Abstract
Brain plasticity is a common phenomenon across animals and in many cases it is associated with behavioral transitions. In social insects, such as bees, wasps and ants, plasticity in a particular brain compartment involved in multisensory integration (the mushroom body) has been associated with transitions between tasks differing in cognitive demands. However, in most of these cases, transitions between tasks are age-related, requiring the experimental manipulation of the age structure in the studied colonies to distinguish age and experience-dependent effects. To better understand the interplay between brain plasticity and behavioral performance it would therefore be advantageous to study species whose division of labor is not age-dependent. Here, we focus on brain plasticity in the bumblebee Bombus occidentalis, in which division of labor is strongly affected by the individual's body size instead of age. We show that, like in vertebrates, body size strongly correlates with brain size. We also show that foraging experience, but not age, significantly correlates with the increase in the size of the mushroom body, and in particular one of its components, the medial calyx. Our results support previous findings from other social insects suggesting that the mushroom body plays a key role in experience-based decision making. We also discuss the use of bumblebees as models to analyze neural plasticity and the association between brain size and behavioral performance.
Key Words: Bombus, Honeybee, Brain size, Cognition
Introduction
The association between brain plasticity and behavioral change is a subject of major interest across the animal kingdom [Kolb and Whishaw, 1998]. Social insects present a system well suited to address this correlation because in many species workers perform different tasks during their lifetime (temporal polyethism). This facilitates analyzing the correlation between behavioral transitions and neuroanatomical changes. For example, in workers of the honeybee Apis mellifera, critical behavioral, physiological and neural changes support the transition between performance of in-hive tasks and foraging activities [discussed by Fahrbach and Dobrin, 2009]. This transition is preceded by an enlargement of the mushroom bodies [Fahrbach et al., 1998], brain structures involved in the integration of multisensory information, learning and memory, initiation of motor activity [reviewed by Strausfeld et al., 1998; Zars, 2000] and sleep [Joiner et al., 2006]. Subsequent orientation flights and foraging experience are accompanied by further enlargement of the mushroom bodies and a decrease in the size of the antennal lobes involved in olfactory processing [Withers et al., 1993], resembling commonly found brain plasticity associated with experience in vertebrates [Kolb and Whishaw, 1998; Fahrbach and Dobrin, 2009]. Similar changes have been shown to occur in other social insect species exhibiting temporal polyethism, such as wasps and ants [Gronenberg et al., 1996; O'Donnell et al., 2004; Kühn-Bühlmann and Wehner, 2006]. Together, these findings show that temporal polyethism is accompanied by neuroanatomical changes, which may be further enhanced by experience. In honeybee workers, these neural changes go along with an increase in learning and memory abilities as they transition toward foraging.
In contrast to temporal polyethism, size-based division of labor is less common in social insects, particularly in bees. However, size-based division of labor provides a different perspective for evaluating hypotheses regarding behavioral specialization, development of cognitive capacities and brain plasticity. Importantly, it avoids having to account for the direct effect of age on an individual's behavior and the necessary manipulations to control for differences associated with age. For instance, in bumblebees, the large intracolony variation in body size among workers [up to 4 times in body mass in Bombus terrestris; Goulson, 2003] correlates much more strongly with division of labor than age does. Large individuals form most of the foraging workforce and may engage in out-of-nest tasks as early as 2 days after emergence [Riveros, pers. observations], whereas small workers engage in in-nest tasks and some may never leave the nest during their entire life. Accordingly, learning and memory performance are not affected by age in bumblebees, and 1-day-old individuals may perform as well as much older bees [Riveros and Gronenberg, 2009a]. However, foraging experience and body size are significantly associated with learning and memory performance in bumblebees [Riveros and Gronenberg, 2009a, b].
Based on these differences, several questions regarding brain plasticity and cognitive maturation emerge: is age associated with brain plasticity or with changes in cognitive capacities in species relying on size-based division of labor? How does foraging experience affect brain structure and cognition in such species? How are changes in body size associated with variation in brain size and cognitive capacities?
Here, we focus on the characterization of brain plasticity in the bumblebee Bombus occidentalis. Specifically, we explore the effect of foraging experience and age on brain plasticity. Taking advantage of the wide variation in body size in bumblebees, their role as models for studying cognition [e.g. Worden et al., 2005; Riveros and Gronenberg, 2009a, b], their advantages for neurophysiological approaches [Paulk et al., 2008, 2009], and their capacity to adapt to laboratory environments, we discuss the implications of our results in a broader context, exploring how the study of bumblebees may contribute to solve more general questions common to evolutionary neurobiology and cognitive ecology.
Materials and Methods
Rearing Conditions and Individual Tracking
All bees were from the same colony of B. occidentalis (Biobest, Inc., USA), which was maintained at 35% relative humidity, 19°C and 12 h/12 h photoperiod. The nest was connected to a foraging flight cage (2.0 × 1.2 × 1.2 m) where the bees collected sugar water (15% w/w), pollen (Bee Healthy Farms, USA) and water from artificial feeders. Only workers were used and all individuals were marked using numbered tags (Betterbee, Inc.) glued to their thorax. Thus, we were able to track age, foraging experience and body size for every bee in the colony. Newly emerged bees were marked every 2 days; hence our age estimation has an error of ±1 day. To assess very young bees, a group of pupae (n = 18) was isolated in individual plastic containers and dissected after eclosion. We collected randomly selected bees (from the entire population) ranging between 1 and 48 days old.
