Abstract
The heparan sulfate proteoglycan glypican-1, the chondroitin sulfate proteoglycan phosphacan/RPTP (receptor protein-tyrosine phosphatase)-ζ/β and the extracellular matrix protein tenascin-C were all found to be expressed by neural stem cells and by neural cells derived from them. Expression of proteoglycans and tenascin-C increased after retinoic acid induction of SSEA1-positive ES (embryonic stem) cells to nestin-positive neural stem cells, and after neural differentiation, proteoglycans and tenascin-C are expressed by both neurons and astrocytes, where they surround cell bodies and processes and in certain cases show distinctive expression patterns. With the exception of tenascin-C (whose expression may decrease somewhat), expression levels do not change noticeably during the following 2 weeks in culture. The significant expression, by neural stem cells and neurons and astrocytes derived from them, of two major heparan sulfate and chondroitin sulfate proteoglycans of nervous tissue and of tenascin-C, a high-affinity ligand of phosphacan/RPTP-ζ/β, indicates that an understanding of their specific functional roles in stem cell neurobiology will be important for the therapeutic application of this new technology in facilitating nervous tissue repair and regeneration.
Keywords: astrocyte, glypican, heparan sulfate, neuron, phosphacan, proteoglycan
Abbreviations: CNS, central nervous system; DMEM, Dulbecco's modified Eagle's medium; ES cell, embryonic stem cell; GFAP, glial fibrillary acidic protein; LIF, leukaemia inhibitory factor; RPTP, receptor protein-tyrosine phosphatase; RT–PCR, reverse transcription–PCR
INTRODUCTION
The potential use of stem cells for restoration of function in the CNS (central nervous system) after injury, or as a result of age-related degenerative and disease processes, is currently an area of intensive investigation in neurobiology (Cao et al., 2002; Lindvall and Kokaia, 2006; Dimos et al., 2008). Stem cell-derived neural cell lines that will be most useful for purposes of implantation include various types of clonally derived cells that are capable of indefinite replication in tissue culture and differentiation into neural cells (Gottlieb, 2002). Approaches for deriving neural cells from mouse ES (embryonic stem) cells are also applicable to human progenitor cells, and it appears that current tissue culture methods can be readily perfected to allow the isolation of highly purified preparations of the major neural cell types. Stem cells are also advantageous for implantation research because they can be used for the preparation of genetically engineered cell lines, for which transfection protocols have recently been optimized (Braam et al., 2008).
Because morphogenic signals co-ordinate major stem cell decisions to regulate the size, shape and cellular diversity in nervous tissue development (Panchision and McKay, 2002; Krencik and Zhang, 2006; Muotri and Gage, 2006), it is clear that the cellular and biochemical environment into which such cells are introduced will play a critical role in determining their survival, migration patterns and expression of differentiated functions. Proteoglycans and their extracellular matrix ligands (for reviews, see Sugahara and Mikami, 2007; Zimmermann and Dours-Zimmermann, 2008) are likely to exert a decisive influence on stem cell behaviour. These macromolecules would be derived both from the implanted cells themselves and from the recipient tissue, possibly resulting in reciprocal effects.
Glypican-1 is largely present as a glycosylphosphatidylinositol-anchored cell-surface heparan sulfate proteoglycan. However, we have previously demonstrated that it contains a nuclear localization signal, is present in the nuclei of CNS neurons and is transported to the nuclei of 293, COS-1, and C6 glioma cells, which show changes in the pattern of glypican nuclear immunoreactivity both during cell division and correlated with different phases of the cell cycle (Liang et al., 1997). These findings suggest that glypican-1 may be involved in the regulation of cell division and survival by directly participating in nuclear processes, possibly by facilitating the transport of growth factors into the nucleus. Recent studies have also shown that glypican-1 regulates anaphase-promoting complex/cyclosome substrates and cell-cycle progression in brain endothelial cells (Qiao et al., 2008), and that glypicans play an essential role in the regulation of morphogen signalling (e.g. by promoting internalization of Hedgehog; Beckett et al., 2008).
Phosphacan is an mRNA splice variant representing the entire extracellular portion of a transmembrane chondroitin sulfate proteoglycan, RPTP (receptor protein-tyrosine phosphatase)-ζ/β, which is a high-affinity ligand of a number of neural cell-adhesion molecules and of the extracellular matrix protein tenascin-C (reviewed in Margolis and Margolis, 1997). Although hyaluronan, the hyaluronan-binding chondroitin sulfate proteoglycans and phosphacan are present in some or all perineuronal nets (Carulli et al., 2007; Galtrey et al., 2008), the dense extracellular matrix structures that develop around many neuronal cell bodies late in development and are thought to be involved in restricting experience-dependent synaptic plasticity in the adult, phosphacan has also been detected very early in CNS development at extrasynaptic sites prior to synapse formation (Dino et al., 2006).
