Summary
Background
The detection of a broad range of bacteria by PCR is applied for the screening of blood and blood products with special attention to platelet concentrates. For practical use it is desirable that detection systems include Gram-positive, Gram-negative and non-Gram-stainable bacteria. It is quite challenging to achieve high sensitivity along with a clear negative control with PCR reagents, because especially Taq polymerase is contaminated with traces of bacterial DNA.
Methods
Bacterial DNA decontamination of Taq polymerase was attempted by two different methods using the restriction enzyme Sau 3A1 and microfiltration. Additionally a commercially available Taq polymerase depleted of bacterial DNA was included. A published real-time PCR specific for Gram-negative bacteria was adapted for Gram-positive bacteria, including certain Staphylococcus species and Mycobacteria, and was used to charge the three Taq polymer-ases depleted of bacterial DNA contamination
Results
Despite published reports about successful DNA decontamination, all three approaches performed poorly in experiments done in this study. Sensitivity ranged at approximately 50–100 colony forming units (CFU) per PCR reaction for Escherichia coli and Staphylococcus epidermidis, corresponding to 1,250–2,500 CFU/ml sample material. Conclusion: It seems unsatisfying to accept detection limits that high for diagnostic bacterial PCR even if highly multiplexed. Reliable methods for DNA decontamination of Taq polymerase are needed and would present one important step towards bacterial DNA detection with high sensitivity.
Key Words: Taq polymerase depleteted of bacterial DNA contamination, DNA decontamination methods
Zusammenfassung
Hintergrund
Die Detektion eines breiten Spektrums verschiedener Bakterien mittels PCR wird bei der Untersuchung von Blut und Blutprodukten eingesetzt und ist bei der Untersuchung von Thrombozytenkonzentraten von besonderem Interesse. Aus praktischen Erwägungen erscheint ein Detektionssystem wünschenswert, das sowohl grampositive und gramnegative, als auch nichtgramfärbbare Bakterien erkennt. Der Wunsch nach hoher Sensitivität bei gleichzeitig eindeutigen Negativkontrollen stellt hierbei eine ganz besondere Herausforderung dar, weil die verwendeten PCR-Reagenzien und ganz besonders die Taq-Polymerase selbst, immer mit Spuren bakterieller DNA verunreinigt sind.
Methoden
Die Abreicherung bakterieller DNA in Taq-Polymerase wurde durch zwei Methoden – den Einsatz des Restriktionsenzyms Sau 3A1 bzw. Mikrofiltration - versucht. Zusätzlich wurde eine kommerziell erhältliche Taq-Polymerase mit angeblich bereits reduziertem Bakterien-DNA-Gehalt in der Studie eingeschlossen. Eine publizierte «Real-time»-PCR-Methode, die vor allem für die Detektion gramnegativer Bakterien geeignet schien, wurde um die Erkennung grampositiver Bakterien erweitert und erlaubte so schlussendlich auch die Detektierung von Spezies der Gattungen Staphylococcus und Mycobacteria. Diese PCR wurde für die Beurteilung der drei verschiedenen Taq-Polymerasen und der Abreicherung bakterieller DNA eingesetzt.
Ergebnisse
Trotz bereits publizierter Berichte über erfolgreiche DNA-Dekontamination enttäuschten alle drei in dieser Studie geprüften Ansätze. Die erreichte Sensitivität lag bei zirka 50-100 Colony Forming Units (CFU) pro PCR-Reaktion für Escherichia coli und Staphylococcus epidermidis, was 1,250-2,500 CFU/ml Probenmaterial entsprach.
Schlussfolgerung
Trotz Mehrfachspezifität der verwendeten diagnostischen PCR erscheinen die erreichten Detektionslimits unbefriedigend hoch. Nach wie vor besteht ein Bedarf an verlässlichen Methoden für die DNA-Dekontamination von Taq-Polymerase, um eine hohe Sensitivität bei der Detektion bakterieller DNA zu erreichen.
Introduction
The detection of a broad range of bacteria by PCR is usually applied for the screening of a variety of sample materials such as blood and blood products to diagnose bacteriemia and to detect the presence of a bacterial contamination, respectively [1,2,3].
As already described extensively, 16S rDNA is a highly homologous target sequence for practically all bacteria, that can be used for detection [1, 2]. Specificity and sensitivity are the classical limiting factors for all test systems. With respect to specificity, a correct choice of the amplified target sequence is essential. In order to obtain an optimal detection, DNA sequence which is most suitable for priming can be defined aligning a representative selection of possibly all bacterial rDNAs. With respect to sensitivity, the main problem of detection of bacterial DNA is the achievement of a high sensitivity along with a clear negative control. Up to this day this is an unresolved problem since the reagents used in the PCR process are contaminated with trace amounts of bacterial DNA, especially the Taq polymerase, where contamination originates from its production in bacterial cultures. No method for an absolute purification is known to date [4, 5]. A number of trials has been carried out to reduce the amount of contaminating DNA within the enzyme preparation with varying success [6,7,8]. However, only very recently successful decontamination of Taq polymerase from bacterial DNA by using DNAse approaches have been reported. [9]
Real-time PCR is the most advanced PCR method available for the detection purpose. In contrast to other PCR methods, it additionally produces quantitative results. Basically a certain fluorescence intensity is defined as a positive signal. The PCR cycle in which this intensity is reached for the first time is called C(t) value. The more target DNA, the sooner a positive signal will be achieved, which means a low C(t) value.