The nest entrance was continuously videorecorded to assess the individual bees’ foraging experience, defined as the number of trips to the foraging cage that exceeded 1 min. We therefore had a continuous record of each bee's foraging behavior (up to 48 days for the older individuals). Head width and fresh body mass (including only head and thorax) were used as measures for individual body size.
Brain Processing and Brain Volume Estimations
After collection, individual bees were chilled in ice for 10 min. Next, the brains were dissected from the head capsule and fixed in 4% phosphate-buffered formaldehyde for 2 h and then rinsed in phosphate buffer. Brains were then stained in the dark using 1% osmium-tetroxide for 2 h at 4°C and an additional 30 min at room temperature. After repeated rinses with distilled water, we dehydrated the brains using 50% ethanol (10 min), acidified 2,2-dimethoxypropane [10 min; Thorpe and Harvey, 1979] and acetone (10 min). Next, brains were plastic-embedded (Spurr's low viscosity medium, RT 14300 Electron Microscopy Sciences) and sectioned on a sliding microtome at 10–20 μm, mounted and coverslipped.
Outlines of the entire brain and individual brain regions were drawn using a camera lucida attached to a light microscope. We specifically assessed the volume of the following brain regions (see also fig. 1): optic lobes (medulla and the lobula, which are involved in processing of visual information), antennal lobes (centers for primary processing of olfactory information), fan-shape body within the central complex (generally considered to integrate multisensory information to coordinate complex motor function), and the mushroom bodies. Next, drawings of these structures were digitized on a flatbed scanner at 300 dpi and areas of the brain and brain components were calculated using the pixel counting routine in Adobe Photoshop v7.0 (Adobe). Volumes were determined by multiplying the area of each region by the section thickness. To estimate volumes, we outlined and measured every second (20 μm) or every third (10 μm) section. This method leads to an error of less than 5% compared to measuring each section [see Mares et al., 2005].
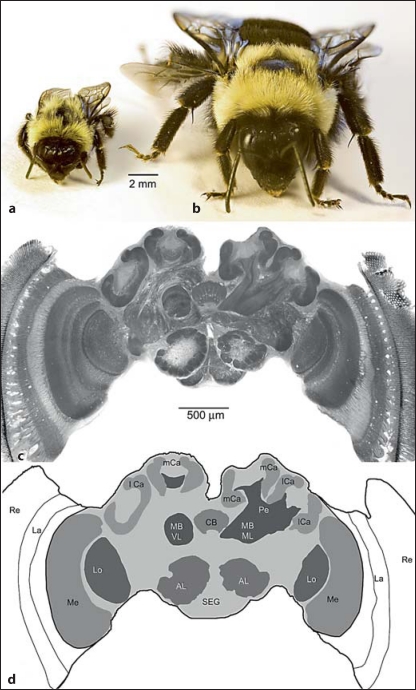
Fig. 1.
Bumblebee size variation and brain composition. A small (a) and a large bumblebee worker (b) approximately representing the body size range covered by the current study. c Bumblebee brain; montage of photomicrographs of three frontal brain sections representing different anteroposterior depths and showing major brain components. d Drawing representing micrograph in c and showing in shades of grey the brain components analyzed in this study; lateral white areas not included in analysis. AL = Antennal lobe; CB = central body; lCa, mCa = lateral and medial mushroom body calyx, respectively; La = lamina; Lo = lobula; Me = medulla; MBVL, MBML = vertical lobe and medial lobe of the mushroom body, respectively; Re = retina; SEG = subesophageal ganglion.
Statistical Analysis
To determine the effect of age, size and foraging experience on specific brain regions, we constructed multilinear regression models including all the bees to predict the volume of each region. The effect of each factor was determined using effect leverage plots, which represent the effect of each variable after accounting for the effect of the other two variables (e.g. effect of age after controlling for the effect of size and foraging experience). In the cases where not all the variables had a significant effect, the leverage plots presented are based on the reduced model. All statistical analyses were performed using the program JMP v 7.0 (SAS Institute, Inc.).
Results
Brain Size and Body Size
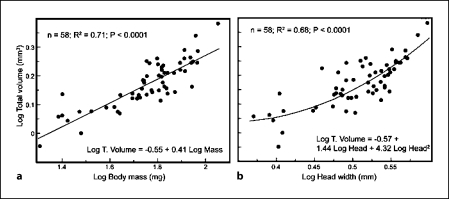
We found a significant allometric correlation between body mass and total brain volume. Total brain volume varied by a factor of almost 3 (0.89–2.41 mm3), whereas body mass varied by almost 6-fold (20.2–112.8 mg; fig. 2a). We also found a nonlinear correlation between brain volume and head width, which varies by a factor of almost 2 (2.35–3.96 mm; fig. 2b). The exponential correlation holds even after correction for differences in dimensionality by raising head width to the third power.
Fig. 2.
Correlation between total brain volume and proxies of body size: body mass (a) and head width (b).