Tenascin-C was the first identified member of a highly conserved family of four large multimodular extracellular matrix glycoproteins found only in vertebrate organisms (for reviews, see Hsia and Schwarzbauer, 2005; Tucker and Chiquet-Ehrismann, 2009). They have distinctive expression patterns (only tenascin-C and tenascin-R are present in nervous tissue) and all of the tenascins share the characteristics of having tightly regulated expression both during development and throughout the life of an organism, and of modulating cell–matrix interactions and mediating cell adhesion in a manner that promotes cell motility. We have previously demonstrated that the fibrinogen-like globe of tenascin-C mediates its high-affinity interactions with the core proteins of two chondroitin sulfate proteoglycans that are characteristic of nervous tissue (neurocan and phosphacan/RPTP-ζ/β) and have potent effects on neural cell adhesion and neurite outgrowth (Milev et al., 1997). Because proteoglycans and their cell surface or extracellular matrix ligands can be expected to have major effects on stem cell behaviour, we have examined the expression of glypican-1, phosphacan/RPTP-ζ/β and tenascin-C by neural stem cells and differentiated neurons and glia derived from them.
EXPERIMENTAL
Antibodies
Rabbit antisera to phosphacan (Milev et al., 1994) and glypican-1 (Liang et al., 1997) have been described previously. Anti-tenascin-C, prepared by immunizing rabbits with tenascin-C purified from the U-251 MG human glioma cell line (Bourdon et al., 1985), was generously provided by Dr Mario Bourdon (La Jolla Institute for Molecular Medicine). The antisera had been adsorbed against serum proteins, fibronectin and laminin and found to be tenascin-C specific on ELISA and in immunoblots of total proteins from 1-day postnatal, 15-day and adult rat brain.
Antibodies to mouse GFAP (glial fibrillary acidic protein; MAB360), β-III tubulin (MAB1637) and the O4 oligodendrocyte marker (MAB345) were all obtained from Chemicon (Temecula, CA, U.S.A.). Secondary antibodies (FITC goat anti-rabbit IgG and Texas Red goat anti-mouse IgG or IgM) were obtained from Jackson ImmunoResearch (West Grove, PA, U.S.A.). The MC-480 antibody to SSEA-1 and the Rat-401 antibody to nestin were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA, U.S.A.).
Cell culture media and other chemicals
DMEM (Dulbecco's modified Eagle's medium) with high glucose (11960-069), MEM (minimal essential medium) non-essential amino acids (11140-050), MEM sodium pyruvate (11360-070), l-glutamine (25030-081), penicillin/streptomycin (15140-122) and trypsin/EDTA (25300-054) were all obtained from Gibco/Invitrogen (Carlsbad, CA, U.S.A.). ES Qualified fetal bovine serum (100-125) was from Gemini Bio-Products (Woodland, CA, U.S.A.). LIF (leukaemia inhibitory factor; ESG1107, ESGRO) was obtained from Chemicon.
Culture of mouse ES cells
Mouse ES and EMFI cells were obtained from the Alexandra Joyner laboratory (NYU Medical Center) and cultured as described by Matise et al. (2000). Mitomycin C-treated primary mouse embryonic fibroblast (EMFI) feeder cells (Doetschman et al., 1985) were plated on to dishes in EMFI medium [DMEM containing 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 15% (v/v) heat-inactivated fetal bovine serum and 50 μg/ml penicillin/streptomycin]. The following day, W4/129S6 ES cells (Auerbach et al., 2000) were suspended in ES cell medium (EMFI medium containing 2-mercaptoethanol, 0.1 mM, and LIF, 1000 units/ml) and plated on the feeder layer. The ES cells (from a stock used to make knockout mice and thus demonstrably normal) were propagated for only a limited time (∼1 month) before starting with new frozen stocks to avoid working with karyotypic variants. After 24 h in culture (65–70% confluent) the medium was replaced, and after a further 24 h, the cells (90–95% confluent) were plated on to 10 cm dishes (Nunc; 1-50679) coated with 0.1% bovine skin gelatin (Sigma; G9391) and maintained in this manner with a further replating over the following 3 days.
Culture of feeder-independent mouse ES cells
The feeder-free E14Tg2a.4 mouse ES cell line was obtained from the Mutant Mouse Regional Resource Center (Davis, CA, U.S.A.) and cultured in a manner very similar to that described above, with some minor differences in medium composition, trypsinization and gelatin coating of plates (Brennan and Skarnes, 1999; Skarnes, 2000). Differentiation and subsequent steps were performed exactly as described below for the W4/129S6 cell line.