To decrease bacterial DNA contamination of Taq polymerase, we compared three different approaches: The elimination of DNA impurities by using the restriction enzyme Sau 3A1, filtration of the polymerase through a microfilter and the use of a ‘Low-DNA-(LD)-Taq polymerase’ which is described as a Taq polymerase with a very low concentration of contaminating bacterial DNA by the manufacturer (Ampli Taq Gold(r) DNA polymerase LD, Applied Biosystems). The rationale behind the usage of a restriction endonuclease for decontamination purposes is the cleavage of DNA contaminants in the Taq polymerase enzyme solution, which consequently should disable specific amplification of the degraded contaminating DNA later in the PCR. Microfiltration on the other hand relies on the molecular weight differences between the Taq polymerase enzyme and contaminating DNA. Using mild centrifugation, the polymerase is expected to pass, whereas contaminating DNA is thought to be withheld by a filter membrane of adequate size exclusion range [10].
The following article reports on our findings in the course of the de novo development of a real-time PCR method to detect bacterial 16S rDNA in anticoagulated whole blood samples. The report addresses two main performance characteristics: specificity improvement for a broad range of bacteria and sensitivity achievable in our hands.
Material and Methods
Extraction of DNA
We used the NucleoSpin® Blood L Kit (Macherey-Nagel, Düren, Germany) according to the manufacturer's instructions, extracting 2 ml of whole blood. Blood samples were spiked with different concentrations of Escherichia coli and Staphylococcus epidermidis. For specificity extension experiments, E. coli and S. epidermidis were suspended in RPMI1640 (Biochrom AG, Berlin, Germany), instead of blood. The extracted DNA was eluted in 200 ml of elution buffer. In the case of Mycobacterium tuberculosis only, purified DNA was used, which has been kindly provided by Prof. W. Prodinger, Department of Hygiene, Microbiology and Social Medicine, Medical University Innsbruck, Austria.
To exclude further bacterial DNA contamination, all working steps must be carried out under sterile conditions.
PCR Mixture
12.5 μl Master Mix (TaqMan® Universal PCR Master Mix; Applied Biosystems, Branchburg, NJ, USA), 1 μl of all primers (5 pmol/μl, final concentration in the reaction of 200 nmol/l each), 0.5 μl of the probe (2.5 pmol/μl, final concentration in the reaction of 50 nmol/l), 4 μl of eluted DNA and water were mixed to a final volume of 25 μl per reaction. The following cycling profile was used: 50 cycles of 50 °C 2 min, 95 °C 10 min, (95 °C 15 s, 60 °C 1 min). E. coli and S. epidermidis were used for sensitivity testing. NucleoSpin® Blood L Kit elution buffer (Macherey-Nagel) was used as negative control. PCRs were run on an ABI Prism 7000 (Applied Biosystems). In our spiking experiments, PCR positivity versus negativity was defined as a difference of at least one C(t) value in the same PCR run.
Bacterial Preparations
Bacterial concentrations are given in colony forming units (CFU), e.g. 50 CFU/PCR means the use of an absolute number of 50 CFU in one PCR reaction, corresponding to a concentration of 1,250 CFU/ml of specimen. CFU were measured using a Spiral System (Spiral System Instruments, Bethesda, MD, USA) as described by the manufacturer. All spiking experiments were performed with bacteria (E. coli and S. epidermidis) from exponentially growing cultures which rather excludes that dead bacteria not counted by the spiral plater might contribute to the overall copy number. Direct conversions from CFU into bacterial genomes are hindered by the fact that the number of 16S rDNA genes per bacterial genome is variable, and exponentially growing bacteria may already include more than only one copy of their bacterial chromosome [11, 12].
