Abstract
Treating cancer with vaccines has been a challenging field of investigation since the 1950s. Over the years, the lack of effective active immunotherapies has led to the development of numerous novel strategies. However, the use of therapeutic cancer vaccines may be on the verge of becoming an effective modality. Recent phase II/III clinical trials have achieved hopeful results in terms of overall survival. Yet despite these encouraging successes, in general, very little is known about the basic immunological mechanisms involved in vaccine immunotherapy. Gaining a better understanding of the mechanisms that govern the specific immune responses (i.e., cytotoxic T lymphocytes, CD4 T helper cells, T regulatory cells, cells of innate immunity, tumor escape mechanisms) elicited by each of the various vaccine platforms should be a concern of cancer vaccine clinical trials, along with clinical benefits. This review focuses on current strategies employed by recent clinical trials of therapeutic cancer vaccines and analyzes them both clinically and immunologically.
1. Introduction
Cancer is the second leading cause of death in the United States, exceeded only by heart disease (23.1% versus 26.0% of total deaths, resp.). Currently, 1 in 4 deaths in the United States is due to cancer. According to American Cancer Society statistics, an estimated 1,479,350 new cases and 562,340 deaths from cancer are expected during 2009, with a slightly higher incidence and death rate in the male population. Prostate, lung, and colorectal cancers are the most common types of cancer in men; breast, lung, and colorectal cancers are most common among women. Altogether, lung, breast, prostate, and colorectal cancers account for 49% of cancer-related deaths in the U.S. population [1]. Overall, except for lung cancer in women, incidence and mortality rates have steadily decreased for all 4 types of cancer in both men and women, probably due to both an increase in early diagnosis and improvements in therapy and combination therapies (surgery, radiotherapy, chemotherapy, and, lately, targeted therapy). But despite these encouraging advances, cancer is still a major public health problem worldwide, requiring new strategies and treatment modalities to optimize patient outcomes.
In this context, immunotherapy has always been an attractive and potentially efficient treatment for cancer patients [19]. Tumor immunotherapy can generally be classified as (a) passive (or adaptive), consisting of administration of cells or antibodies ex vivo, and (b) active, represented by vaccines, aimed at eliciting a specific immune response against tumor-specific antigens (TSAs) and tumor-associated antigens (TAAs). Prophylactic and therapeutic vaccines represent one of the most intriguing approaches in the multidisciplinary treatment of cancer patients. Compared to all other standard modalities (surgery, chemotherapy, radiotherapy, and adaptive immunotherapy), an effective vaccine-based immune response against tumor may be the only cancer treatment with the potential to last a lifetime. Theoretically, vaccinated patients could mount an immune response able to either cure tumor or keep it under constant restraint (i.e., immune surveillance), delaying tumor recurrence and prolonging survival.
One of the major problems in developing an efficient cancer vaccine is the lack of TSAs and the weakness of immune responses against TAAs, usually recognized by the immune system as self-antigens. During the last decades, various strategies for therapeutic cancer vaccines have been proposed to overcome this weak immune response against TAAs, including cell-based vaccines, DNA- or RNA-based vaccines, protein- or peptide-based vaccines, and vector-based vaccines [20]. The common rationale for all these modalities is the activation of antigen-presenting cells (APCs) and the stimulation of an antigen-specific cytotoxic T lymphocyte-(CTL-) mediated immune response. Dendritic cells (DCs) are the most potent APCs, and various strategies have been used to enhance their ability to activate T cells. This review focuses on the state of the art of these modalities and analyzes the most promising phase II/III clinical trials, emphasizing vaccines directed against carcinomas (Table 1). Despite recent achievements, one criticism of some of these clinical trials has been the lack of immunological data supporting the significant improvements in time to progression and overall survival (OS) observed. An effort should be made to define the specific components of each immune response as a consequence of anticancer vaccination. In this context, both the specificity and the identification of potential escape mechanisms (i.e., increase of Treg number or function, balance between positive and negative regulators of antitumor responses, such as CD28, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed death-1 molecule (PD-1) and its ligands PD-L1 and PD-L2) should be investigated. Increasing our understanding of how these modalities modulate the CTL response is vital to developing novel and effective antitumor vaccines.
Table 1.
