below the poverty line was reported by 11.0% of participants.
Individuals with low household income were more likely to be older, female, and black (table 1). These individuals were more likely to have hypertension, diabetes, coronary heart disease, or a previous stroke, to be a current smoker, and to report a lower attained level of education (table 1). A GFR of <60 ml/min/1.73 m2 was present in 11.0% of participants, and individuals with household incomes below poverty were more likely to have a GFR of <60 ml/min/1.73 m2 (table 1).
Table 1.
Association between participant characteristics and household income below poverty
| Characteristic | All | Below poverty level | Unadjusted odds ratio (95% CI)1 |
|---|---|---|---|
| All | 23,538 (100%) | 2,472 (11.0%) | – |
| Age, years | |||
| 45-54 | 2,923 (13.0%) | 244 (8.4%) | Ref |
| 55-64 | 8,765 (38.9%) | 849 (9.7%) | 1.18 (1.01,1.37) |
| 65-74 | 7,174 (31.8%) | 828 (11.6%) | 1.44 (1.24, 1.67) |
| 75-84 | 3,271 (14.5%) | 478 (14.6%) | 1.88 (1.60,2.22) |
| >85 | 405 (1.8%) | 68 (16.8%) | 2.23 (1.7,3.0) |
| Gender | |||
| Male | 10,568 (46.9%) | 686 (6.5%) | Ref |
| Female | 11,970 (53.1%) | 1,786 (14.9%) | 2.56 (2.30,2.77) |
| Race | |||
| White | 13,147 (58.3%) | 874 (6.6%) | Ref |
| Black | 9,391 (41.7%) | 1,598 (17.0%) | 2.88 (2.64,3.14) |
| Hypertension | 13,127 (58.2%) | 1,722 (13.1%) | 1.74 (1.59, 1.91) |
| Diabetes | 4,702 (20.9%) | 742 (15.8%) | 1.74 (1.59,1.91) |
| Current smoker | 3,338 (14.8%) | 578 (17.3%) | 1.84 (1.64,2.05) |
| CHD | 3,159 (14.0%) | 437 (13.8%) | 1.37 (1.22, 1.53) |
| Stroke | 1,384 (6.1%) | 288 (20.8%) | 2.28 (2.0,2.6) |
| Education | |||
| Less than high school | 2,606 (11.5%) | 889 (33.9%) | 34.0 (25.7,44.9) |
| High school graduate | 5,694 (25.3%) | 864 (15.2%) | 11.8 (9.0, 15.6) |
| Post-high school | 10,522 (46.7%) | 670 (6.4%) | 4.48 (3.4, 5.9) |
| Professional | 3,716 (16.5%) | 55 (1.5%) | Ref |
| GFR, ml/min/1.73m2 | |||
| >60 | 19,944 (88.9%) | 2,086 (10.4%) | Ref |
| 50-59 | 1,292 (5.8%) | 178 (13.7%) | 1.36 (1.16,1.61) |
| 40-49 | 699 (3.1%) | 104 (14.8%) | 1.50 (1.21, 1.85) |
| 30-39 | 306 (3.8%) | 51 (16.6%) | 1.71 (1.26,2.32) |
| 20-29 | 141 (0.6%) | 36 (25.5%) | 2.95 (2.01,4.32) |
| 10-19 | 60 (0.2%) | 17 (25.0%) | 2.87 (1.65,4.97) |
For total population.
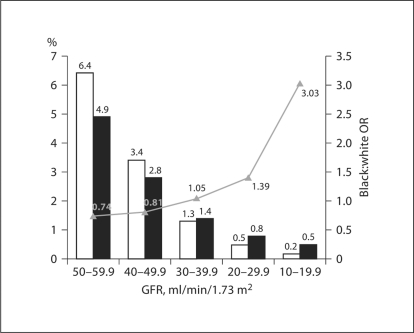
The association between race and GFR was complex (fig. 1). Among individuals with a GFR of <60 ml/min/1.73 m2, the proportion of subjects who were black increased relative to the proportion of subjects who were white as GFR level declined. The black:white OR (95% CI) among individuals with a GFR between 50 and 59 ml/min/1.73 m2, compared to a GFR of ≥60 ml/min/1.73 m2, was 0.74 (0.66, 0.84), increasing to 2.96 (1.72, 5.11) among individuals with a GFR between 10 and 19 ml/min/ 1.73 m2.