Supplemental Primer Design for Detection of Bacterial 16S rRNA Genes
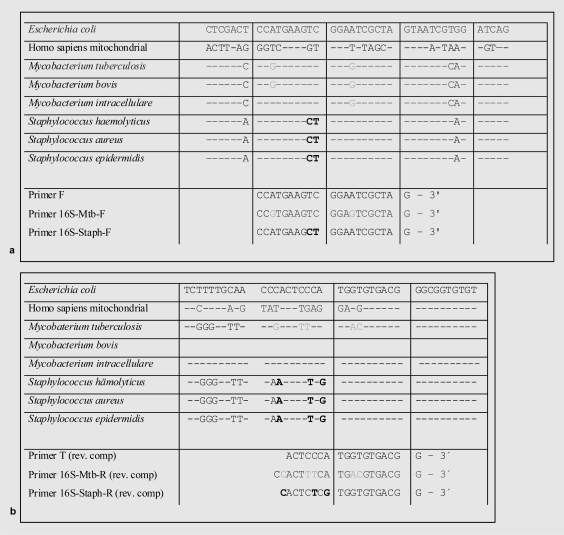
For optimal positioning of the primers, an alignment containing a total of 110 different bacterial 16S rRNA genes (table 1) and homologous regions of the human mitochondrial DNA was elaborated using GeneRunner Software (Version 3.05, Hastings Software Inc., Westwood, NJ, USA, 1994). We used Gram-positive, Gram-negative and non-Gram-stainable bacteria for our alignment and compared already published primers in the alignment for their detection ability of bacterial DNA [4]. Supplemental primer pairs for the additional detection of M. tuberculosis and Staphylococcus ssp. were added and tested for their specificity. All forward primers used showed at least 52% (11 of 21 bases) and all reverse primers at least 32% (6 of 19 bases) mismatching to the most homologous human mitochondrial DNA sequence. Therefore, combined specificities were thought to be restrictive of all homologous human mitochondrial DNA sequences. This assumption was not tested independently. Sequences of the primers used and the probe are shown in table 2. The position of the different primers are given in figure 1.
Table 1.
List of bacterial species along with their NCBI accession numbers used for alignment and in silico analysis of the 16S rRNA genes
| Bacterium | Access-No. |
|---|---|
| Homo sapiens mitochondrial DNA | NC_012920.1 |
| Bartonella bacilliformis | M65249 |
| Bartonella grahamii | Z31349 |
| Bartonella henselae | AJ223778 |
| Bartonella quintana | AJ250247 |
| Bartonella taylorii | Z31350 |
| Bartonella vinsonii | U26258 |
| Brucella abortus | X13695 |
| Brucella canis | L37584 |
| Brucella melitensis | L26166 |
| Brucella neotomae | L26167 |
| Brucella ovis | L26168 |
| Brucella sius | L26169 |
| Campylobacter coli | M59073 |
| Campylobacter concisus | L06977 |
| Campylobacter curvus | L06976 |
| Campylobacter fetus | L14633 |
| Campy lob acter fetus fetus | M65012 |
| Campylobacter fetus veneralis | M65011 |
| Campylobacter hyointestinalis | M65009 |
| Campylobacter jejuni | M59298 |
| Campylobacter rectus | L06973 |
| Campylobacter showae | L06975 |
| Candidatus Rickettsia tarasevichiae | AF503168 |
| Citrobacter farmeri | AF025371 |
| Citrobacter freundii | M59291 |
| Citrobacter rodentium | AF025363 |
| Citrobacter sedlakii | AF025364 |
| Citrobacter werkmanii | AF025373 |
| Chlamydophila pneumoniae | L06108 |
| Chlamydophila psittaci | E17342 |
| Chlamydophila trachomatis | D85719 |
| Eikenella corrodens (strain FDC1073) | M22515 |
| Enterobacter aerogenes | AF395913 |
| Enterobacter agglomérons | AF024613 |
| Enterobacter cancerogenus | Z96078 |
| Enterobacter cloacae | AF157695 |
| Enterobacter dissolvens | Z96079 |
| Enterobacter nimipressuralis | Z96077 |
| Escherichia coli | J01859 |
| Francisella philomiragia | Z21933 |
| Francisella tularensis | Z21932 |
| Francisella tularensis var. novicida | L26084 |
| Helicobacter pylori | AY366424 |
| Helicobacter pylori | U00679 |
| Klebsiella oxytoca | AB053117 |
| Klebsiella pneumoniae | AY369139 |
| Klebsiella pneumoniae | X80684 |
| Klebsiella pneumoniae strain CC-88170 | AY315447 |
| Klebsiella pneumoniae subsp. ozaenae | AF228919 |
| Klebsiella rhinoscleromatis | AF009169 |
| Klebsiella sp. CC-88168 | AY315448 |
| Leptospira biflexa | Z12821 |
| Leptospira illini | M88719 |
| Leptospira interrogans | M71241 |
| Leptospira kirschneri | Z21628 |
| Morganella morganii | AJ301681 |
| Mycobacterium bovis | AY360331 |
| Mycobacterium tuberculosis | AJ536031 |
| Mycobacterium paratuberculosis | M61680 |
| Neisseria gonorrhoeae | X07714 |
| Proteus mirabilis | AF128840 |
| Proteus vulgaris | AJ233425 |
| Pseudomonas aeruginosa | M34133 |
| Pseudomonas cepacia | M22518 |
| Pseudomonas diminuta | M59064 |
| Pseudomonas flavescens | U01916 |
| Pseudomonas mendocina | M59154 |
| Pseudomonas putida | L28676 |
| Pseudomonas testosteroni | M11224 |
| Rickettsia conorii strain Malish 7 | AF541999 |
| Rickettsia prowazekii | M21789 |
| Rickettsia rickettsii | U11021 |
| Rickettsia sibirica | D38628 |