Effect of Age, Size and Experience on Individual Brain Components
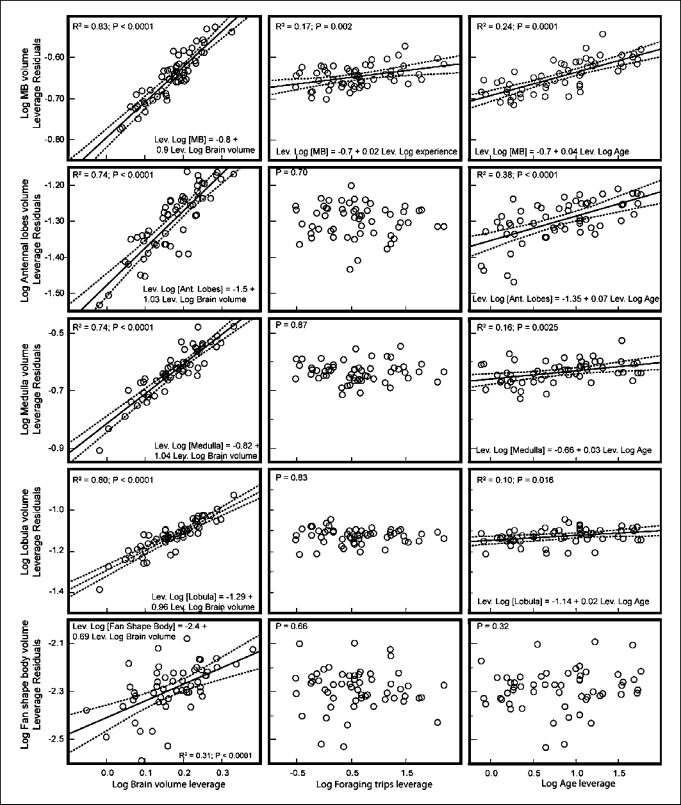
Including all individuals, our model showed a significant effect of total brain volume, age and experience on the volume of individual brain regions. However, not all the regions were equally affected by each factor. As expected, larger total brain volume explained most of the variation (31–83%) and was associated with increases in all individual regions (fig. 3). The slope of the regression lines varied between 0.69 (fan-shaped body) and 1.04 (medulla; fig. 3) after accounting for the effects of experience and age, but in all cases the 95% confidence interval included 1, showing that all the regions on average increase isometrically with respect to the total volume of the brain. Age was associated with increases in brain component volume, with the exception of the fan-shaped body. However, changes associated with age were slighter (explaining between 4–40% of the variation after accounting for the effect of brain volume and experience) and were statistically significant only due to the inclusion of the 1-day-old bees (see below). Foraging experience only affected the volume of the mushroom bodies (fig. 3), leading to a slight increase.
Fig. 3.
Effect of total brain volume, age and foraging experience on total size of individual brain components in bees of all ages.
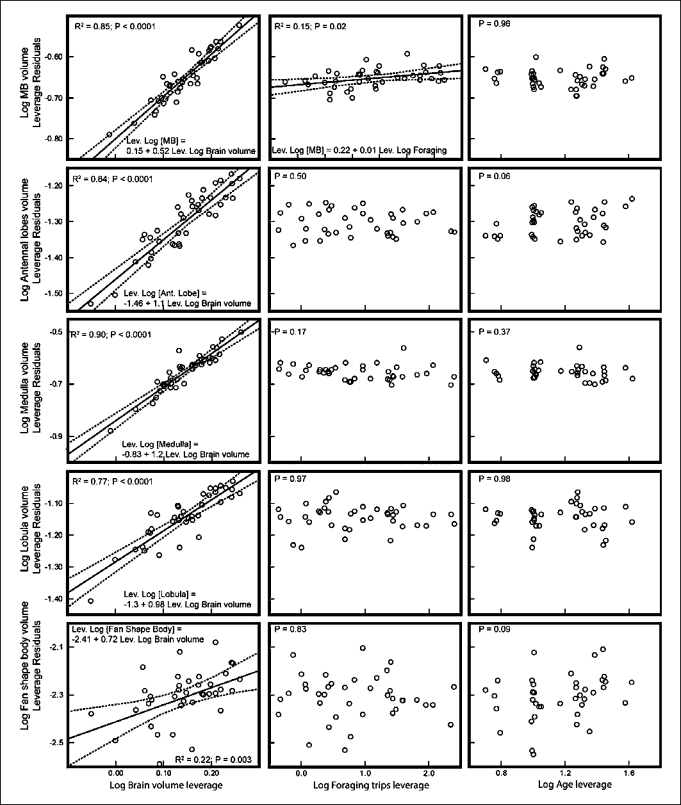
Given the effect of the 1-day-old bees on our first model (fig. 3), we constructed a second model excluding this group of bees (fig. 4). Similar to the first model, most of the variation (77–90%) in brain component volume was explained by changes in total brain volume, with the exception of the fan-shaped body (R2 = 0.22; fig. 4). Also consistent with the first model, the volume of the mushroom bodies increased along with foraging experience. In contrast, our second model showed that age did not affect the volume of the brain regions evaluated. Thus, 1-day-old bees seemed to differ from the other individuals, but age did not significantly affect the brain of older adults, suggesting that age-dependent brain volume changes occur only during the first 1–2 days.
Fig. 4.
Effect of total brain volume, age and foraging experience on total volume of individual brain components in bees older than 1 day.