Fig. 1.
Prevalence of REGARDS participants by race and level of GFR. The proportion of REGARDS subjects are denoted by bars, whites (□) and blacks (█), and the unadjusted black:white prevalence odds ratio is denoted by the triangles and line.
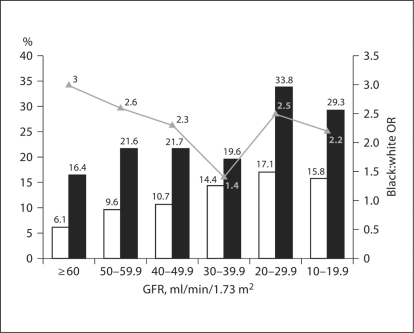
The prevalence of low household incomes was substantially greater among blacks at every level of kidney function (fig. 2). As GFR declined the proportion of both whites and blacks with a household income below the poverty level increased. As shown in table 2 the proportion of whites with household incomes below poverty increased substantially more among whites than blacks as GFR declined, rising nearly threefold from 6.1 to 15.8% among whites and from 16.4 to 29.3% among blacks (p < 0.0001). The prevalence of low household income among blacks compared to whites was thus attenuated as GFR declined, with the black:white OR for low household income declining from 3.0 (2.74, 3.32) to 2.2 (1.11, 5.45) as GFR declined from ≥60 to 10–19 ml/min/1.73 m2 (fig. 2).
Fig. 2.
Proportion of REGARDS participants with household incomes below poverty by race and level of GFR. The proportion of whites (□) and blacks (█) and unadjusted black:white odds ratio.
Table 2.
Association between level of GFR and family income below the poverty line and community poverty
| White |
Black |
|||||
|---|---|---|---|---|---|---|
| n (%) | Low family income, % | Community poverty >25%, % | n (%) | Low family income, % | Community poverty >25%, % | |
| All subjects | 13,147 (58.3) | 6.6 | 13.7 | 9,391 (41.7) | 17.0 | 43.4 |
| GFR ml/min/1.73m2 | ||||||
| >60 | 11,598 (88.2) | 6.1 | 13.6 | 8,405 (89.7) | 16.4 | 43.0 |
| 50-59 | 843 (6.4) | 9.5 | 13.3 | 457 (4.9) | 21.4 | 44.9 |
| 40-49 | 442 (3.4) | 10.6 | 17.0 | 259 (2.8) | 22.0 | 44.8 |
| 30-39 | 174 (1.3) | 14.4 | 16.1 | 133 (1.4) | 19.6 | 50.4 |
| 20-29 | 70 (0.5) | 17.1 | 10.0 | 71 (0.8) | 33.8 | 53.5 |
| 10-19 | 20 (0.2) | 15.8 | 5.0 | 48 (0.5) | 29.2 | 58.5 |
| p value | <0.0001 | 0.0625 | <0.0001 | 0.2149 | ||
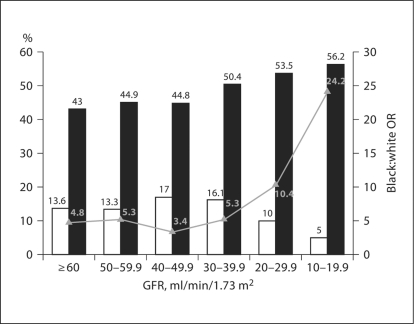
There were substantial racial differences with respect to community poverty as well, with 43.4% of black and 13.7% of white subjects living in census tracts with more than 25% of households having incomes below poverty (fig. 3). As GFR declined from ≥60 to 10–19 ml/min/ 1.73 m2, the proportion of blacks living in low income communities increased from 43 to 56.2%. In contrast, among whites the proportion of individuals living in low income communities increased from 13.6% for individuals with a GFR of ≥60 ml/min/1.73 m2 to 17.0% among those with a GFR of 40–49 ml/min/1.73 m2 and then declined to 5.0% among those with a GFR of 10–19 ml/min/1.73 m2 (fig. 3). Overall, blacks were nearly fivefold more likely to live in low income communities (OR 4.84; 95% CI 4.54, 5.16). However, despite these racial differences there was no association between the degree of community poverty and the level of GFR among blacks and whites (table 2).
Fig. 3.
Proportion of REGARDS participants living in high poverty communities by race and level of GFR. The proportion of whites (□) and blacks (█) and unadjusted black:white odds ratio.