Neural induction
Neural differentiation of ES cells was performed by the general procedure of Bain et al. (1998), but using the slightly simplified EMFI medium described above. ES cells were detached by mild trypsinization in EMFI medium and placed in suspension culture in dishes (Corning; 430591) coated with 0.1% agar (Gibco/BRL; M00391B). After 48 h they were transferred to fresh EMFI medium after gravity sedimentation (10 min in 15 ml conical tubes) and replated in the same dish. Two days later they were transferred by sedimentation to EMFI medium containing 0.6 μM all-trans-retinoic acid (Sigma; R2625), and the medium was changed after a further 2 days. (During the 4-day period when cells were cultured in suspension with retinoic acid, the second day is referred to as day 2+ and the fourth and final day of the retinoic acid treatment as day 4+.) At days 2+ and 4+, and 9 days after beginning the retinoic acid induction, cells were seeded on to glass coverslips coated with 0.1 mg/ml poly-d-lysine (molecular mass >300000 Da; Sigma; P1024) in a 24-well plate (Corning; 3527; 0.5–1×106 cells per well). Differentiated cells were maintained for up to 2 weeks for immunocytochemistry.
Immunocytochemistry
Semi-quantitative immunofluorescence microscopy was employed to evaluate proteoglycan expression, due to its greater resolution (at essentially the single cell level) compared with biochemical procedures based on total cell protein, and in view of substantial technical obstacles to accurate quantification of large proteoglycan core proteins by techniques such as immunoblotting. Cells on coverslips were washed with PBS and fixed for 30 min at room temperature (25°C) with 3% (w/v) paraformaldehyde. After washing for 3×5 min with PBS, cells were treated for 1 h at room temperature with 5% BSA in PBS, and for immunocytochemical detection of intracellular markers (e.g. tubulin and GFAP) cells were permeabilized by inclusion of 0.2% Triton X-100 in the BSA blocking solution. Cells were treated overnight at 4°C in a humidified chamber with primary antibodies in BSA blocking solution, washed for 3×5 min with PBS and treated for 1 h at room temperature (25°C) with FITC- or Texas Red-conjugated secondary antibodies in blocking solution. After 3×5 min washes with PBS, nuclei were stained by treatment for 30 min at room temperature with TO-PRO-3 (Molecular Probes/Invitrogen, 1 μg/ml in PBS), washed for 3×5 min with PBS and the coverslips were mounted on slides using Vectashield (Vector Laboratories, Burlingame, CA, U.S.A.). Confocal imaging was performed using a Zeiss LSM 510 microscope with a Plan-Neofluar ×40 oil immersion objective.
RESULTS
Studies were performed using both the W4/129S6 cell line and a mouse ES cell line (E14Tg2a.4) that does not require a feeder layer. Examination of proteoglycan and tenascin-C expressions gave identical results in both the cases (e.g. most results were obtained with the W4 cells, but those shown in Figures 1 and 3(D)–3(F) were obtained with the feeder-independent E14T cell line).
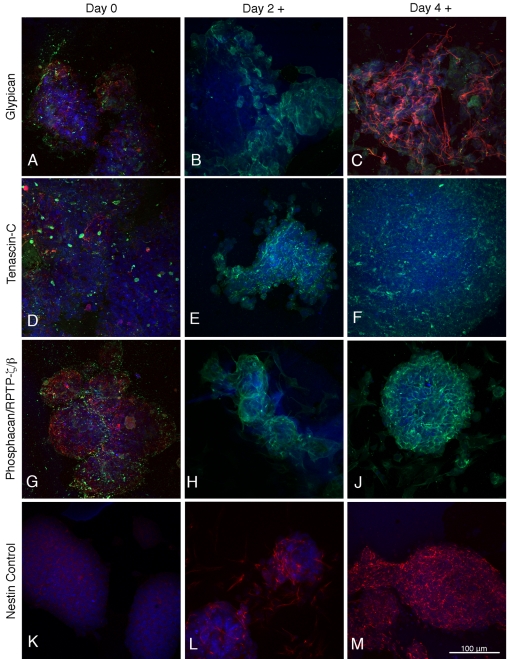
Figure 1. Expression of glypican-1, tenascin-C and phosphacan/RPTP-ζ/β in mouse ES cells (day 0) and on the second and fourth days of retinoic acid treatment during the neural induction period.
The green fluorescence in (A–C, D–F and G–J) represents glypican-1, tenascin-C and phosphacan/RPTP-ζ/β respectively. ES cells and neural stem cells are identified in red by their expression of SSEA-1 (A, D, G) and nestin (K, L, M) respectively. β-III tubulin staining (red) is shown in (C) and nuclear staining with TO-PRO-3 is blue.