Overview of 4 different vaccination strategies employed in clinical trials.
| VACCINE | PHASE | TUMOR | PTS* | NOTE | REF. |
|---|---|---|---|---|---|
| Vaccines with viral vectors | |||||
| PSA-TRICOM | II | Prostate | 122 | 8.5 mos OS improvement versus placebo. | [2–4] |
| II | Prostate | 32 | >16.4 mos OS improvement in HPS>18 mos group. | [5] | |
| PANVAC-VF | III | Pancreatic | 255 | Failed >OS. Pts with life expectancy <3 mos. | [6] |
| Vaccines with peptides | |||||
| Provenge | III | Prostate | 512 | 4.1 mos OS improvement versus placebo. | [7, 8] |
| Oncophage | III | Melanoma | 322 | Prolonged OS in M1a or M1b subpopulation. | [9] |
| III | Renal | 818 | No difference in DFS and OS. | [10] | |
| gp100 : 209-217(210 M) | III | Melanoma | 185 | Significant improvement in RR and PFS. | [11] |
| Stimuvax | IIB | Lung | 171 | 17.3 mos OS improvement versus BSC in locoregional stage IIIB. | [12] |
| Vaccines with tumor cells or tumor-cell lysates | |||||
| OncoVAX | III | Colon | 254 | Significant improvement in DFS and OS in stage II. | [13–15] |
| Reniale | III | Renal | 558 | Significant improvement in DFS and OS. | [16, 17] |
| GVAX | III | Prostate | 626 | Failed to improve OS versus docetaxel. | [6] |
| III | Prostate | 408 | Failed. Higher death rate in combination arm (vaccine + docetaxel) versus docetaxel alone. | [6] | |
| Vaccines with RNA | |||||
| mRNA from PCa cell lines | I/II | Prostate | 19 | Immunological responses. | [18] |
*PTS: patients enrolled.
The goal of therapeutic cancer vaccines is to “teach” the patient's own immune system to specifically recognize and eliminate tumor cells. The potential target for the immune response can be either TSAs (antigens present only on tumor cells) or TAAs (antigens present mostly on tumor cells but also on some normal cells). Theoretically, TSAs are the ideal target for cancer immunotherapy because of their specificity. They are largely composed of mutant proteins caused by somatic mutations in the original sequence of the protein. A major advantage of targeting TSAs is that many of these proteins have been demonstrated to be essential for tumorigenesis and cancer progression [21]. On the other hand, a major drawback of targeting TSAs is the fact that most of the mutations identified are unique to each tumor, potentially requiring the development of personalized immunotherapy for individual patients. In contrast, TAAs are commonly expressed on tumors with the same histology and are shared among tumors of different origin. A major limitation of targeting TAAs is that they are weakly immunogenic due to the tolerance for self-antigens acquired by the immune system in its developmental stages [22].
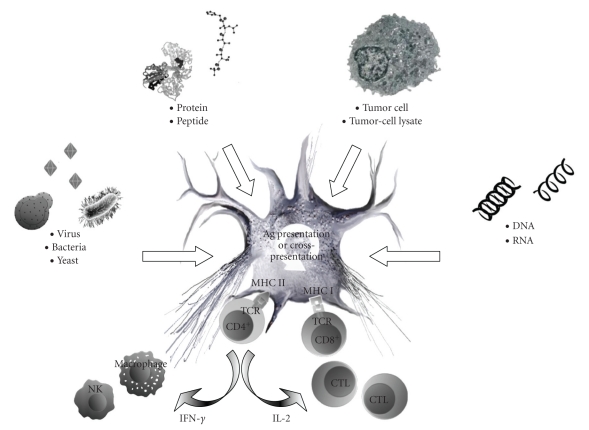
In the last decades, several different mechanisms have been proposed to “instruct” DCs, the most potent APCs known, to induce Th and CTL responses against tumor antigens, thus breaking immune tolerance. Antigen-loading techniques include (a) infecting DCs with viral, bacterial, or yeast vectors, (b) pulsing DCs with proteins or peptides, (c) loading DCs with tumor cells or tumor-cell lysates, and (d) transfecting DCs with DNA or RNA (Figure 1) [20].
Figure 1.
Various modalities employing dendritic cells for the development of therapeutic cancer vaccines.
Encouraged by positive preclinical and clinical data [23–27], further studies are currently ongoing to evaluate the possibility to enhance vaccine-induced immunity by combining vaccines with low doses of chemotherapeutic agents (i.e., cyclophosphamide, doxorubicin, docetaxel) or radiation therapy, that showed synergistic immunotherapeutic effects when given in proper sequence.