The first model in table 3 presents the unadjusted black:white OR for successively severe levels of impaired kidney function with a GFR of ≥60 ml/min/1.73 m2 as the reference level. The next model adjusts for household poverty, the third includes community poverty, and the final model adds personal characteristics to the fully adjusted model. The association between race and GFR persisted after controlling for low household income, community poverty and personal characteristics. The addition of household poverty somewhat attenuated the black:white OR for CKD (model 2) and the addition of community poverty (model 3) did not further change the GFR stratum-specific ORs.
Table 3.
Association between race and severity of kidney disease, with and without adjustments for age, gender, comorbidities, and household income
| eGFR | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| ≥60 | Ref | Ref | Ref | Ref |
| 59-50 | 0.74 (0.66, 0.84) | 0.71 (0.63,0.80) | 0.72 (0.63,0.81) | 0.65 (0.57, 0.74) |
| 49-40 | 0.81 (0.69,0.94) | 0.76 (0.65,0.89) | 0.74 (0.62, 0.87) | 0.68 (0.57,0.81) |
| 39-30 | 1.05 (0.84, 1.32) | 0.98 (0.78, 1.24) | 0.93 (0.73, 1.19) | 0.76 (0.59,0.99) |
| 29-20 | 1.39 (1.00,1.94) | 1.20 (0.86, 1.68) | 1.19 (0.83,1.70) | 0.97 (0.67,1.40) |
| 19-10 | 3.03 (1.96,5.57) | 2.97 (1.75,5.05) | 2.79 (1.61,4.87) | 2.21 (1.25,3.93) |
Black:white ratio (95% CI) for CKD. Model 1 = Unadjusted association between black race and level of GFR. Model 2 = Model 1 controlling for household income below poverty. Model 3 = Model 2 and controlling for community poverty. Model 4 = Model 3 and controlling for age, gender, and comorbidities.
In the fully adjusted model there was a further attenuation of the black:white OR for CKD (model 4). In the fully adjusted model the black:white OR for impaired kidney function increased from 0.65 (95% CI 0.57, 0.74) to 2.21 (1.26, 3.93) as GFR declined from 50–59 to 10–19 ml/min/1.73 m2. In these models we tested the interaction between race and either low household income (p = 0.1987) or community poverty (p = 0.2423). There was no interaction between low household income and community poverty in these models (p = 0.1402). The addition of education and having health insurance and an identified source of care to this final model did not change these GFR-specific black:white OR (data not shown).
Characteristics in the fully adjusted model other than race (p < 0.0001) that were associated with the level of GFR included: increasing age (p < 0.0001), female gender (p < 0.0001), diabetes mellitus (p < 0.0001), hypertension (p < 0.0001), coronary heart disease (p < 0.0001), previous stroke (p < 0.0001), and household income below poverty (p = 0.0052). When education, rather than poverty, was included in the fully adjusted model there was no association between education and kidney function (p = 0.1907), and when the three SES measures were included in the fully adjusted model household income below poverty (p = 0.02), but neither community poverty (p = 0.9131) nor education (p = 0.3715) were significantly associated with GFR level.
Discussion
Our main finding is that race and family income were independently associated with GFR level and this effect was similar for whites and blacks in our study. In contrast, neighborhood poverty was not associated with GFR. Further, accounting for household and community poverty attenuated some, but not all, of the unexpectedly higher prevalence of CKD among whites compared to blacks. These observations suggest that (1) household poverty increases the likelihood that both black and whites will have more severe reductions in GFR; (2) this effect of poverty is comparable or greater among whites, and (3) poverty as measured in our study cannot fully explain racial disparities in the prevalence of early stages of CKD in the this population.
The increased prevalence of CKD (GFR <60 ml/min/1.73 m2) among whites compared to blacks was first identified in the National Health and Nutrition III (NHANES) Study [12,13] and previously reported for the REGARDS cohort [14]. Even after including the presence of proteinuria as well as a GFR of <60 ml/min/1.73 m2 in the definition of CKD, analysis of later NHANES surveys conducted between 1999 and 2004, found the prevalence of all stages of CKD to be 16.1% (95% CI 16.6–18.7%) among whites and 19.9% (95% CI 18.2–21.8%) among blacks [22], clearly unexpected in view of the fourfold increased incidence of ESRD among blacks consistently reported for the US population. Of note, the 30% increased prevalence among blacks reflected an increased prevalence of persistent albuminuria among individuals with a GFR of ≥60 ml/min/1.73 m2 and, as with earlier NHANES reports, the prevalence for whites and blacks of a GFR between 30 and 59 ml/min/1.73 m2 was 5.8 and 4.7% and of a GFR between 15 and 29 ml/min/1.73 m2, 0.3 and 1.1% [22].