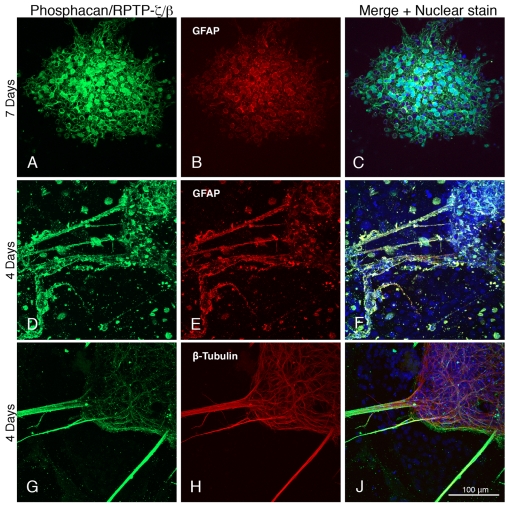
Figure 3. Expression of phosphacan/RPTP-ζ/β by neural cells derived from ES cells.
Days after differentiation are indicated on the left, and astrocytes and neurons are identified by their expression of GFAP and β-III tubulin. The green fluorescence indicates the presence of phosphacan/RPTP-ζ/β.
Glypican-1, phosphacan/RPTP-ζ/β and tenascin-C were weakly expressed in the SSEA-positive, nestin-negative ES cells (Figures 1A, 1D and 1G), and on the second day of retinoic acid treatment, SSEA-1 staining decreased and nestin staining first appears (Figure 1L), but neither tubulin nor GFAP is detectable. Proteoglycan and tenascin-C staining (Figures 1B, 1E and 1H) has, however, increased in intensity as compared with the ES cells. On day 4 of the retinoic acid treatment, only a small proportion of cells still show SSEA-1 staining, whereas almost all are strongly nestin positive (Figure 1M). There is also significant proteoglycan and tenascin-C expression in these neural stem cells (Figures 1C, 1F and 1J), as well as the first appearance of β-tubulin, although GFAP is still absent (Figure 1C). By 1 day after replating (‘differentiated day 1’ after the retinoic acid neural induction) most cells are strongly nestin-positive and there is similarly strong staining of β-tubulin, whereas significant staining of GFAP did not usually appear until day 4 of differentiation (the next time point examined), at which time nestin has greatly decreased. The neural induction protocol used in the present study yielded only a very small proportion of oligodendrocytes expressing the O4 marker. For the Figures, we selected fields that illustrate the range of neuronal and glial morphologies observed in our study. Thus, not all neurons or glia (identified by appropriate differentiation markers) will have the same appearance.
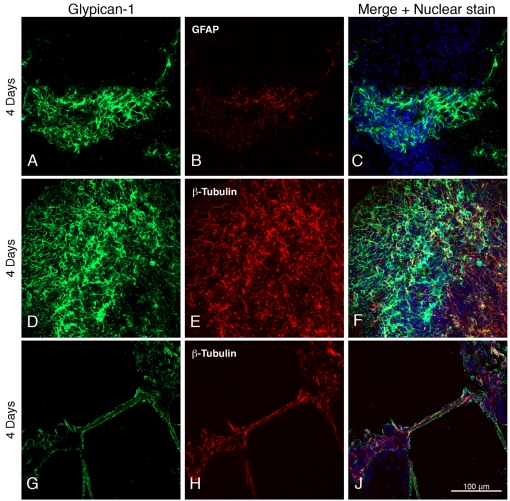
Staining of the heparan sulfate proteoglycan glypican-1 is seen around astroglial and neuronal cell bodies identified by the presence of GFAP and β-tubulin (Figures 2A–2C and 2D–2F) and on neuronal processes (Figures 2G–2J).
Figure 2. Expression of glypican-1 by neural cells derived from ES cells.
Days after differentiation are indicated on the left, and astrocytes and neurons are identified by their expression of GFAP and β-III tubulin. The green fluorescence indicates the presence of glypican-1.
Antibodies to the chondroitin sulfate proteoglycan phosphacan, which recognizes both RPTP-ζ/β and its extracellular domain (phosphacan), also showed staining surrounding astroglial cell bodies and their processes (Figures 3A–3C and 3D–3F), as well as on β-tubulin-expressing neuronal processes (Figures 3G–3J).
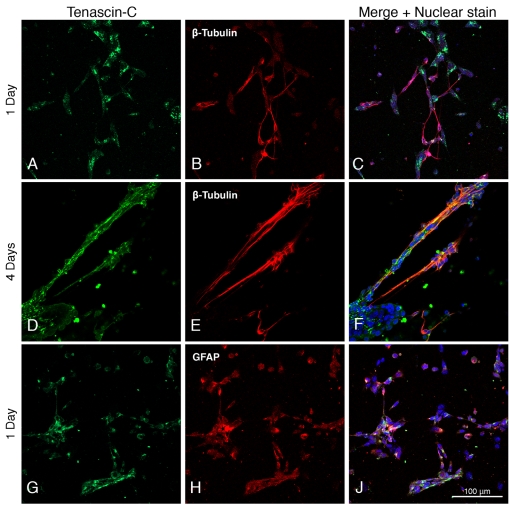
Tenascin-C appears as clumps concentrated on neurons (Figures 4A–4C) and covering neuronal processes connecting widespread groups of cells (Figures 4D–4F). It is also associated with astrocytes (Figures 4G–4J).