2. Vaccines with Viral, Bacterial, or Yeast Vectors
As mentioned above, one of the major difficulties in cancer immunotherapy is to develop a strategy to overwhelm the characteristically weak immune response of the host against TAAs. Several vectors can be used to deliver recombinant genes (including genes expressing TAAs, costimulatory molecules, or cytokines) into APCs. Recombinant vector-based vaccines may induce the immune system to generate a strong inflammatory response, directed mainly towards vector proteins. In turn, this inflammatory response may lead to an increased immune response against the genes of interest that have been inserted into the vector. One advantage of using vectors as vehicles for TAAs is that this type of delivery of a recombinant protein is much more immunogenic than administering the protein with adjuvants [28, 29].
Vectors used in cancer immunotherapy include viral, bacterial, and yeast vectors. The choice of vector can have important consequences for the subsequent immune response against TAAs because each vector has its own characteristics and is potentially able to uniquely stimulate the host immune system. A further factor that must be taken into account in the development of an efficient vector-based vaccine strategy is the balance between the stimulation of innate versus adaptive responses, Th1 versus Th2 responses, or the preferred activation of subsets of cells mainly committed to regulatory (Tregs, Tr1, and Th3) or proinflammatory functions (Th17).
Poxviral vectors are among the most heavily exploited in vaccine development. The prototype is vaccinia virus, which was used successfully to eradicate smallpox [30]. The poxvirus family is composed of double-stranded DNA viruses that replicate within the cytoplasm of infected cells. This feature is important for the safe use of poxviruses as recombinant vaccines, since no genetic sequence from the virus will be inserted into the host cell genome. However, owing to concerns about the use of replicating vectors in potentially immunocompromised patients and immune responses generated against the vector by immunocompetent patients, developing safe, nonreplicating viral vectors has been the focus of extensive research. Other attenuated poxviruses have been identified and are currently available for clinical use. Fowlpox, an avipoxvirus, can infect mammalian cells abortively, but recombinant-encoded genes are transcribed [31]. The drawback is that recombinant fowlpox usually generates a weaker immune response in humans than vaccinia and is thus often used for booster vaccinations after a primary vaccination with recombinant vaccinia. Modified vaccinia Ankara (MVA) is a highly attenuated strain of vaccinia that was developed by hundreds of passages of vaccinia virus in chick embryo fibroblasts. MVA can infect mammalian cells and undergo DNA replication in them but has lost the ability to produce infective viral particles [32]. Preclinical and clinical studies have demonstrated the superiority of a priming vaccination with recombinant vaccinia followed by multiple boosts with recombinant fowlpox, over different dosing schedules or the continuous use of either vector alone [33–36].
The large genome of poxviruses (approximately 130 kb for mammalian poxviruses and 300 kb for avian poxviruses) allows for insertion of more than 10 kb of foreign DNA.
Moreover, gene products are usually expressed at high levels, resulting in a potent cellular immune response. As mentioned previously, poxviruses can also be modified to express one or more T-cell costimulatory molecules along with the transgene for a TAA, or cytokines such as GM-CSF. Tumor recognition by CTLs is a complex mechanism that requires several different signals. DCs provide T cells with antigenic signal 1 through the specific interaction between the peptide-MHC I complex and the T-cell receptor. A costimulatory signal 2 is needed for the activation and expansion of T cells. Finally, DCs provide an additional polarizing signal 3 through the release of different cytokines, driving the immune response toward type-1 or type-2 immunity. Therefore, costimulatory molecules are critical in the generation of potent T-cell responses, particularly toward weak antigens such as TAAs. The most studied costimulatory signals involve the interaction between B7.1 (CD80) expressed on APCs and CD28 or CTLA-4 on T cells, between intercellular adhesion molecule-1 (ICAM-1 or CD54) on APCs and leukocyte function-associated antigen-1 (LFA-1) on T cells, and between LFA-3 (CD58) on APCs and CD2 on T cells [37].
PSA-TRICOM vaccine (prostate-specific antigen plus a TRIad of COstimulatory Molecules; PROSTVAC) consists of a priming vaccination with recombinant vaccinia- (rV-) PSA-TRICOM and booster vaccinations with recombinant fowlpox- (rF-) PSA-TRICOM. Each vaccine consists of the transgenes for PSA, including an agonist epitope [38], and 3 immune costimulatory molecules (B7.1, ICAM-1, and LFA3; designated TRICOM). The efficacy of PSA-TRICOM has been evaluated in 2 phase II clinical trials in patients with metastatic hormone-refractory prostate cancer (mHRPC). In the first multicenter clinical trial, 122 patients with Gleason scores of ≤ 7 were randomized 2 : 1 to receive PSA-TRICOM plus GM-CSF (n = 82) versus an empty-vector placebo (n = 40). A vaccinia-based vector was used as prime, followed by 6 boosts with a fowlpox-based vector. Vaccinated patients had a greater 3-year OS compared to the placebo arm (30% versus 17%, resp.) and an improvement in median OS of 8.5 months (24.5 months versus 16 months, resp.; P = .016). T-cell responses to vaccine or vector were not evaluated in this trial [2–4].