It has been suggested that blacks may experience more rapid progression to ESRD, thus contributing to higher incidence rates but not the prevalence of lower GFR. Factors that might mediate more rapid progression may include genetic susceptibility to kidney injury like the recently reported polymorphisms in the nonmuscle myosin heavy chain-9 (MYH9) gene which are associated with increased risk of ESRD among blacks [23], environmental exposures, and barriers to healthcare [24,25]. Another possible contributing factor is that differential survival among individuals with CKD might contribute to these racial disparities [26].
There is growing interest in the role of SES as a modifying influence on racial disparities in the risk of progressive kidney disease and ESRD [27,28]. Risk factors associated with poverty that might accelerate the progression of CKD associated with SES include perinatal factors and low birth weight, behavioral exposures like smoking, analgesic and other drug use, lead exposure due to illicit alcohol or pica consumption, and access and adequacy of healthcare. It is possible that poverty-related exposures might interact with genetic factors predisposing to rapid loss of kidney function or increased mortality rates among individuals with lower GFR and thus contribute to racial disparities in the prevalence of CKD.
There is increasing evidence to support a role of poverty and SES in a more rapid decline in GFR. Peralta et al. [29] used a genetic admixture score based on 24 genetic markers to estimate the degree of African ancestry in the Cardiovascular Health Study, and found that the degree of African ancestry was not associated with either baseline or change in kidney function. Decreasing income, in contrast, was associated with an increased prevalence of impaired kidney function and this association persisted after controlling for African ancestry, age, gender, smoking status, diabetes, hypertension, education and occupation. The ARIC cohort study found increasing neighborhood poverty was associated with an increased 9-year risk of a composite outcome measured by an increase in serum creatinine level of 0.4 mg/dl or more, hospitalization for CKD, or death [15]. A decreasing neighborhood SES score was associated with higher age-adjusted incidence rates among white males but not other race-gender groups.
These observations were repeated for elderly white participants in the Cardiovascular Health Study [30] where the age-adjusted incidence rate for a composite outcome was 60% higher in the lowest versus the highest quartile of SES, and was unchanged after controlling for other covariates. Finally, a report using NHANES III data by Martins et al. [31] found that blacks were 25% more likely than whites (OR 1.25, 95% CI 1.10, 1.43) to have a urinary albumin to creatinine ratio (ACR) between 30 and 300 mg/g and 80% more likely (OR 1.80, 95% CI 1.31, 2.49) to have an ACR in excess of 300 mg/g. When subjects were stratified into low and high poverty levels based on reported income, the black:white OR was lower, reflecting a diminished effect of race on prevalence of proteinuria, in the higher compared to lower poverty level [31].
We recently reported that increasing census tract poverty level was strongly associated with a higher ESRD incidence for both blacks and whites [10]. We found that, while the magnitude of the rate difference between black and white ESRD incidence rates increased, the black:white relative risk declined as community poverty increased. These opposite effects were due to a greater rate of rise in white compared to black ESRD incidence rates as community poverty increased, raising the possibility that the impact of lower socioeconomic conditions on ESRD risk may be greater in whites. Our current study is consistent with that possibility as controlling for the effect of lower personal income on racial disparities in the prevalence of decreased GFR reduced the impact of declining GFR on black:white prevalence differences, leaving the unresolved issue of why, despite a substantial fourfold increase in risk of all-cause ESRD among blacks, they are substantially less likely to have prevalent CKD.
It should be noted in this comparison that the majority of blacks live in census tracts with high community poverty. As the level of GFR declined the proportions with low household income increased tenfold in blacks and sevenfold in whites. Thus, larger proportions of black as compared to whites with more severe CKD have low family incomes. Previously reported racial disparities in incident ESRD associated with community poverty may reflect these population differences. However, we cannot reconcile these observations until we have accumulated sufficient follow-up time to examine the risks associated with personal income and community poverty in the REGARDS cohort.