Figure 4. Expression of tenascin-C by neural cells derived from ES cells.
Days after differentiation are indicated on the left, and neurons and astrocytes are identified by their expression of β-III tubulin and GFAP. The green fluorescence indicates the presence of tenascin-C.
Cells were examined at 1, 4, 7, 10 and 14 days post-differentiation, and although most of the fields selected to show expression of proteoglycans were from 4-day post-differentiation cultures, there was no clear trend of increased or decreased proteoglycan expression as a function of time after differentiation. However, expression of tenascin-C appeared to be greatest early after differentiation.
DISCUSSION
Most previous studies of glycoconjugates in neural stem/progenitor cells have concerned glycolipids and glycoproteins, with relatively little information being available on proteoglycans and associated extracellular matrix molecules (reviewed by Yanagisawa and Yu, 2007). However, microarray studies comparing acutely isolated human brain A2B5+ progenitor cells with an unsorted white matter dissociate from which they were obtained demonstrated a 5–10-fold greater expression of the gene for phosphacan/RPTP-ζ/β in the progenitor cells (Sim et al., 2006). In other studies based on SDS/PAGE and immunoblotting, mouse neural precursor cells grown as neurospheres have been reported to secrete phosphacan and aggrecan into the medium, and genes for aggrecan, neurocan, brevican, phosphacan and tenascin-C could be amplified from mouse neurospheres by RT–PCR (reverse transcription–PCR; Kabos et al., 2004). Immunocytochemical studies also indicated that chondroitin sulfate surrounded nestin-positive cells or neural stem/progenitor cells in the rat ventricular zone of the telencephalon at embryonic day 14, and the chondroitin sulfate proteoglycans neurocan, phosphacan/RPTP-ζ/β and neuroglycan C, were detected in the ventricular zone (Ida et al., 2006). Neurospheres formed by cells from the fetal telencephalon also expressed these chondroitin sulfate proteoglycans. However, all of these studies have concerned neurosphere-forming stem and progenitor cells isolated from fetal tissue, which are known to differ in many significant respects from neural stem cells derived from ES cells (Shin et al., 2007). These latter cells, which are derived from the inner cell mass of preimplantation mammalian embryos, are a unique population of pluripotent cells that can differentiate into the embryonic precursors of all adult tissues. Moreover, most of these studies examined only gene expression, which may not reflect the actual expression of the corresponding protein(s).
The glycosaminoglycan chains of cell-surface heparan sulfate proteoglycans are ubiquitous elements of the stem cell ‘niche’ (i.e. the histologically defined microenvironment for stem cell regulation), and there is evidence that they play an important role in regulating extrinsic signalling pathways required for ES cell self-renewal and pluripotency (Nurcombe and Cool, 2007; Sasaki et al., 2008). Insofar as large changes in glycosaminoglycan biosynthesis and fine structure occur as murine ES cells differentiate to embryoid bodies and extraembryonic endoderm (Nairn et al., 2007) or to neural progenitor cells (Johnson et al., 2007), early developmental alterations in, for example, glypican-1 heparan sulfate fine structure, may modulate growth-factor binding, and thereby affect morphogenetic signalling processes critical for neural stem cell proliferation and differentiation.
Major ligands of glypican-1 in the CNS are the Slit proteins, which regulate axonal guidance, branching, dendritic development and neural migration, and with which glypican-1 co-localizes in brain (Liang et al., 1999; Ronca et al., 2001). In addition to earlier evidence for the role of cell-surface heparan sulfate in the repulsive guidance activities of Slit-2 protein, it is known that both Slit-2 and glypican-1 mRNAs are strongly up-regulated and co-expressed in the reactive astrocytes of injured adult brain, suggesting a possible function of Slit proteins and glypican-1 in the adult CNS (where few axon guidance events occur) as significant components of the inhibitory environment after injury. It is therefore possible that glypican-1 and Slit proteins, either acting alone or as a complex, are a significant factor in inhibiting axonal regeneration after spinal cord injury (Zhang et al., 2004; Lau and Margolis, 2010), a condition for which there has been much interest in the possible therapeutic use of ES cells.
Studies of neural stem cells in the subependymal zone of adult mouse brain have shown that the neural stem cell niche functions properly in the absence of tenascin-C, suggesting that this major extracellular matrix protein does not play a significant role in adult neurogenesis (Kazanis et al., 2007). However, recent induction gene trap studies of tenascin-C in neural stem cells indicate that its functions may be instructive rather than permissive or redundant, and that its absence leads to only marginal defects that the stem cell niche can compensate for as a functional, integrative entity (von Holst, 2008). Of the 64 theoretically possible tenascin-C isoforms that could be generated by alternative splicing, 20 have been described in neural stem cells maintained as neurospheres, where their expression is differentially regulated by Pax6 (von Holst et al., 2007). Although the functional significance of these various alternatively spliced tenascin-C domains is not yet clear, they may contribute to the formation of slightly different microenvironments within the neural stem cell niche (von Holst, 2008). It is also of interest that the transcript level of tenascin-C increased ∼100-fold after the retinoic acid-induced differentiation of ES cells to extra-embryonic endoderm (Nairn et al., 2007).