| Rickettsia sp. (Kytorhinus sharpianus symbiont) | AB021128 |
| Rickettsia sp. Bar29-like isolate 249 | AF487650 |
| Rickettsia sp. Chad | AF510102 |
| Rickettsia typhi | M20499 |
| Salmonella entérica subsp. entérica (serovar Shomron) | X80678 |
| Salmonella enteritidis | U90318 |
| Salmonella give | X80683 |
| Salmonella paratyphi A | X80682 |
| Salmonella paratyphi B | U88547 |
| Salmonella paratyphi C | U88548 |
| Salmonella sofia | X80677 |
| Salmonella typhi | U88545 |
| Salmonella typhimurium | X80681 |
| Serratia entomophila | AJ233427 |
| Serratia ficaria | AJ233428 |
| Serratia fonticola | AJ233429 |
| Serratia grimesii | AJ233430 |
| Serratia marcescens | M59160 |
| Serratia odorífera | AJ233432 |
| Serratia plymuthica | AJ233433 |
| Serratia proteamaculans | AJ233435 |
| Serratia rubidaea | AJ233436 |
| Shigella boydii | X96965 |
| Shigella dysenteriae | X80680 |
| Shigella dysenteriae | X80680 |
| Shigella flexneri | X80679 |
| Shigella sonnei | X80726 |
| Staphylococcus aureus | L37597 |
| Staphylococcus epidermidis | L37605 |
| Streptococcus sanguis | AF003928 |
| Streptococcus pyogenes | X59029 |
| Streptococcus, oralis | X58308 |
| Vibrio aspartigenicus | M98446 |
| Vibrio cholerae | L05178 |
| Vibrio parahaemolyticus | M59161 |
| Yersinia entero eolítica | AF366378 |
| Yersinia pseudotuberculosis | Z21939 |
Table 2.
Sequences of the primers and the TflijrMan-probea
| Primer name | Origin | Sequence |
|---|---|---|
| Primer F (forward) | Corless et al., 2000 [4] | 5′-CCATGAAGTCGGAATCGCTAG-3′ |
| Primer T (reverse) | Corless et al., 2000 [4] | 3′-ACTCCCATGGTGTGACGG-3′ |
| Primer 16S-Mtb-F (forward) | This work | 5′-CCGTGAAGTCGGAGTCGCTAG-3′ |
| Primer 16S-Mtb-R (reverse) | This work | 5′-CCACTTTCATGACGTGACGG-3′ |
| Primer 16S-Staph-F (forward) | This work | 5′-CCATGAAGCTGGAATCGCTAG-3′ |
| Primer 16S-Staph-R (reverse) | This work | 5′-CACTCTCGTGGTGTGACGG-3′ |
| Probe | Corless et al., 2000 [4] | FAM-5′-CGGTGAATACGTTCCCGGGCCTTGTAC-3′-TAMRA |
The TaqMan probe was marked with 6-carboxyfluorescein (FAM) at the 5′-terminus and with 6-carboxytetramethylrhodamine (TAMRA) at the 3′-terminus.
Fig. 1.
Positioning of a the forward- and b reverse primers: The sequence of parts of the 16S rRNA gene of E. coli is shown. Genetic differences to Mycobacteriae (gray) and Staphylococci (bold) are shown. Homologous regions of human mitochondrial DNA are displayed for illustration. Modifications of primers T lead to an improved detection of mycobacterial DNA (primer 16S-Mtb-F and -R, gray) and gram-positive bacteria (primer 16S-Staph-F and -R, bold).
DNA Decontamination Using the Restriction Enzyme Sau 3A1
Since definitive Taq polymerase concentration in the TaqMan Universal PCR Master Mix was not provided by the manufacturer, we estimated that 20 μl of Master Mix contained approximately 1 U of Taq polymerase. PCR reaction mixtures containing 1 U, 3 U and 6 U of Sau 3A1 (Roche Diagnostics, Mannheim, Germany) per 1 U of Taq polymerase were prepared. The complete mixture, containing the primers, the TaqMan probe, the water and the TaqMan Universal PCR Master Mix were incubated at 37 °C for 30 min. Inactivation of the restriction enzyme was done by heating at 96 °C for 2 min, following the protocol of Carroll et al. [7]. After inactivation, 4 μl of eluted bacterial test DNA were added.
Usage of a Commercially Available Taq Polymerase Depleted of Bacterial DNA
We also used AmpliTaq Gold® DNA polymerase LD (Low DNA, 250 U with gold buffer and MgCl2 solution; Applied Biosystems). This lot, according to the manufacturer, contains a maximum of 10 copies of 16S rDNA per 5 U of polymerase.
DNA Decontamination Using a Microfilter
Furthermore, a microfilter with a cut-off molecular size of about 100 kDa (Amicon Microcon YM-100 Centrifigal Filter Unit; Millipore Corporation, Bedford, MA, USA) was used to diminish the amount of contaminating DNA of the polymerase. Using a centrifugal device, the polymerase and the water were filtered through the filter's membrane at 100 g for 30 min according to the protocol of Yang et al. [10].