Foraging Experience and Enlargement of the Mushroom Bodies
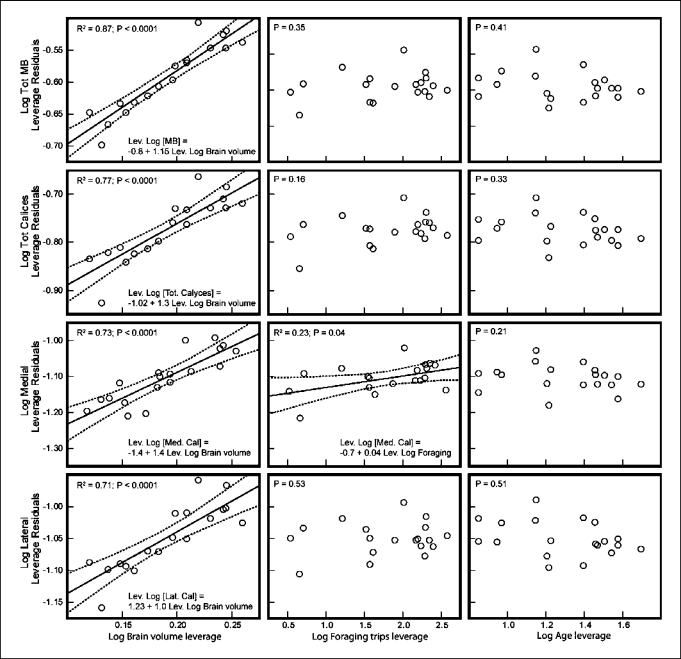
Given the consistent effect of foraging experience on the volume of the mushroom bodies, we conducted a more detailed evaluation of the bees that actively foraged (range of foraging trips = 1–297). We examined potential changes in the volume of the entire mushroom bodies, both calyces and each calyx (medial and lateral) separately. This analysis showed that increased foraging experience did not significantly affect the total volume of the mushroom bodies (fig. 5), the total volume of the calyces (fig. 5) or the volume of the lateral calyx (fig. 5) after accounting for the effect of the total brain volume. In contrast, we found that foraging experience significantly explained 23% of the variation in the volume of the medial calyx after accounting for the effect of the total brain volume (fig. 5; table 1). The slope of this correlation was significantly higher than 1 only in foragers (table 1), whereas the slope of the correlation for the lateral calyx was lower and did not differ from 1 (table 1).
Fig. 5.
Effect of total brain volume, foraging experience and age on the volume of individual components of the mushroom bodies of bees that foraged.
Table 1.
Values of slopes for the correlation between total brain volume and mushroom body calyces
| Group | Medial calyx slope (95% CI) | Lateral calyx slope (95% CI) |
|---|---|---|
| 1-day-old | 0.6 (0.4–0.8)a | 0.6 (0.3–0.9)a |
| Nonforagers | 1.2 (0.8–1.5) | 1.0 (0.7–1.3) |
| Foragers | 1.6 (1.2–2.l)b | 1.0 (0.6–1.3) |
Negative allometry.
Positive allometry.
Discussion
Here, we have presented an analysis of brain plasticity associated with age and foraging experience in bumblebees. We have quantified these variables in much greater detail than previous approaches, by recording precisely the foraging effort and age of each bee in the colony, rather than broadly defining foragers versus in-hive workers. The analysis of continuous variables associated with brain plasticity enabled the use of multivariate linear models that also included the effect of total brain volume. This latter aspect is very important because of the variation in brain volume in bumblebees associated with the large variation in body size [Mares et al., 2005].
Our results demonstrate that the variation in brain volume goes along with the variation in body mass and head width, as expected from studies comparing different mammalian species [Jerison, 1973]. Interestingly, the association between total brain volume and head width is almost equally well described as an exponential (r2 = 0.68) or a linear (r2 = 0.65) correlation. This might suggest the existence of two slopes within the correlation (fig. 2b). Within ‘small’ bees, the correlation does not deviate from isometry and exhibits a large variance (slope = 1.1; 95% CI for slope = 0.4 −1.8). Within large bees, the correlation exhibits positive allometry and less variation (slope = 1.6; 95% CI for slope = 1.1–2.2). The significance of this result cannot be inferred from our data but certainly warrants further investigations considering the association between body size and behavior in the context of division of labor in bumblebees.
Importantly, our results provide evidence for brain plasticity in specific areas within the brain after statistically controlling for the effect of the variation in brain volume as described above. Thus, our models for individual brain regions included total brain volume as a factor (fig. 3, 4). Excluding the effect of total brain volume (directly affected by variation in body size) allows determining potential significant changes in a brain component's volume associated with age or foraging experience. This showed a positive effect of age on most structures, with the exception of the fan shape body (fig. 3). However, this effect seemed to result from the inclusion of 1-day-old workers, as it was not significant within mature older adults (fig. 4). This may suggest a considerable change within the 1st week of life, although our results do not enable us to determine the nature of the change. However, even if bumblebee brains require a few days in order to fully develop, such differences do not seem to be crucial at least for some aspects of their behavior. Young bumblebee workers may start foraging within 1 or 2 days after emergence [Riveros, unpubl. data; O'Donnell et al., 2000] and, when trained using pavlovian olfactory conditioning, they exhibit adult-like levels of learning and memory as early as 2 h after emergence [Riveros and Gronenberg, 2009a].