In the work reported here, we have demonstrated that ES cells differentiated to neural stem cells and then along neuronal and astroglial pathways produce chondroitin sulfate and heparan sulfate proteoglycans, as well as tenascin-C, a high-affinity extracellular matrix ligand of phosphacan. In a recent study demonstrating structural alterations in heparan sulfate during the differentiation of ES cells to Sox1-expressing neural progenitor cells, their transcription profiles were also compared by screening total RNA isolated from the cell populations for the presence of mRNA from a wide range of glycosaminoglycan synthetic enzyme and proteoglycan core protein genes (Johnson et al., 2007). Consistent with our results, RT–PCR detected expression of proteoglycan core proteins in both undifferentiated cells and in those expressing Sox1 (the earliest known specific marker of neuroectodermal precursors, before their differentiation into neurons and glia). Therefore even proteoglycans such as phosphacan that are considered as characteristic of nervous tissue are expressed at very early developmental stages. It should also be noted in this connection that the transcript abundance of phosphacan/RPTP-ζ/β increased ∼10-fold after the differentiation of ES cells to embryoid bodies, but was essentially absent when the stem cells were differentiated to extra-embryonic endoderm (Nairn et al., 2007). Moreover, recent studies have revealed a novel mechanism of axonal degeneration after spinal cord injury, involving protein tyrosine phosphatases that can be targeted with small molecule inhibitors that exert a neuroprotective effect (Nakashima et al., 2008). These results suggest that phosphacan/RPTP-ζ/β expressed by neural stem cells and neural cells derived from them may be significant in relation to the potential use of implanted neural stem cells for the treatment of spinal cord injury. It can be expected that future studies will clarify the specific neurodevelopmental roles of these proteoglycans and their associated ligands in stem cell populations, and how these interactions can be used to advantage in the therapeutic application of neural stem cells for the treatment of injury, degeneration and aging in the CNS.
ACKNOWLEDGMENTS
The MC-480 antibody to SSEA-1 developed by D. Solter and B.B. Knowles and the Rat-401 antibody to nestin developed by S. Hockfield were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD (National Institute of Child Health and Human Development) of the National Institutes of Health and maintained by the Department of Biological Sciences, University of Iowa (Iowa City, IA, U.S.A.).
Footnotes
This work was supported by the National Institutes of Health [grant numbers AG022590 and AG024969].
REFERENCES
- Auerbach W, Dunmore JH, Fairchild-Huntress V, Fang Q, Auerbach AB, Huszar D, Joyner AL. Establishment and chimera analysis of 129/SvEv- and C57BL/6-derived mouse embryonic stem cell lines. BioTechniques. 2000;29:1024–1032. doi: 10.2144/00295st04. [DOI] [PubMed] [Google Scholar]
- Bain G, Yao M, Huettner J, Finley M, Gottlieb D. Neuronlike cells derived in culture from P19 embryonal carcinoma and embryonic stem cells. In: Banker G, Goslin K, editors. In Culturing Nerve Cells. MIT, Cambridge, MA: 1998. pp. 189–212. [Google Scholar]
- Beckett K, Franch-Marro X, Vincent JP. Glypican-mediated endocytosis of Hedgehog has opposite effects in flies and mice. Trends Cell Biol. 2008;18:360–363. doi: 10.1016/j.tcb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Bourdon MA, Coleman RE, Blasberg RG, Groothius DR, Bigner DD. Monoclonal antibody localization in subcutaneous and intracranial human glioma zenografts: paired-label and imaging analysis. Anticancer Res. 1985;4:133–140. [PubMed] [Google Scholar]
- Braam SR, Denning C, van den Brink S, Kats P, Hochstenbach R, Passier R, Mummery CL. Improved genetic manipulation of human embryonic stem cells. Nat Methods. 2008;5:389–392. doi: 10.1038/nmeth.1200. [DOI] [PubMed] [Google Scholar]
- Brennan J, Skarnes WC. Gene trapping in mouse embryonic stem cells. Methods Mol Biol. 1999;97:123–138. doi: 10.1385/1-59259-270-8:123. [DOI] [PubMed] [Google Scholar]
- Cao Q, Benton RL, Whittemore SR. Stem cell repair of central nervous system injury. J Neurosci Res. 2002;68:501–510. doi: 10.1002/jnr.10240. [DOI] [PubMed] [Google Scholar]