A major strength of our study is that of a large, random sample of the older US population which provides sufficient numbers of cases to examine the full range of impaired kidney function below a GFR of 60 ml/min/ 1.73 m2. It is unlikely that the racial differences in the prevalence of CKD we report are due to biased selection of subjects. Further, the possibility of misspecification of kidney function is reduced as we used an appropriately calibrated serum creatinine and the MDRD equation, which has been extensively validated for both whites and blacks, to estimate GFR [18]. We only had complete information on 74.6% of our subjects and this raises a concern that the subjects included in our analyses may not be representative of our entire cohort leading to a biased assessment of association between individual and community SES measures and the distribution of kidney function blacks and whites. We have addressed this in several ways. First, when we included subjects with missing income information in our models using an indicator for poverty status that accounted for missing income our results were essentially unchanged (data not shown). Second, we compared the key attributes in our analyses (GFR, household income, and community poverty status) for the whole cohort, those included in our analyses and those who we excluded. Mean age, race, the proportions with low household incomes and living in poor communities, and the distribution of CKD are comparable for all subjects, those included in our analyses and those with one or more missing data element. In contrast, compared to men, women were more likely to have missing data. However, our main observation of an independent association between GFR and race that persists in sequential models controlling first for GFR, next for income, then community poverty, and finally demographic characteristics is consistent across models and we feel this reduces the likelihood of substantial selection bias. Thus, while we cannot exclude the possibility that the subjects excluded for our analyses may have altered our conclusions, we feel that the comparability of the excluded and included subjects and the consistency of our crude and adjusted results lessens this possibility.
A weakness of our study is that our measure of household income is by self-report and was not validated, and previous studies suggest that considerable misclassification of income status may occur with self-reported income data [30], although the misclassification and failure to report seems to be consistent across all income ranges. It should also be noted that the income threshold that we used to identify low income homes failed to account for household size, which was unavailable in our data, and thus is an imperfect measure of actual household poverty. However, inasmuch as individual poverty was associated with increased CKD prevalence we suspect that more precise estimates of individual poverty would give results consistent with those we report here.
Further, although its use as a measure of neighborhood impoverishment has been extensively validated for studies like ours [31,32,33], it should be noted that census tract poverty might not accurately reflect the neighborhood environment as experienced by our subjects. It is also possible that, as kidney disease is a chronic process, residential mobility might confound the associations between race and kidney disease within communities as persons experiencing the economic burden of chronic diseases move to less affluent neighborhoods [34,35].
As we had only a single measure of serum creatinine we cannot exclude the possibility that some individuals were misclassified with respect to CKD. There is no reason to expect that this misclassification would occur differently for blacks and whites, and thus it should attenuate, rather than amplify, the racial disparities we observed. Also, we cannot exclude the possibility that some degree of misclassification of individual and neighborhood poverty may be possible sources of bias, but we feel that this is unlikely to significantly obscure the observation that the increased prevalence of CKD among whites compared to blacks among REGARDS subjects cannot be attributed to differences in SES. Finally, and importantly, we cannot exclude the possibility of reverse causation in the current analysis and it is possible that the association between income and CKD is due to the impact of disease on family earning potential. Finally, the relevance of lower GFR as a risk factor for progression of CKD among both older blacks and whites remains unsettled and follow-up studies to ascertain the independent role of poverty in progression to ESRD are needed.
Finally, a recent publication by Crews et al. [36]noted that blacks sampled from a single community in Maryland had an increased prevalence of eGFR of <30 ml/min/1.73 m2. Lower SES in this population, measured by self-reported income, was associated with an increased prevalence of CKD among blacks but not whites. This observation is consistent with the failure of individual SES to account for racial disparities in the prevalence of CKD in our analysis [36].
In conclusion, family income below the poverty level is a risk factor for increased prevalence of CKD in both blacks and whites. Low income did not explain the unexpected increased CKD prevalence among whites. The factors associated with poverty that contribute to these disparities remain to be identified by prospective studies of the REGARDS and other cohort populations.
Acknowledgements
This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data.