These patterns of behavior and brain maturation in B. occidentalis contrast with observations from the honeybee A. mellifera, and might reflect divergences in the social structure of their colonies. A primary difference is the temporal pattern that follows the transition toward foraging activity. In honeybees, engagement in foraging typically starts when workers are about 2 weeks old, and once foragers, workers tend to perform much less or no work at all inside the hive. Following this pattern, the transition toward foraging behavior in honeybees is preceded by a significant increase in neuropil volume in the mushroom bodies, which has been regarded as an example of experience-expectant brain maturation [Fahrbach et al., 1998]. Further increases in the mushroom body neuropil are experience-dependent and are particularly large following foraging activity [Fahrbach et al., 2003; Ismail et al., 2006; Maleszka et al., 2009]. The antennal lobes are the only structures, besides the mushroom bodies, showing changes associated with behavioral maturation. In this case, however, changes point toward a reduction in the volume of the antennal lobes. Thus, nurses have bigger antennal lobes than foragers, even when foragers are older [Withers et al., 1993, but see Brown et al., 2004].
In contrast to honeybees, bumblebee workers may start foraging much earlier in life [O'Donnell et al., 2000 for Bombus bifarius; Riveros, pers. observations for B. occidentalis] and engagement in foraging does not preclude performance of activities inside the nest [O'Donnell et al., 2000; Jandt et al., 2009]. However, our results suggest that similarly to honeybee brains, bumblebee brain plasticity goes along with behavioral maturation and is affected by foraging experience and by changes during the first days of life. Age showed a significant effect on the volume of the structures within the brain only when the 1-day-old bees were included in the analysis, which suggests dramatic changes occurring very early in the adult life of workers. Whether such changes are analogous to the experience-expectant reorganization of the honeybee brain is unknown, yet not likely as our results showed differences in all the structures within the brain rather than only in the mushroom bodies. Furthermore, an experience-expectant reorganization of the bumblebee brain should occur relatively fast given the early performance of foraging activities. Assuming that the general developmental mechanisms are comparable in honeybees and bumblebees, our results suggest that an interesting heterochrony exists regarding both, brain and behavioral maturation. We currently do not know what mechanisms may underlie the apparently accelerated development of young adult bumblebees. In fact, we assume that bumblebees might represent a more basal developmental time course that one would also expect in solitary bees that need to be completely ‘functional’ soon after eclosure. In contrast, the delayed maturation of young honeybees may represent an adaptation for the highly social life of honeybees. While bumblebees appear to be precocious when compared to honeybees, they still mature and gain experience during their first days as foragers, as has also been shown for solitary bees (Osmia lignaria), whose mushroom bodies also increase with experience [Withers et al., 2007].
Unlike the apparent effect of age, plasticity associated with foraging experience was evident independently of the inclusion of 1-day-old bees in the models, yet it was limited to a specific region in the bumblebee brain, the mushroom bodies. The fact that the mushroom bodies exhibited plasticity associated with foraging experience is not unexpected, as it has been previously reported in both solitary [Withers et al., 2007] and social hymenopteran species, including honeybees [Withers et al., 1993; Durst et al., 1994], ants [Gronenberg et al., 1996; Kühn-Bühlmann and Wehner, 2006] and wasps [O'Donnell et al., 2004]. In our case, a more detailed analysis of the mushroom bodies including only foragers showed that the effect of experience was limited to the medial calyx (fig. 5; table 1). Previous reports on honeybees and ants have shown that experience does not always lead to a global increase in the volume of the mushroom body, but that the reorganization consistently involves the calyces [Durst et al., 1994; Kühn-Bühlmann and Wehner, 2006; Maleszka et al., 2009]. Furthermore, morphological plasticity can be localized in distinct subcompartments within the calyx. Thus, honeybee nurses have smaller collars (which receive visual input) and lips (which receive olfactory input) than foragers, but do not differ in the size of their basal ring [Durst et al., 1994]. Our current results suggest further exploration of the development of the lip, collar and basal ring regions in bumblebees, in particular in the medial calyx, which we found to be significantly affected by experience. This finding is unexpected as we are not aware of previous accounts suggesting functional differences between the calyces. In general, the two calyces are viewed as very similar, receiving identical or almost identical input and serving the same functions. This view is evident from the fact that most quantitative studies do not discriminate between the two calyces. Only a single study suggests a slight difference in the innervation pattern of the lateral and median calyx in honeybees [Abel et al., 2001]. However, like other studies, this study [Abel et al., 2001] implies that both calyces receive identical input. We therefore cannot explain our finding that the medial calyx appears to correspond more with foraging experience than the lateral one, but the problem may be relevant for understanding the evolution of double-calyx mushroom bodies in advanced hymenoptera, cockroaches and beetles and some other insects [Farris, 2005]. The increased size of only the medial calyx may also suggest a certain degree of functional subdivision, as is associated with brain region expansion in vertebrates [discussed by Kaas, 2005].
Also in contrast to honeybees, we did not find evidence of plasticity in the volume of the antennal lobes. As mentioned above, honeybee nurses exhibit bigger antennal lobes than foragers, presumably because of the enriched chemical environment within the hive [Withers et al., 1993]. It is likely that such differences are not observed or are more difficult to detect because bumblebee foragers are also active within the nest, thus sharing the enriched chemical experience of in-nest workers. Higher levels of activity inside and outside of the nest are expected because of the smaller colony size in bumblebees relative to honeybees, which also partially explains the fast engagement of workers in foraging behavior and probably their fast maturation of learning and memory abilities [Riveros and Gronenberg, 2009a].