Results
Detection of Gram-Positive Bacterial and Mycobacterial 16S rDNA
According to our in silico analysis, primers used by Corless et al. [4] were well suitable for the detection of Gram-negative bacteria but less qualified to detect Gram-positive bacteria, especially certain Staphylococcus spp. Additionally - judging from our alignment - Corless' primers also seemed to be inappropriate for the detection of mycobacterial DNA. Therefore, we considered to use Corless' primers for the detection of Gram-negative bacteria only and added two additional primer pairs to increase the detection range to Gram-positive, non-Gram-stainable M. tuberculosis and especially certain Staphylococcus spp. The primer sequences for the detection of Gram-positive bacteria (primer 16S-Staph-F and primer 16S-Staph-R), Gram-negative bacteria (primer F and primer T from the publication of Corless et al. [4]) and non-Gram-stainable bacteria (primer 16S-Mtb-F and primer 16S-Mtb-R) are shown in figure 1.
In order to validate our in silico findings, the primers and TaqMan probe were tested for their specificity with DNA preparations of E. coli, S. epidermidis and M. tuberculosis suspended in RPMI1640. Primers used by Corless et al. [4] showed pooror even negative signals for M. tuberculosis, and Gram-positive bacteria were only detectable in very high concentrations (>10,000 CFU), as exemplified by S. epidermidis when compared to E. coli. After addition of our newly designed two primer pairs (fig. 1), specificity for Gram-positive bacteria increased clearly as exemplified using DNA of S. epidermidis. Even mycobacterial DNA was now detectable as exemplified by M. tuberculosis. C(t) values before adaptation of the system were 32.2 for E. coli (20 CFU) and 32.1 for S. epidermidis (1,000 CFU); M. tuberculosis showed comparable values to the negative control (C(t) = 33.5. After adaptation C(t) values for 50 CFU of E. coli were still 32.2, but the detection limit for S. epidermidis was improved to a value of 50 CFU (C(t) = 32.2), and DNA of M. tuberculosis was now detectable in several concentrations like 2 ng (C(t) = 22.3), 0.2 ng (C(t) = 26.1) and 0.02 ng (C(t) = 29.5). Whereas 0.02 ng of DNA of M. tuberculosis corresponds to approximately 4,000 single bacterial genomes, the low C(t) value of only 29.5 still indicated a higher potential with respect to sensitivity [13].
Detection Limit without Taq Polymerase Decontamination Procedures
Each experiment included NucleoSpin elution buffer as a negative control at least in duplicates, and mean values of them were used for calculations. C(t) values of these reagent controls were remarkably reproducible and amount to 33.1–35.0 for all controls in all 6 experiments performed (n = 15 single controls in total; mean C(t) = 33.9; standard deviation C(t) = 0.64). Due to the presence of contaminating bacterial DNA, as expected, we never observed a completely negative amplification result, even in our planned negative controls. Therefore and in order to avoid general sensitivity reduction by competitive internal controls, we also refrained from considering a separate positive amplification control in our experiments.
Positive samples were 8-step serial dilutions of E coli and S. epirdermidis in whole blood in all experiments calculated to a final concentration of 5,000, 1,000, 500, 250, 100, 50, 10 and 1 CFU/PCR. For illustration, an experimental PCR run of E. coli with 1,000, 500, 100, 50 CFU/PCR and a negative control is shown in figure 2. CFU concentrates of 1 CFU/PCR were identical to our negative control in all experiments performed, with C(t) values of 33.1–34.7 (n = 6 single tests with 1 CFU/ PCR in total; mean C(t) = 33.6; standard deviation C(t) = 0.32). The 1 CFU/sample setup can be regarded as negative control also for whole blood and as a clear indication that the PCR does not cross-react with human mitochondrial DNA.
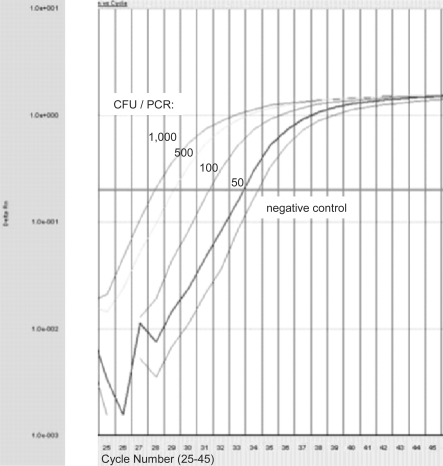
Fig. 2.
Exemplary amplification plot of different E. coli dilutions. Amplification plots of 1,000, 500, 100, 50 CFU per PCR and negative control (no template control) for PCR cycles 25 to 45 are shown. C(t) = 27.90, C(t) = 29.08, C(t) = 31.21 and C(t) = 33.30 represent 1,000, 500, 100, and 50 CFU/PCR, respectively. C(t) = 34.21 represents the negative control.