- Carulli D, Rhodes KE, Fawcett JW. Upregulation of aggrecan, link protein 1, and hyaluronan synthases during formation of perineuronal nets in the rat cerebellum. J Comp Neurol. 2007;501:83–94. doi: 10.1002/cne.21231. [DOI] [PubMed] [Google Scholar]
- Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321:1218–1221. doi: 10.1126/science.1158799. [DOI] [PubMed] [Google Scholar]
- Dino MR, Harroch S, Hockfield S, Matthews RT. Monoclonal antibody Cat-315 detects a glycoform of receptor protein tyrosine phosphatase beta/phosphacan early in CNS development that localizes to extrasynaptic sites prior to synapse formation. Neuroscience. 2006;142:1055–1069. doi: 10.1016/j.neuroscience.2006.07.054. [DOI] [PubMed] [Google Scholar]
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985;87:27–45. [PubMed] [Google Scholar]
- Galtrey CM, Kwok JC, Carulli D, Rhodes KE, Fawcett JW. Distribution and synthesis of extracellular matrix proteoglycans, hyaluronan, link proteins and tenascin-R in the rat spinal cord. Eur J Neurosci. 2008;27:1373–1390. doi: 10.1111/j.1460-9568.2008.06108.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb DI. Large-scale sources of neural stem cells. Annu Rev Neurosci. 2002;25:381–407. doi: 10.1146/annurev.neuro.25.112701.142904. [DOI] [PubMed] [Google Scholar]
- Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem. 2005;280:26641–26644. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- Ida M, Shuo T, Hirano K, Tokita Y, Nakanishi K, Matsui F, Aono S, Fujita H, Fujiwara Y, Kaji T, Oohira A. Identification and functions of chondroitin sulfate in milieu of neural stem cells. J Biol Chem. 2006;281:5982–5991. doi: 10.1074/jbc.M507130200. [DOI] [PubMed] [Google Scholar]
- Johnson CE, Crawford BE, Stavridis M, Ten Dam G, Wat AL, Rushton G, Ward CM, Wilson V, van Kuppevelt TH, Esko JD, Smith A, Gallagher JT, Merry CL. Essential alterations of heparan sulfate during the differentiation of embryonic stem cells to Sox1-enhanced green fluorescent protein-expressing neural progenitor cells. Stem Cells. 2007;25:1913–1923. doi: 10.1634/stemcells.2006-0445. [DOI] [PubMed] [Google Scholar]
- Kabos P, Matundan H, Zandian M, Bertolotto C, Robinson ML, Davy BE, Yu JS, Krueger Jr RC. Neural precursors express multiple chondroitin sulfate proteoglycans, including the lectican family. Biochem Biophys Res Commun. 2004;318:955–963. doi: 10.1016/j.bbrc.2004.04.114. [DOI] [PubMed] [Google Scholar]
- Kazanis I, Belhadi A, Faissner A, Ffrench-Constant C. The adult mouse subependymal zone regenerates efficiently in the absence of tenascin-C. J Neurosci. 2007;27:13991–13996. doi: 10.1523/JNEUROSCI.3279-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krencik R, Zhang S-C. Stem cell neural differentiation: a model for chemical biology. Curr Opin Chem Biol. 2006;10:592–597. doi: 10.1016/j.cbpa.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Lau E, Margolis RU. Inhibitors of Slit protein interactions with the heparan sulfate proteoglycan glypican-1: potential agents for the treatment of spinal cord injury. Clin Exp Pharmacol Physiol. 2010;37:417–421. doi: 10.1111/j.1440-1681.2009.05318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Häring M, Roughley P, Margolis RK, Margolis RU. Glypican and biglycan in the nuclei of neurons and glioma cells: presence of functional nuclear localization signals and dynamic changes in glypican during the cell cycle. J Cell Biol. 1997;139:851–864. doi: 10.1083/jcb.139.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Annan RS, Carr SA, Popp S, Mevissen M, Margolis RK, Margolis RU. Mammalian homologues of the Drosophila slit protein are ligands of the heparan sulfate proteoglycan glypican-1 in brain. J Biol Chem. 1999;274:17885–17892. doi: 10.1074/jbc.274.25.17885. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans as mediators of axon growth and pathfinding. Cell Tissue Res. 1997;290:343–348. doi: 10.1007/s004410050939. [DOI] [PubMed] [Google Scholar]
- Matise MP, Auerbach W, Joyner AL. Production of targeted embryonic stem cell clones. In: Joyner A L, editor. In Gene Targeting – A Practical Approach., 2nd edn. New York: Oxford University Press; 2000. pp. 101–132. [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J Cell Biol. 1994;127:1703–1715. doi: 10.1083/jcb.127.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Fischer D, Häring M, Schulthess T, Margolis RK, Chiquet-Ehrismann R, Margolis RU. The fibrinogen-like globe of tenascin-C mediates its interactions with neurocan and phosphacan/protein-tyrosine phosphatase-ζ/β. J Biol Chem. 1997;272:15501–15509. doi: 10.1074/jbc.272.24.15501. [DOI] [PubMed] [Google Scholar]