A final difference in behavior between honeybees and bumblebees is the mode of foundation of new colonies. Although we have not explored it here, bumblebee queens, unlike honeybee queens, must forage during early stages of the colony. Thus, brain plasticity and learning and memory abilities are likely to have a stronger effect in the bumblebee queen brain than that observed in honeybee queens [Fahrbach et al., 1995].
Implications of Bumblebee Brain Allometry in Studies of Cognition
A remarkable aspect of the neurobiology of bumblebees is the large intraspecific variation in brain volume, which is clearly observed at the intracolony level [Mares et al., 2005]. We consider that such variation, together with our increasing knowledge of the bumblebees’ cognitive capacities, suggests that they can be used as models to approach the longstanding problem of the relationship between brain size and behavior [Rensch, 1956; Aboitiz, 1996; Healy and Rowe, 2007]. Typically introduced as a ‘bigger = better’ (‘B = B’) problem, the hypothesis is most often associated with two closely related predictions:
-
(1)
Behavioral complexity positively correlates with brain size.
-
(2)
Larger brains should perform particularly well in demanding cognitive tasks or provide higher ‘cognitive power’.
However, the evaluation of these two predictions has been repeatedly challenged due to methodological and semantic problems in defining appropriate measures of ‘cognition’ [which can also be affected by anthropomorphic estimations of animal cognition; Andrews, 2008, 2009] or ‘behavioral complexity’ and correlating them with proper measures of ‘brain size’ [Deaner et al., 2007; Healy and Rowe, 2007].
These problems are in part a consequence of the use of interspecific comparisons. Comparing different species allows the inclusion of larger ranges of brain sizes and the exploration of evolutionary trends, being more appropriate to approach prediction (1). However, regarding prediction (2), interspecific comparisons carry the problem of generalized measures of cognition and the difficulty to control for species-specific adaptations [Tinbergen, 1951, p. 12]. Thus, intraspecific comparisons should be much more appropriate to test prediction (2). On the other hand, it is important to remember that it is not brain size per se that underlies predictions (1) and (2), but the changes in internal organization and structure associated with variation in brain size [Striedter, 2005]. Also, brains are organized in anatomically segregated functional modules [Redies and Puelles, 2004; Sporns et al., 2004]. Thus, the identification and measurement of specific areas involved in particular cognitive processes, and the inclusion of measures of connectivity across brain regions and the ratio between volume and neuronal density need to be taken into account when available [Tononi et al., 1994; Striedter, 2005; Herculano-Houzel et al., 2007] in order to obtain a proper interpretation of the meaning of ‘brain size’. Focusing on a single species can ameliorate these problems.
We argue that the large variation of brain size in bumblebees supports their use to approach the ‘B = B’ question in neurobiology and cognition. Our results also highlight two main elements that support the use of bumblebees as models in future neurobiological studies. First, our results show a significant selective increase in the mushroom bodies as a consequence of foraging experience (fig. 3; table 1). Interestingly, our previous results evaluating learning and memory performance in bumblebees showed a significant improvement in learning associated with increased foraging experience [Riveros and Gronenberg, 2009a]. Whether these two correlates of foraging experience exhibit a causal relationship is currently unknown, but, given the central role of mushroom bodies in learning and memory, certainly warrants further investigation.
A second important result is the difference in brain allometry between 1-day-old bees and older adults. When compared with our previous results on learning, the remarkable learning performance of the youngest bees, which learned as well as the older workers [Riveros and Gronenberg, 2009a], is intriguing. Thus, it seems that the change in brain allometry found here is not associated with changes in our measures of behavioral performance. However, the fact that inexperienced young bees can be reliably tested in a learning and memory paradigm opens up new opportunities to examine in more detail correlations between brain composition and behavioral performance without the confounding effect of experience.
It is also important to consider the effect of the variation in size at the level of the sensory organs. In bumblebees, larger body size correlates with longer antenna [and greater number of olfactory receptors; Spaethe et al., 2007] and larger eyes [and greater number of ommatidia; Kapustjanskij et al., 2007], which leads to higher sensory sensitivity in large bees. Thus, differences in performance are not exclusively associated with differences in the brain but may be strongly influenced by the amount of information being captured by the sensory organs.
Finally, it is important to consider the link between brain composition, size and behavior in the context of the social organization of the bumblebees. Large bees compose most of the foraging workforce, which probably relies on their higher performance in resource detection and exploitation [Spaethe et al., 2001; Spaethe and Weidenmüller, 2002]. Would one expect small workers to experience more or fewer critical changes in the brain because of foraging activity? In a different social insect, the desert ant Cataglyphis, changes in mushroom body size associated with foraging experience are relatively larger in small than in large workers [Kühn-Bühlmann and Wehner, 2006], suggesting that within the social group larger individuals are better equipped than smaller ones for foraging tasks. As maintenance of neural tissue is energetically very expensive [Laughlin et al., 1998; Laughlin, 2001], small individuals might face larger costs of foraging if greater investment in brain development is required. At the colony level, however, such costs might be minute unless there were only small foragers available. In bumblebees, further research on the potential differences in brain plasticity between large and small individuals is needed. Also, if smaller bees, indeed, undergo more dramatic changes in their brain composition, it will be interesting to determine whether learning and memory performance also increase and whether changes in the mushroom bodies are localized in a particular region of the calyces.