The authors acknowledge the participating investigators and institutions for their valuable contributions. University of Alabama at Birmingham, Birmingham, Alabama (Study PI, Statistical and Data Coordinating Center, Survey Research Unit): George Howard, PhD; Leslie McClure, PhD; Virginia Howard, PhD; Libby Wagner, MA; Virginia Wadley, PhD; Rodney Go, PhD; Monika Safford, MD; Ella Temple, PhD; Margaret Stewart, MSPH, and J. David Rhodes, BSN; University of Vermont (Central Laboratory): Mary Cushman, MD; Wake Forest University (ECG Reading Center): Ron Prineas, MD, PhD; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): Camilo Gomez, MD, and Susana Bowling, MD; University of Arkansas for Medical Sciences (Survey Methodology): LeaVonne Pulley, PhD; University of Cincinnati (Clinical Neuroepidemiology): Brett Kissela, MD, and Dawn Kleindorfer, MD; Examination Management Services, Incorporated (In-Person Visits): Andra Graham; Medical University of South Carolina (Migration Analysis Center): Daniel Lackland, PhD; Indiana University School of Medicine (Neuropsychology Center): Frederick Unverzagt, PhD; National Institute of Neurological Disorders and Stroke, National Institutes of Health (funding agency): Claudia Moy, PhD. The authors acknowledge the contribution of Dr. Ron Cantrell for providing programming and technical support in geocoding.
Additional funding was provided by an investigator-initiated grant-in-aid from Amgen Corporation. Amgen did not play any role in the design and conduct of the study, the collection, management, analysis, and interpretation of the data, or the preparation or approval of the manuscript. The manuscript was sent to Amgen for review prior to submission for publication.
References
- 1.US Renal Data System, USRDS 2004 Annual Data Report . Atlas of End-Stage Renal Disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2004. [Google Scholar]
- 2.Whittle JC, Whelton PK, Seidler AJ, Klag MJ. Does racial variation in risk factors explain black-white differences in the incidence of hypertensive end-stage renal disease? Arch Intern Med. 1991;151:1359–1364. [PubMed] [Google Scholar]
- 3.Brancati FL, Whittle JC, Whelton PK, Seidler AJ, Klag MJ. The excess incidence of diabetic end-stage renal disease among blacks. A population-based study of potential explanatory factors. JAMA. 1992;268:3079–3084. [PubMed] [Google Scholar]
- 4.Powe NR, Tarver-Carr ME, Eberhardt MS, Brancati FL. Receipt of renal replacement therapy in the United States: a population-based study of sociodemographic disparities from the Second National Health and Nutrition Examination Survey (NHANES II) Am J Kidney Dis. 2003;42:249–255. doi: 10.1016/s0272-6386(03)00649-8. [DOI] [PubMed] [Google Scholar]
- 5.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J. End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA. 1997;277:1293–1298. [PubMed] [Google Scholar]
- 6.Tarver-Carr ME, Powe NR, Eberhardt MS, LaVeist TA, Kington RS, Coresh J, Brancati FL. Excess risk of chronic kidney disease among African-American versus white subjects in the United States: a population-based study of potential explanatory factors. J Am Soc Nephrol. 2002;13:2363–2370. doi: 10.1097/01.asn.0000026493.18542.6a. [DOI] [PubMed] [Google Scholar]
- 7.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs. whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159:1777–1783. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 8.Perneger TV, Whelton PK, Klag MJ. Race and end-stage renal disease. Socioeconomic status and access to health care as mediating factors. Arch Intern Med. 1995;155:1201–1208. [PubMed] [Google Scholar]
- 9.Young EW, Mauger EA, Jiang KH, Port FK, Wolfe RA. Socioeconomic status and end-stage renal disease in the United States. Kidney Int. 1994;45:907–911. doi: 10.1038/ki.1994.120. [DOI] [PubMed] [Google Scholar]
- 10.Volkova N, McClellan W, Klein M, Flanders D, Kleinbaum D, Soucie JM, Presley R. Neighborhood poverty and racial differences in ESRD incidence. J Am Soc Nephrol. 2008;19:356–364. doi: 10.1681/ASN.2006080934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrne C, Nedelman J, Luke RG. Race, socioeconomic status, and the development of end-stage renal disease. Am J Kidney Dis. 1994;23:16–22. doi: 10.1016/s0272-6386(12)80806-7. [DOI] [PubMed] [Google Scholar]
- 12.Clase CM, Garg AX, Kiberd BA. Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2002;13:1338–1349. doi: 10.1097/01.asn.0000013291.78621.26. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 14.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, Cushman M, Howard G. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710–1715. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 15.Merkin SS, Coresh J, Roux AV, Taylor HA, Powe NR. Area socioeconomic status and progressive CKD: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2005;46:203–213. doi: 10.1053/j.ajkd.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 16.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke Study: objectives and design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 17.Warnock DG, McClellan W, McClure LA, Newsome B, Campbell RC, Audhya P, Cushman M, Howard VJ, Howard G. Prevalence of chronic kidney disease and anemia among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study: baseline results. Kidney Int. 2005;68:1427–1431. doi: 10.1111/j.1523-1755.2005.00553.x. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F. Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766–772. doi: 10.1373/clinchem.2006.077180. [DOI] [PubMed] [Google Scholar]
- 19.http://www.census.gov/geo/www/cen_tract.html
- 20.Kleinbaum DG, Klein M. Logistic Regression – A Self-Learning Text. ed 2. New York: Springer; 2002. [Google Scholar]
- 21.SAS Institute Inc., Version 8. Cary, SAS Institute Inc., 2000.