- Muotri AR, Gage FH. Generation of neuronal variability and complexity. Nature. 2006;441:1087–1093. doi: 10.1038/nature04959. [DOI] [PubMed] [Google Scholar]
- Nairn AV, Kinoshita-Toyoda A, Toyoda H, Xie J, Harris K, Dalton S, Kulik M, Pierce JM, Toida T, Moremen KW, Linhardt RJ. Glycomics of proteoglycan biosynthesis in murine embryonic stem cell differentiation. J Proteome Res. 2007;6:4374–4387. doi: 10.1021/pr070446f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima S, Arnold SA, Mahoney ET, Sithu SD, Ping Zhang Y, D'Souza SE, Shields CB, Hagg T. Small-molecule protein tyrosine phosphatase inhibition as a neuroprotective treatment after spinal cord injury in adult rats. J Neurosci. 2008;28:7293–7303. doi: 10.1523/JNEUROSCI.1826-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurcombe V, Cool SM. Heparan sulfate control of proliferation and differentiation in the stem cell niche. Crit Rev Eukaryot Gene Expr. 2007;17:159–171. doi: 10.1615/critreveukargeneexpr.v17.i2.50. [DOI] [PubMed] [Google Scholar]
- Panchision DM, McKay RD. The control of neural stem cells by morphogenic signals. Curr Opin Genet Dev. 2002;12:478–487. doi: 10.1016/s0959-437x(02)00329-5. [DOI] [PubMed] [Google Scholar]
- Qiao D, Yang X, Meyer K, Friedl A. Glypican-1 regulates anaphase promoting complex/cyclosome substrates and cell cycle progression in endothelial cells. Mol Biol Cell. 2008;19:2789–2801. doi: 10.1091/mbc.E07-10-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronca F, Andersen JS, Paech V, Margolis RU. Characterization of Slit protein interactions with glypican-1. J Biol Chem. 2001;276:29141–29147. doi: 10.1074/jbc.M100240200. [DOI] [PubMed] [Google Scholar]
- Sasaki N, Okishio K, Ui-Tei K, Saigo K, Kinoshita-Toyoda A, Toyoda H, Nishimura T, Suda Y, Hayasaka M, Hanaoka K, Hitoshi S, Ikenaka K, Nishihara S. Heparan sulfate regulates self-renewal and pluripotency of embryonic stem cells. J Biol Chem. 2008;283:3594–3606. doi: 10.1074/jbc.M705621200. [DOI] [PubMed] [Google Scholar]
- Shin S, Sun Y, Liu Y, Khaner H, Svant S, Cai J, Xu QX, Davidson BP, Stice SL, Smith AK, Goldman SA, Reubinoff BE, Zhan M, Rao MS, Chesnut JD. Whole genome analysis of human neural stem cells derived from embryonic stem cells and stem and progenitor cells isolated from fetal tissue. Stem Cells. 2007;25:1298–1306. doi: 10.1634/stemcells.2006-0660. [DOI] [PubMed] [Google Scholar]
- Sim FJ, Lang JK, Waldau B, Roy N, Schwartz TE, Pilcher WH, Chandross KJ, Natesan S, Merrill JE, Goldman SA. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann Neurol. 2006;59:763–779. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- Skarnes WC. Gene trapping methods for the identification and functional analysis of cell surface proteins in mice. Methods Enzymol. 2000;3285:592–615. doi: 10.1016/s0076-6879(00)28420-6. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Mikami T. Chondroitin/dermatan sulfate in the central nervous system. Curr Opin Struct Biol. 2007;17:536–545. doi: 10.1016/j.sbi.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta. 2009;1793:888–892. doi: 10.1016/j.bbamcr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- von Holst A. Tenascin C in stem cell niches: redundant, permissive or instructive? Cells Tissues Organs. 2008;188:170–177. doi: 10.1159/000112848. [DOI] [PubMed] [Google Scholar]
- von Holst A, Egbers U, Prochiantz A, Faissner A. Neural stem/progenitor cells express 20 tenascin C isoforms that are differentially regulated by Pax6. J Biol Chem. 2007;282:9172–9181. doi: 10.1074/jbc.M608067200. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17:57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- Zhang F, Ronca F, Linhardt RJ, Margolis RU. Structural determinants of heparan sulfate interactions with Slit proteins. Biochem Biophys Res Commun. 2004;317:352–357. doi: 10.1016/j.bbrc.2004.03.059. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmermann MT. Extracellular matrix of the central nervous system: from neglect to challenge. Histochem Cell Biol. 2008;130:635–653. doi: 10.1007/s00418-008-0485-9. [DOI] [PubMed] [Google Scholar]