Acknowledgements
We thank Fabiola Santos, Renden Sullivan, and Chirag Patel for the analysis of video recordings. This manuscript was also greatly improved thanks to comments by one anonymous reviewer and the editor. This work was supported by the National Science Foundation (IOS-0724591 grant to W.G.), a Sigma-Xi Grant-in-Aid to A.J.R. and by the Interdisciplinary Graduate Program in Insect Science at the University of Arizona (A.J.R.).
References
- Abel R, Rybak J, Menzel R. Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J Comp Neurol. 2001;437:363–383. doi: 10.1002/cne.1289. [DOI] [PubMed] [Google Scholar]
- Aboitiz F. Does bigger mean better? Evolutionary determinants of brain size and structure. Brain Behav Evol. 1996;47:225–245. doi: 10.1159/000113243. [DOI] [PubMed] [Google Scholar]
- Andrews K (2008): Animal cognition; in Zalta EN (ed): The Stanford Encyclopedia of Philosophy. http://plato.stanford.edu/
- Andrews K. Politics or metaphysics? On attributing psychological properties to animals. Biol Philos. 2009;24:51–63. [Google Scholar]
- Brown SM, Napper RM, Mercer AR. Foraging experience, glomerulus volume, and synapse number: a stereological study of the honey bee antennal lobe. J Neurobiol. 2004;60:40–50. doi: 10.1002/neu.20002. [DOI] [PubMed] [Google Scholar]
- Durst C, Eichmuller S, Menzel R. Development and experience lead to increased volume of subcompartments of the honeybee mushroom body. Behav Neural Biol. 1994;62:259–263. doi: 10.1016/s0163-1047(05)80025-1. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Dobrin S. The how and why of structural plasticity in the honey bee brain. In: Dukas R, Ratcliffe J, editors. Cognitive Ecology II. Chicago: University of Chicago Press; 2009. [Google Scholar]
- Fahrbach SE, Farris SM, Sullivan JP, Robinson GE. Limits on volume changes in the mushroom bodies of the honey bee brain. J Neurobiol. 2003;57:141–151. doi: 10.1002/neu.10256. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Giray T, Robinson GE. Volume changes in the mushroom bodies of adult honey bee queens. Neurobiol Learn Mem. 1995;63:181–191. doi: 10.1006/nlme.1995.1019. [DOI] [PubMed] [Google Scholar]
- Fahrbach SE, Moore D, Capaldi EA, Farris SM, Robinson GE. Experience-expectant plasticity in the mushroom bodies of the honeybee. Learn Mem. 1998;5:115–123. [PMC free article] [PubMed] [Google Scholar]
- Farris SM. Evolution of insect mushroom bodies: old clues, new insights. Arthropod Struct Dev. 2005;34:211–234. [Google Scholar]
- Gronenberg W, Heeren S, Holldobler B. Age-dependent and task-related morphological changes in the brain and the mushroom bodies of the ant Camponotus floridanus. J Exp Biol. 1996;199:2011–2019. doi: 10.1242/jeb.199.9.2011. [DOI] [PubMed] [Google Scholar]
- Goulson D. Bumblebees: Behaviour and Ecology. Oxford: Oxford University Press; 2003. [Google Scholar]
- Healy SD, Rowe C. A critique of comparative studies of brain size. Proc Biol Sci. 2007;274:453–464. doi: 10.1098/rspb.2006.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proc Natl Acad Sci USA. 2007;104:3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N, Robinson GE, Fahrbach SE. Stimulation of muscarinic receptors mimics experience-dependent plasticity in the honey bee brain. Proc Natl Acad Sci USA. 2006;103:207–211. doi: 10.1073/pnas.0508318102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandt JM, Huang E, Dornhaus A. Weak specialization of workers inside a bumblebee (Bombus impatiens) nest. Behav Ecol Sociobiol. 2009;63:1829–1836. [Google Scholar]
- Jerison HJ. Evolution of the Brain and Intelligence. New York: Academic Press; 1973. [Google Scholar]
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature. 2006;441:757–760. doi: 10.1038/nature04811. [DOI] [PubMed] [Google Scholar]
- Kaas JH. From mice to men: the evolution of large, complex human brain. J Biosci. 2005;30:155–165. doi: 10.1007/BF02703695. [DOI] [PubMed] [Google Scholar]
- Kapustjanskij A, Streinzer M, Paulus HF, Spaethe J. Bigger is better: implications of body size for flight ability under different light conditions and the evolution of alloethism in bumblebees. Funct Ecol. 2007;21:1130–1136. [Google Scholar]
- Kolb B, Whishaw IQ. Brain plasticity and behavior. Annu Rev Psychol. 1998;49:43–64. doi: 10.1146/annurev.psych.49.1.43. [DOI] [PubMed] [Google Scholar]
- Kühn-Bühlmann S, Wehner R. Age-dependent and task-related volume changes in the mushroom bodies of visually guided desert ants, Cataglyphis bicolor. J Neurobiol. 2006;66:511–521. doi: 10.1002/neu.20235. [DOI] [PubMed] [Google Scholar]
- Laughlin SB. Energy as a constraint on the coding and processing of sensory information. Curr Opin Neurobiol. 2001;11:475–480. doi: 10.1016/s0959-4388(00)00237-3. [DOI] [PubMed] [Google Scholar]