Defining a difference of at least 1 C(t) as threshold between PCR positivity and negativity, 3 out of 6 (50%) E. coli and 4 out of 6 (67%) S. epidermidis dilutions would have been typed positive at concentrations as low as 50 CFU/PCR, and all 6 (100%) E. coli and 6 (100%) S. epidermidis dilutions of 100 CFU/PCR would have been found correctly positive. These data would correspond to a detection limit of our method of 1,250 and 2,500 bacterial CFU/ml sample material for E. coli and S. epidermidis, respectively.
DNA Decontamination of Taq Polymerase Using the Restriction Enzyme Sau 3A1
At a mixing ratio of TaqMan Universal PCR Master Mix to Sau 3A1 of 1 unit : 1 unit restriction enzyme, no raise of C(t) values, was observed compared to the tests without Sau 3A1, indicating a successful removal of contaminating bacterial DNA in the negative controls. When using E.coli (10,000 CFU) as a positive control, increasing the PCR Master Mix to Sau 3A1 ratio from 1:1 to 1:3 or 1:6, C(t) values raised, at first view indicating a decrease of contaminating bacterial DNA. However, the C(t) values of the negative control were also raised accordingly (table 3). Furthermore, the C(t) value difference between positive and negative control also decreased (table 3), indicating a desensitization of the detection system in general. Comparable data were observed using S. epidermidis as a positive control. The above mentioned observations indicate that the treatment of the TaqMan Universal PCR Master Mix with the restriction enzyme Sau 3A1 in our protocol seemed to deteriorate Taq polymerase activity only and did not have any beneficial effect on the general test system sensitivity.
Table 3.
Bacterial DNA decontamination trial of Taq polymerase using the restriction enzyme Sau 3 Ala
| Untreated |
Sau 3 Al |
||
|---|---|---|---|
| 1:3 | 1:6 | ||
| E. coli (10,000 CFU) | 24.51 | 30.95 | 33.26 |
| Negative control (elution buffer) | 32.96 | 38.62 | 39.68 |
| Delta C(t) negative control – E. coli | 8.45 | 7.67 | 6.42 |
C(t) values of untreated and mixing ratios of TaqMan Universal PCR Master Mix to Sau 3A1 of 1 unit : 3 units restriction enzyme and 1:6 ratio are shown.
Testing the Commercially Available Taq Polymerase Depleted of Bacterial DNA
We were unable to observe a pronounced difference between the mean C(t) values for the negative control duplicate performed with AmpliTaq Gold DNA polymerase LD (C(t) = 32.5) and the ‘regular’ TaqMan Universal PCR Master Mix (C(t) = 33.1). The same observations were made for the 8-step serial dilution (5,000, 1,000, 500, 250, 100, 50, 10 and 1 CFU/ PCR) of both positive controls, E. coli and S. epidermidis. Therefore, we could not confirm a lower bacterial DNA contamination in the depleted DNA polymerase compared to those commonly used in real-time PCR master mixes.
DNA Decontamination by the Use of a Microfilter
We were unable to observe a pronounced difference between the mean C(t) values for the negative control duplicate performed with filtered (C(t) = 34.6) and unfiltered Taq polymerase (C(t) = 33.7). The same observations were made for a 8-step serial dilution (5,000, 1,000, 500, 250, 100, 50, 10 and 1 CFU) of both positive controls, E. coli and S. epidermidis. Thus, we were unable to proof a pronounced effect of the DNA decontamination by microfiltration.
Discussion
Even if real-time PCR is used as a tool, detection of microbial DNA is difficult. Current PCR reagents - especially the Taq polymerase itself - is contaminated with bacterial DNA originating from its bacterial production host, and the Taq polymerase showed a high natural affinity to DNA, both complicating the enzyme purification process.
One possibility to improve the sensitivity may be an optimization of the bacterial DNA preparation and PCR setup. An increase of DNA concentration in the final PCR may be achieved by rising the quantity of sample material for one single DNA preparation, reducing the elution volume of e.g. column-based DNA preparation procedures and increasing the absolute volume of DNA eluate per PCR.
Another way to improve the system's general detection sensitivity would be to reduce the contaminating bacterial DNA input mainly thought to be found in Taq polymerase enzyme preparations. Therefore, in this study two methods for DNA decontamination of Taq polymerase and a commercially available Taq polymerase depleted of bacterial DNA were tested to increase sensitivity of bacterial DNA detection using 16S rRNA gene real-time PCR. However, treatment of PCR master mix with a Sau 3AI restriction enzyme in order to degrade contaminating DNA only reduced Taq polymerase activity. This has been also found by others [7]. Neither did microfiltration of the PCR master mix lower the ‘false’ positivity of the negative control in our hands. Other reports on this method are controversial: Yang et al. [10] described an improvement, whereas Mohammadi et al. [14] found deterioration of sensitivity after filtration. With respect to commercially available Taq polymerase depleted of bacterial DNA, as already suggested by Rand et al. [5], again in our tests this approach did not show any influence on the total sensitivity. Additionally variations in bacterial DNA contamination found in different batches of Taq polymerase have been reported by Petershofen et al. [15]. Therefore, our discouraging findings may be due to the fact that only one batch of one specific distributor has been tested. Other Taq polymerases depleted of bacterial DNA are currently available, but we did not aim to perform a market survey of changing qualities of Taq polymerase suppliers. If users wish to become independent of batch-dependent bacterial DNA contamination in Taq polymerase, our preliminary conclusion is that other methods will be needed to address this problem. Currently, at least two other methods are reported to be effective: one of them uses ethidium monoazide [16], the other DNAse I to decontaminate Taq polymerase before its use [17].