In a concurrent phase II clinical trial at the National Cancer Institute employing the identical PSA-TRICOM vaccine, 32 patients (representing all Gleason scores) were randomized to 1 of 4 cohorts. Cohort 1 received no immune adjuvant; cohort 2 received recombinant human GM-CSF protein; cohort 3 received 107 plaque-forming units (pfu) rF-GM-CSF; cohort 4 received 108 pfu rF-GM-CSF. All patients were primed with rV-PSA-TRICOM s.c. on day 1 and then received monthly boosts of rF-PSA-TRICOM until progression. Patients who remained on-study after 12 months had booster vaccinations every 3 months. With a median follow-up of 44.6 months, the median OS for all 32 patients on-study was 26.6 months, compared to a median Halabi nomogram-predicted survival of 17.4 months (an improvement of 9.2 months) [5]. No major differences were observed among the 4 cohorts. The subanalysis of patients with a Halabi-predicted survival (HPS) of <18 months showed a minimal difference between actual OS and HPS. However, patients with HPS >18 months had a significant increase in actual OS (>37.3 months, median not reached, with 8 of 15 patients still alive at 44.6 months) compared to HPS (20.9 months). PBMCs from patients pre and post vaccination were analyzed by ELISPOT assay to evaluate the specific immune response against the HLA-A2 PSA peptide PSA-3 [39]. Thirteen of 29 patients analyzed had an enhanced (≥2-fold) PSA-3-specific T-cell immune response post vaccination. Furthermore, patients with a postvaccination ELISPOT response to PSA epitope >6-fold seemed to live longer, compared to patients with a postvaccination ELISPOT response to PSA epitope <6-fold (P = .055). We also analyzed Treg function pre and post vaccination. Among patients who survived longer than predicted, Treg suppressive function decreased in 10/13 (77%) after 3 vaccinations versus pre vaccination. In contrast, among patients whose survival was less than predicted, Treg function increased in 6/8 (75%) after 3 vaccinations versus pre vaccination. These data strongly suggest that Tregs play a significant role in the modulation of antitumor immune response [40].
PANVAC-VF, another poxviral-based vaccine, consists of a priming vaccination with rV encoding CEA(6D), MUC1(L93), and TRICOM plus booster vaccinations with rF expressing the identical transgenes. CEA(6D) and MUC1(L93) represent carcinoembryonic antigen and mucin 1 glycoprotein, respectively, with a single amino acid substitution designed to enhance their immunogenicity [41, 42]. A phase III study in patients with advanced pancreatic cancer treated with PANVAC-VF as second-line therapy showed no improvement in survival [6]. The vaccine is currently under evaluation in several different types of CEA- or MUC1-expressing carcinomas and in patients with a life expectancy >3 months. In our experience, PANVAC-VF was well tolerated in a pilot study enrolling 25 patients with metastatic carcinomas. After vaccination, CAP1(6D)-specific CD8 immune responses were detected in 3/8 patients by ELISPOT, CAP1(6D)-tetramer, and intracellular IFN-γ staining. We also evaluated CD4 immune responses in 15 patients included in the study, using CEA protein as antigen. Six of 15 patients with undetectable levels of IFN-γ pre vaccination showed measurable levels in response to CEA protein. Four of 14 patients were positive for the generation of MUC1-specific T cells post vaccination [43].
The rationale for the use of microbes such as yeast as delivery vehicles for TAAs is based on the ability of these agents to activate a proinflammatory response through the interaction of pathogen-associated molecular patterns with pattern-recognition receptors, such as Toll-like receptors, expressed on APCs. These interactions play a central role in the activation of innate and adaptive immunity [44]. Over the years, several different bacterial and yeast vectors, such as Escherichia coli, Salmonella, Shigella, Yersinia, Listeria monocytogenes, and Saccharomyces cerevisiae, have been investigated for use as vaccine vectors.