- Laughlin SB, de Ruyter van Steveninck RR, Anderson JC. The metabolic cost of neural information. Nat Neurosci. 1998;1:36–41. doi: 10.1038/236. [DOI] [PubMed] [Google Scholar]
- Maleszka J, Barron AB, Helliwell PG, Maleszka R. Effect of age, behaviour and social environment on honey bee brain plasticity. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2009;195:733–740. doi: 10.1007/s00359-009-0449-0. [DOI] [PubMed] [Google Scholar]
- Mares S, Ash L, Gronenberg W. Brain allometry in bumblebee and honeybee workers. Brain Behav Evol. 2005;66:50–61. doi: 10.1159/000085047. [DOI] [PubMed] [Google Scholar]
- O'Donnell S, Donlan NA, Jones TA. Mushroom body structural change is associated with division of labor in eusocial was workers (Polybia aequatorialis, Hymenoptera: Vespidae) Neurosci Lett. 2004;356:159–162. doi: 10.1016/j.neulet.2003.11.053. [DOI] [PubMed] [Google Scholar]
- O'Donnell S, Reichardt M, Foster R. Individual and colony factors in bumble bee division of labor (Bombus bifarius nearcticus Handl; Hymenoptera, Apidae) Insectes Soc. 2000;47:164–170. [Google Scholar]
- Paulk A, Dacks A, Gronenberg W. Color processing in the medulla of the bumblebee (Apidae: Bombus impatiens) J Comp Neurol. 2009;513:441–456. doi: 10.1002/cne.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulk AC, Phillips-Portillo J, Dacks AM, Fellous JM, Gronenberg W. The processing of color, motion, and stimulus timing are anatomically segregated in the bumblebee brain. J Neurosci. 2008;28:6319–6332. doi: 10.1523/JNEUROSCI.1196-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redies C, Puelles L. Central nervous system development: from embryonic modules to functional modules. In: Schlosser G, Wagner GP, editors. Modularity in Development and Evolution. Chicago: University of Chicago Press; 2004. pp. 154–182. [Google Scholar]
- Rensch B. Increase of learning capability with increase of brain-size. Am Nat. 1956;90:81–94. [Google Scholar]
- Riveros AJ, Gronenberg W. Olfactory learning and memory in the bumblebee Bombus occidentalis. Naturwissenschaften. 2009a;96:851–856. doi: 10.1007/s00114-009-0532-y. [DOI] [PubMed] [Google Scholar]
- Riveros AJ, Gronenberg W. Learning from learning in bumblebees. Commun Integr Biol. 2009b;2:437–440. doi: 10.4161/cib.2.5.9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethe J, Brockmann A, Halbig C, Tautz J. Size determines antennal sensitivity and behavioral threshold to odor in bumblebee workers. Naturwissenschaften. 2007;94:733–739. doi: 10.1007/s00114-007-0251-1. [DOI] [PubMed] [Google Scholar]
- Spaethe J, Tautz J, Chittka L. Visual constraints in foraging bumblebee: flower size and color affect search time and flight behavior. Proc Natl Acad Sci USA. 2001;98:3898–3903. doi: 10.1073/pnas.071053098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaethe J, Weidenmüller A. Size variation and foraging rate in bumblebees (Bombus terrestris) Insect Soc. 2002;49:142–146. [Google Scholar]
- Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci. 2004;8:418–425. doi: 10.1016/j.tics.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Strausfeld NJ, Hansen L, Li Y, Gomez RS, Ito K. Evolution, discovery and interpretation of arthropods mushroom bodies. Learn Mem. 1998;5:11–37. [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. Principles of Brain Evolution. Sunderland: Sinauer; 2005. [Google Scholar]
- Thorpe JR, Harvey DMR. Optimization and investigation of the use of 2,2-dimethoxy-propane as a dehydration agent for plant tissue in TEM. J Ultrastruct Res. 1979;68:186–194. doi: 10.1016/s0022-5320(79)90153-9. [DOI] [PubMed] [Google Scholar]
- Tinbergen N. The Study of Instinct. London: Oxford University Press; 1951. [Google Scholar]
- Tononi G, Sporns O, Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc Natl Acad Sci USA. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers GS, Day NF, Talbot EF, Dobson HEM, Wallace CS. Experience-dependent plasticity in the mushroom bodies of the solitary bee Osmia lignaria (Megachillidae) Dev Neurobiol. 2007;68:73–82. doi: 10.1002/dneu.20574. [DOI] [PubMed] [Google Scholar]
- Withers GS, Fahrbach SE, Robinson GE. Selective neuroanatomical plasticity and division of labour in the honeybee. Nature. 1993;364:238–240. doi: 10.1038/364238a0. [DOI] [PubMed] [Google Scholar]
- Worden BD, Skemp AK, Papaj DR. Learning in two contexts: the effects of interference and body size in bumblebees. J Exp Biol. 2005;208:2045–2053. doi: 10.1242/jeb.01582. [DOI] [PubMed] [Google Scholar]
- Zars T. Behavioral functions of the insect mushroom bodies. Curr Opin Neurobiol. 2000;10:790–795. doi: 10.1016/s0959-4388(00)00147-1. [DOI] [PubMed] [Google Scholar]