Another approach could be to develop ‘multiplex’ PCRs specific for all bacterial species of interest but the Taq polymerase-producing host. However, since E. coli is both a pathogen which has to be detected and an organism widely used for Taq polymerase production, this seems illogical. Still, if Taq polymerase could be produced in a bacterial host, non pathogenic for humans and with unique 16S rDNA genes, this would offer an ‘all but host’ priming specificity for diagnostic test systems. An additional problem is that the pharmaceutical companies do not seem to be eager to reveal the species (and its 16S rDNA) of the Taq polymerase-producing host. Declaration of such 16S rRNA gene sequences of production bacteria would certainly help to develop more specific and contamination-independent PCR assays.
Although earlier descriptions of bacterial DNA decontamination of Taq polymerase were described as effective, taken together none of them proved functional in our hands. We were unable to achieve an improvement in sensitivity lower than for a total of 100 CFU per PCR reaction, which corresponds to a concentration of 2,500 CFU/ml sample material. Comparable detection limits, e.g. 238 fg corresponding to 48 E. coli cells per PCR, which is comparable to 50–100 CFU/PCR in our study, have already been described elsewhere [18]. However, even much lower detection limits, e.g. 175 CFU/ml specimen of Propionibacterium spp. [14] and 150 E. coli and 20 S. epidermidis CFU/ml whole blood [19], have been reported.
We are unable to explain the sensitivity reached by Mohammadi et al. [14].
However, highly variable number of rRNA operon numbers in the genomes of different bacterial species and possible aggregation of bacteria might offer some explanations for variable sensitivity reports [11, 12]. To compare different protocols, standardized bacterial reference material with known copy numbers of rRNA operons would be needed. Sensitivity reached by Mühl et al. [19] may partially be explained by the usage of an optimized DNA preparation strategy as concentration of starting specimens improved their sensitivity by a calculative factor of 6.25 compared to our method. The capacity of this potential improvement was not tested during our experiments.
In other settings, e.g. single cells in preimplantation diagnosis, PCR has proven its enormous potential already decades ago [20]. It is therefore unsatisfying to accept detection limits as high as 100 copy numbers/PCR in the course of bacterial PCR.
Alternatively, instead of bacterial DNA detection, direct microbiological methods, such as Scansystem™ (Hemosystem, Marseille, France) and BacT/ALERT® (bio-Mérieux, Nürtingen, Germany), can be taken into consideration for routine testing of leukoreduced platelet-rich plasma-derived platelets and apheresis platelets [21]. Last but not least, blood and blood products void of bacterial contamination may also be granted, not only by means of diagnosis but also by pathogen inactivation applications [22, 23]. Hence, detection of bacterial contamination of blood and blood products is not only a technical challenge, but a rather multifactorial topic in transfusion medicine, also dependent on medical, scientific and organizational parameters, best managed by experts in the field.
Disclosure
The authors declared no conflict of interest.
Acknowledgments
DNA of M. tuberculosis was kindly provided by Prof. Dr. W. Prodinger, Institute of Microbiology and Social Medicine, Medical University Innsbruck, Austria.