The development of genetic engineering technology and efficient fermentation procedures has made large-scale, cost-effective production of these vectors possible and is one of the major advantages of their use in antitumor vaccines. Unfortunately, development of yeast-based vaccines has lagged behind that of cell-, protein-, and viral-based vaccines, and clinical experience has been limited to phase I/II studies [45]. One such vector currently being evaluated is a whole, heat-killed, recombinant S. cerevisiae yeast (Tarmogens GI-4000, GlobeImmune) intended to generate a T-cell response to eliminate tumor cells expressing the 7 most common mutations in the ras oncogene product. A randomized, double-blind, placebo-controlled phase IIa clinical trial has enrolled 100 patients with resected pancreatic cancer, with half receiving adjuvant gemcitabine plus placebo and half receiving adjuvant gemcitabine plus GI-4000 [46].
3. Vaccines with Proteins or Peptides
The use of proteins or peptides to stimulate a specific immune response against cancer has long been investigated and covers a broad spectrum of possibilities employing single agents or combinations of proteins, heat-shock proteins (HSPs) [47], peptides and agonist peptides [48–51], anti-idiotype antibodies [52, 53], and fusion proteins [54]. These protein- or epitope-based vaccines have 2 main advantages over the use of tumor cells or lysates: (a) production, storage, and distribution are faster and more cost-effective, and (b) the identification and administration of TSAs is preferable since tumor-cell preparations mostly contain self-proteins with no therapeutic benefit and are potentially capable of generating an autoimmune response. On the other hand, this approach has certain drawbacks: (a) first is the weak immunogenicity of a single protein or, especially, a single epitope; (b) tumors can easily escape immune recognition through antigen mutation or loss; (c) their use is HLA-restricted (mainly for epitope-based vaccines) and limited to a subset of patients (usually HLA-A2+); (d) they have a poor ability to induce balanced activation of CD4 and CD8 subsets, which is thought to be essential for effective, long-lasting antitumor immunity. To date, in fact, most epitope-based vaccines induce HLA-A2-restricted responses that efficiently kill tumor cells but are characterized by a limited lifespan in the absence of CD4 helper T cells. Protein-based vaccines are capable of generating stronger CD4 responses (MHC class II-restricted), but at the cost of less effective induction of CTLs [55, 56]. Most of the issues described above could be easily overcome by the use of longer peptides or the combination of several different epitopes in the same vaccine, while the relatively poor immunogenicity of peptides could necessitate that they be administered with adjuvants or loaded onto DCs [57, 58].
The use of specific proteins or peptides as targets for immunotherapy clearly requires a careful choice of the targeted TAAs and their epitopes, involving knowledge of their structural and functional characteristics. Single-peptide epitopes composed of 8 to 10 amino acids are able to induce a CTL response by binding to MHC class I molecules expressed on APCs. Each epitope is composed of conserved anchor residues (mostly at position 2 and the C-terminal position) needed to bind to the cleft of MHC I molecules and residues that are specific for T-cell recognition. Theoretically, changes in the former do not affect the specificity of the latter, and they have been used as a strategy to increase the immunogenicity of several different epitopes (agonist epitopes) [38, 41, 42, 50, 59]. Furthermore, the ideal TAA should be widely expressed in different tumor types and also play a central role in oncogenic processes or in cancer cell survival, to avoid immune escape by mutations or loss of antigens by tumor cells.
Identification of novel TAAs can be achieved through 2 experimental processes: direct immunology (starting from patient-derived autologous tumor-specific CTL clones specific for an unknown epitope) and reverse immunology (starting from a predicted epitope). The former has been used since the discovery of the first tumor-specific CTL epitope, MAGE-1 [60]. Direct immunology is further subdivided into genetic or biochemical approaches. Briefly, in the genetic approach, a patient-derived CTL clone is screened by using target cells transfected with tumor-derived cDNA libraries. Subsequently, the increased release of cytokines in the supernatant due to the recognition by the tumor-specific CTL clone allows one to select the cells that contain the antigen-encoding cDNA; these are then subcloned and rescreened to finally identify the cDNA that encodes the specific antigen. The biochemical approach consists of the purification of peptides eluted from MHC class I molecules of antigen-expressing cells by high-performance liquid chromatography fractionation. Antigen-negative target cells expressing the appropriate HLA molecule are used to load these peptides and tested for CTL recognition. Positive fractions are analyzed by mass spectrometry to identify the amino acid sequence of the epitope recognized by CTLs [61]. The need for expensive specialized equipment, plus the labor-intensive method, probably accounts for the increasing use of reverse immunology. Over the years, a growing understanding of HLA-specific peptide-binding motifs has led to the development of several computer algorithms for amino acid sequences with predicted binding capacity. Reverse immunology consists of two different phases: in the epitope prediction phase, proteins are analyzed for the presence of potential epitopes by the use of prediction algorithms. Subsequently, in the epitope validation phase, the candidate peptides are tested by binding and stability assays in vitro. Nevertheless, differences between the processing machinery in normal and tumor cells might be liable for the lack of activity against tumor cells of several CTLs raised against high-affinity binding TAAs [62]. Nowadays, indeed, the most recent algorithms also take into account the proteasomal processing and transporters associated with antigen processing- (TAP-)translocation, 2 other fundamental processes in the antigen-presentation pathway. Despite many efforts, the use of epitope-based vaccines has not advanced beyond phase I or II clinical trials, probably due to the drawbacks described above. To date, the best results have been achieved with the use of fusion protein- or HSP-based vaccines.