- 22.Centers for Disease Control and Prevention (CDC) Prevalence of chronic kidney disease and associated risk factors – United States, 1999–2004. MMWR Morb Mortal Wkly Rep. 2007;56:161–165. [PubMed] [Google Scholar]
- 23.Freedman BI, Parekh RS, Kao WH. Genetic basis of nondiabetic end-stage renal disease. Semin Nephrol. 2010;30:101–110. doi: 10.1016/j.semnephrol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perneger TV, Brancati FL, Whelton PK, Klag MJ. Studying the causes of kidney disease in humans: a review of methodologic obstacles and possible solutions. Am J Kidney Dis. 1995;5:722–731. doi: 10.1016/0272-6386(95)90548-0. [DOI] [PubMed] [Google Scholar]
- 25.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14:2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 26.Choi AI, Rodriguez RA, Bacchetti P, Bertenthal D, Hernandez GT, O'Hare AM. White/black racial differences in risk of end-stage renal disease and death. Am J Med. 2009;122:672–678. doi: 10.1016/j.amjmed.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoham DA, Vupputuri S, Kshirsagar AV. Chronic kidney disease and life course socioeconomic status: a review. Adv Chronic Kidney Dis. 2005;12:56–63. doi: 10.1053/j.ackd.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Franks P, Muennig P, Lubetkin E, Jia H. The burden of disease associated with being African-American in the United States and the contribution of socio-economic status. Soc Sci Med. 2006;62:2469–2478. doi: 10.1016/j.socscimed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Peralta CA, Ziv E, Katz R, Reiner A, Burchard EG, Fried L, Kwok PY, Psaty B, Shlipak M. African ancestry, socioeconomic status, and kidney function in elderly African Americans: a genetic admixture analysis. J Am Soc Nephrol. 2006;17:3491–3496. doi: 10.1681/ASN.2006050493. [DOI] [PubMed] [Google Scholar]
- 30.Merkin SS, Diez Roux AV, Coresh J, Fried LF, Jackson SA, Powe NR. Individual and neighborhood socioeconomic status and progressive chronic kidney disease in an elderly population: the Cardiovascular Health Study. Soc Sci Med. 2007;65:809–821. doi: 10.1016/j.socscimed.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 31.Martins D, Tareen N, Zadshir A, Pan D, Vargas R, Nissenson A, Norris K. The association of poverty with the prevalence of albuminuria: data from the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2006;47:965–971. doi: 10.1053/j.ajkd.2006.02.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Epstein WM. Response bias in opinion polls and American social welfare. Soc Sci J. 2006;43:99–110. [Google Scholar]
- 33.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Choosing area based socioeconomic measures to monitor social inequalities in low birth weight and childhood lead poisoning: The Public Health Disparities Geocoding Project (US) J Epidemiol Community Health. 2003;57:186–199. doi: 10.1136/jech.57.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krieger N, Waterman PD, Chen JT, Soobader MJ, Subramanian SV. Monitoring socioeconomic inequalities in sexually transmitted infections, tuberculosis, and violence: geocoding and choice of area-based socioeconomic measures – the public health disparities geocoding project (US) Public Health Rep. 2003;118:240–260. doi: 10.1093/phr/118.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156:471–482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 36.Crews DC, Charles RF, Evans MK, Zonderman AB, Powe NR. Poverty, race, and CKD in a racially and socioeconomically diverse urban population. Am J Kidney Dis. 2010;55:992–1000. doi: 10.1053/j.ajkd.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]