References
- 1.Mothershed EA, Whitney AM. Nucleic acid-based methods for the detection of bacterial pathogens: present and future considerations for the clinical laboratory. Clin Chim Acta. 2006;363(1-2):206–20. doi: 10.1016/j.cccn.2005.05.050. [DOI] [PubMed] [Google Scholar]
- 2.Barken KB, Haagensen JA, Tolker-Nielsen T. Advances in nucleic acid-based diagnostics of bacterial infections. Clin Chim Acta. 2007;384(1-2):1–11. doi: 10.1016/j.cca.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Zhang T, Fang HH. Applications of real-time polymerase chain reaction for quantification of microorganisms in environmental samples. Appl Microbiol Biotechnol. 2006;70(3):281–9. doi: 10.1007/s00253-006-0333-6. [DOI] [PubMed] [Google Scholar]
- 4.Corless CE, Guiver M, Borrow R, Edwards-Jones V, Kaczmarski EB, Fox AJ. Contamination and sensitivity issues with a real-time universal 16S rRNA PCR. J Clin Microbiol. 2000;38(5):1747–52. doi: 10.1128/jcm.38.5.1747-1752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rand KH, Houck H. Taq polymerase contains bacterial DNA of unknown origin. Mol Cell Probes. 1990;4(6):445–50. doi: 10.1016/0890-8508(90)90003-i. [DOI] [PubMed] [Google Scholar]
- 6.Corless CE, Guiver M, Borrow R, Edwards-Jones V, Fox AJ, Kaczmarski EB. Simultaneous detection of Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae in suspected cases of meningitis and septicemia using real-time PCR. J Clin Microbiol. 2001;39(4):1553–8. doi: 10.1128/JCM.39.4.1553-1558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll NM, Adamson P, Okhravi N. Elimination of bacterial DNA from Taq DNA polymerases by restriction endonuclease digestion. J Clin Microbiol. 1999;37(10):3402–4. doi: 10.1128/jcm.37.10.3402-3404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klaschik S, Lehmann LE, Raadts A, Hoeft A, Stuber F. Comparison of different decontamination methods for reagents to detect low concentrations of bacterial 16S DNA by real-time-PCR. Mol Biotechnol. 2002;22(3):231–42. doi: 10.1385/MB:22:3:231. [DOI] [PubMed] [Google Scholar]
- 9.Silkie SS, Tolcher MP, Nelson KL. Reagent decontamination to eliminate false-positives in Escherichia coli qPCR. J Microbiol Methods. 2008;72(3):275–82. doi: 10.1016/j.mimet.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Yang S, Lin S, Kelen GD, Quinn TC, Dick JD, Gaydos CA, Rothman RE. Quantitative multiprobe PCR assay for simultaneous detection and identification to species level of bacterial pathogens. J Clin Microbiol. 2002;40(9):3449–54. doi: 10.1128/JCM.40.9.3449-3454.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bremer H, Dennis PP. Escherichia coli and Salmonella typhimurium; In: Neidhardt FC, Curtiss R III, Ingraham JL, Lin ECC, Low KB, et al., editors. Cellular and Molecular Biology. Washington, DC: American Society for Microbiology; 1996. pp. 1553–1569. [Google Scholar]
- 12.Klappenbach JA, Saxman PR, Cole JR, Schmidt TM. rrndb: the Ribosomal RNA Operon Copy Number Database. Nucleic Acids Res. 2001;29(1):181–4. doi: 10.1093/nar/29.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393(6685):537–44. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadi T, Pietersz RN, Vandenbroucke-Grauls CM, Savelkoul PH, Reesink HW. Detection of bacteria in platelet concentrates: comparison of broad-range real-time 16S rDNA polymerase chain reaction and automated culturing. Transfusion. 2005;45(5):731–6. doi: 10.1111/j.1537-2995.2005.04258.x. [DOI] [PubMed] [Google Scholar]
- 15.Petershofen EK, Fislage R, Faber R, Schmidt H, Roth WK, Seifried E. Detection of nucleic acid sequences from bacterial species with molecular genetic methods. Transfus Sci. 2000;23(1):21–7. doi: 10.1016/s0955-3886(00)00051-5. [DOI] [PubMed] [Google Scholar]
- 16.Rueckert A, Morgan HW. Removal of contaminating DNA from polymerase chain reaction using ethidium monoazide. J Microbiol Methods. 2007;68(3):596–600. doi: 10.1016/j.mimet.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Tondeur S, Agbulut O, Menot ML, Larghero J, Paulin D, et al. Overcoming bacterial DNA contamination in real-time PCR and RT-PCR reactions for LacZ detection in cell therapy monitoring. Mol Cell Probes. 2004;18(6):437–41. doi: 10.1016/j.mcp.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 18.Nadkarni MA, Martin FE, Jacques NA, Hunter N. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148(Pt 1):257–66. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 19.Mühl H, Kochem AJ, Disqué C, Sakka SG. Activity and DNA contamination of commercial polymerase chain reaction reagents for the universal 16S rDNA real-time polymerase chain reaction detection of bacterial pathogens in blood. Diagn Microbiol Infect Dis. 2008 doi: 10.1016/j.diagmicrobio.2008.07.011. doi:10.1016/j.diagmicrobio. 2008.07.011. [DOI] [PubMed] [Google Scholar]
- 20.Erlich HA, Gelfand D, Sninsky JJ. Recent advances in the polymerase chain reaction. Science. 1991;252(5013):1643–51. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt M, Hourfar MK, Heck J, Weis C, Montag T, Nicol SB, Seifried E. Scansystem(tm) enables rapid and sensitive bacterial detection in platelets stored in additive solution with implementation of standard positive control capsules. Transfus Med Hemother. 2006;33(3):274–8. [Google Scholar]
- 22.Corash L. Inactivation of viruses, bacteria, protozoa and leukocytes in platelet and red cell concentrates. Dev Biol (Basel) 2000;102:115–23. [PubMed] [Google Scholar]
- 23.van Rhenen D, Gulliksson H, Cazenave JP, et al. Transfusion of pooled buffy coat platelet components prepared with photochemical pathogen inactivation treatment: the euroSPRITE trial. Blood. 2003;101:2426–33. doi: 10.1182/blood-2002-03-0932. [DOI] [PubMed] [Google Scholar]