Provenge (sipuleucel-T, Dendreon Corporation) is in late-stage development for the treatment of mHRPC. Sipuleucel-T is an immunotherapy product designed to stimulate T-cell immunity against prostatic acid phosphatase (PAP). It consists of autologous APCs isolated by leukapheresis, cultured with a PAP-GM-CSF fusion protein, and reinfused into the patient. The time from apheresis to infusion of final product is approximately 48 hours. The efficacy of Provenge was evaluated in 2 randomized, double-blind, placebo-controlled phase III clinical trials (D9901 and D9902A) [7, 8]. D9901 enrolled 127 patients with asymptomatic mHRPC, who were randomly assigned 2 : 1 to receive 3 infusions of Provenge (n = 82) or placebo (n = 45) every 2 weeks. Enrollment in D9902A was stopped at 98 patients after D9901 showed encouraging results in terms of disease progression, and the study was amended to become D9902B (IMPACT), enrolling 512 patients with OS as the primary endpoint. An integrated analysis of 225 patients in D9901 and D9902A (147 in the vaccine arm and 78 in the placebo arm) demonstrated a survival benefit for patients treated with Provenge versus placebo (23.2 months versus 18.9 months, resp.), with a 33% reduction in the risk of death. The only immunological data to emerge from these studies are limited to the correlation between the upregulation of CD54 molecules on the cell surface of sipuleucel-T-treated APCs and OS, whereas no data are available about a specific immune response against PAP. At the American Urological Association 2009 Annual Meeting, Dendreon Corporation announced that the phase III IMPACT clinical trial had met its primary endpoint of significantly improving OS by 4.1 months compared to placebo {25.8 months versus 21.7 months, respectively, P = .032, HR = 0.775 [95% CI: 0.614, 0.979]}. The U.S. Food and Drug Administration (FDA) will respond to the existing Dendreon's amended Biologics License Application (BLA) for the licensing of Provenge in men with metastatic castrate-resistant prostate cancer (CRPC) by May 2010. If approved, Provenge will be the first active cellular immunotherapy to decisively demonstrate a survival benefit for cancer patients.
Oncophage (vitespen, Antigenics), an autologous tumor-derived HSP gp96 peptide complex, has been evaluated in 2 phase III clinical trials in stage IV melanoma patients and in renal cell carcinoma (RCC) patients at high risk of recurrence after nephrectomy [9, 10]. Oncophage consists of a purified preparation of the HSP gp96 from tumor. HSPs are noncovalently bound to peptides derived from self- and tumor-specific proteins. Immunization with gp96 peptide complexes leads to their uptake by DCs through CD91 (an HSP receptor) and stimulation of cognate T cells. In the first phase III clinical trial, 322 patients with stage IV melanoma were randomized 2 : 1 to receive Oncophage or a treatment of the physician's choice. The first 4 injections were administered weekly and subsequent injections were given every other week. Results from this trial suggested a survival benefit in the subpopulation of patients with M1a or M1b disease who were able to receive 10 or more doses of vaccine. In the second phase III trial of 818 patients with postnephrectomy RCC, no difference in recurrence-free survival or OS was observed between patients receiving Oncophage versus no treatment, although a trend toward a decrease in recurrence-free survival was reported in stage I or II disease in the experimental arm.
In a prospective randomized multicenter phase III trial, 185 patients with locally advanced stage III or stage IV melanoma were randomized to receive high-dose (HD) IL-2 alone (94 patients) or a synthetic peptide from the gp100 melanoma-associated antigen [gp100 : 209-217(210M)] plus an adjuvant (Montanide ISA) followed by HD IL-2 (91 patients). Overall response rate (RR, 22.1% versus 9.7%, P = .0223) and progression-free survival (PFS) (2.9 months versus 1.6, P = .0101) were significantly improved in the experimental arm compared with the HD IL-2 arm, respectively. Median OS was 17.6 months in the HD IL-2 + vaccine arm versus 12.8 in the HD IL-2 alone arm (P = .0964) [11].
Stimuvax (BLP25 liposome vaccine, L-BLP25, Oncothyreon partnered with Merck KGaA) is a cancer vaccine designed to induce an immune response against the extracellular core peptide of MUC1, a type I membrane glycoprotein widely expressed on many tumors (i.e., lung cancer, breast cancer, prostate cancer, and colorectal cancer). Stimuvax consists of MUC1 lipopeptide BLP25 [STAPPAHGVTSAPDTRPAPGSTAPPK(Pal)G], an immuno-adjuvant monophosphoryl lipid A, and three lipids (cholesterol, dimyristoyl phosphatidylglycerol, and dipalmitoyl phosphatidylcholine), capable of enhancing the delivery of the vaccine to APCs.
A randomized phase IIB clinical trial evaluated the effect of Stimuvax on survival and toxicity in 171 patients (88 in the L-BLP25 arm and 83 in the best supportive care arm (BSC)) with stage IIIB and IV nonsmall-cell lung cancer (NSCLC), after stable disease or response to a first-line chemotherapy [12]. Median OS was 17.4 months in the L-BLP25 arm and 13.0 months in the BSC arm, respectively, with a nonsignificant improvement of 4.4 months in the experimental arm (P = .112). T-cell proliferation assays were conducted on 78 of 88 patients enrolled in the L-BLP25 group, before and after immunization. Sixteen patients showed a MUC1-specific T-cell response (only two with a locoregional stage IIIB disease). No severe toxicities were reported. After a median follow-up of 53 months, updated survival data reported a median OS of 30.6 months in the Stimuvax arm versus 13.3 months in the BSC arm, in the subgroup of patients with locoregional stage IIIB (65 patients, of whom 35 were randomized to the vaccine arm and 30 were randomized to the BSC arm) (P = .09) [63]. Although nonsignificant, considering the magnitude of the difference and the prolonged follow-up, these results suggest a signal of efficacy for the vaccine.
Based on these data, Merck is currently conducting three large phase III clinical trials of Stimuvax. START (Stimulating Targeted Antigenic Responses To NSCLC) is a double-blind, placebo-controlled, randomized, multicenter phase III clinical trial that will enroll patients with unresectable stage IIIA or IIIB NSCLC, after stable disease or response to a platinum-based chemo-radiotherapy. This study will involve more than 1,300 patients.
The INSPIRE study (Stimuvax trial In Asian NSCLC Patients: stimulating Immune REsponse) will enroll approximately 420 patients with unresectable stage III NSCLC across China, Hong Kong, South Korea, Singapore, and Taiwan. STRIDE (STimulating immune Response In aDvanced brEast cancer) is a randomized, double-blind, controlled, multicenter Phase III study designed to evaluate the efficacy of Stimuvax, in combination with hormonal therapy, in patients with inoperable, locally advanced, recurrent, or metastatic breast cancer.
4. Vaccine with Tumor Cells or Tumor-Cell Lysates
Theoretically, tumor-cell vaccines have at least 3 advantages over the single-target approaches discussed above in terms of eliciting an immune response: (a) different and unknown antigens can be targeted at the same time; (b) the immune response is not HLA-restricted; (c) the variety of both MHC class I and class II epitopes processed is likely to be able to stimulate both an innate (NK cells, macrophages, and eosinophils) and adaptive (CD8+ and CD4+ T cells) response.
The first important distinction is between vaccines using autologous (patient-specific) or allogeneic (nonpatient-specific) tumor cells. Second, these cells may be unmodified, modified for expression of MHC, costimulatory molecules, or cytokines, or used in combination with adjuvants such as GM-CSF and Bacille Calmette-Guerin (BCG). Third, these cells can be used in the form of tumor-cell lysates [64].
The mechanism for priming naïve T cells in response to whole-cell or lysate vaccination is still unclear. Tumor antigens are probably phagocytosed by DCs and cross-presented to CD8+ cells by MHC class I molecules. In some models, a CD4+ response seems to be required for effective tumor rejection [65, 66]. A mesothelin-specific CD8+ T cell response has been shown in a clinical trial employing vaccination with GM-CSF-secreting pancreatic cancer cell lines. The results of this study provide the first direct evidence that a cross-priming mechanism mediated by professional APCs is involved in a postvaccination induction of CD8+ T cell response [51].
In the past 20 years, several different vaccines derived from whole tumor cells or tumor-cell lysates have been evaluated in preclinical models and clinical trials. OncoVAX (Vaccinogen) is composed of autologous irradiated tumor cells, with or without BCG as an adjuvant. In a multicenter phase III clinical trial, 254 patients with stage II and III colon cancer were randomly assigned, after curative resection for primary tumor, to receive OncoVAX or no adjuvant treatment [13]. The 5.8-year median follow-up showed a 20.4% reduction in risk of disease progression in patients receiving OncoVAX compared to the control group. Analysis by stage showed no significant benefit of OncoVAX in stage III disease, whereas a statistically significant improvement in recurrence-free survival in stage II was reported, with a 41.4% reduction in relative risk of disease progression (P = .018) in the OncoVAX arm. The OS rate for the OncoVAX-treated group was higher compared to control, with an 11.1% and a 33.3% relative risk reduction in all patients and stage II patients, respectively [14]. Besides the clinical data and a prospective study of medical and economic benefits, the only immunological mechanism proposed by the authors was the presence of a significant delayed cutaneous hypersensitivity response to tumor cells after the third and fourth OncoVAX treatments (which lack BCG), as a measure of the immugenicity of the treatment, potentially correlated with long-term survival [15].
Reniale (LipoNova) is a vaccine designed to treat RCC. It is based on a lysate of autologous tumor cells, preincubated with IFN-γ to increase the antigenicity of these cells, and tocopherol acetate to protect cell membranes during the incubation process. A randomized, open-label, multicenter phase III clinical trial compared adjuvant treatment with Reniale after radical nephrectomy versus radical nephrectomy alone in nonmetastatic RCC (pT2-3b, pN0-3, M0) [16]. Prior to surgery, 558 patients at 55 institutions in Germany were enrolled in the trial and were randomized to receive 6 s.c. vaccinations at 4-week intervals, or no adjuvant therapy (control group). The intention-to-treat (ITT) population consisted of 379 patients in the primary analysis (177 patients in the vaccine group and 202 patients in the control group). Progression-free survival at 5 years for patients at all tumor stages was 77.4% in the Reniale group and 67.8% in the control group (P = .0204). Interestingly, patients with a higher risk (T3 subgroup) showed greater benefit from adjuvant treatment with Reniale, with a 5-year PFS of 67.5% in the vaccine group and 49.7% in the control group. A secondary ITT analysis on 477 patients (233 patients in the Reniale group and 244 patients in the control group) showed a statistically significant advantage in the experimental arm in terms of PFS (P = .0476); there was no statistically significant difference in OS between the 2 arms (P = .1185). However, a per-protocol analysis of 352 patients revealed a statistically significant increase in PFS (P = .024) and OS (P = .0356) in the vaccine arm [17]. No immunological data from this study have been reported.
GVAX for prostate cancer (Cell Genesys) is an allogeneic vaccine composed of 2 irradiated human prostate cancer cell lines, LNCaP and PC-3, modified by ex vivo transduction with an adenoassociated viral vector encoding the human GM-CSF gene. A preclinical study has demonstrated that s.c. administration of these cells invokes a local immune response, characterized by a local infiltration of neutrophils, CD4+ T cells, and apoptotic cells. The irradiated tumor cells persist and secrete high levels of GM-CSF at the injection site for >21 days. Theoretically, secretion of GM-CSF by allogeneic tumor cells improves the antigen presentation of TSAs and TAAs through recruitment and maturation of DCs at the site of immunization. DCs then migrate to draining lymph nodes and activate antigen-specific CD4+ T cells, characterized by the production of both Th1 and Th2 cytokines. Moreover, DCs may efficiently capture apoptotic tumor cells and cross-present multiple TAAs on MHC class I molecules for recognition by host CD8+ T cells, as demonstrated by the ability of GM-CSF-secreting tumor cells to generate T-cell responses to multiple TAAs capable of targeting antigenically related but distinct tumors [67]. Based on encouraging clinical and immunological responses in 5 phase I/II clinical trials with nearly 200 prostate cancer patients [68–70], 2 phase III trials were initiated. VITAL-1 completed patient accrual in 2007, enrolling 626 patients with mHRPC randomized to receive GVAX as monotherapy for up to 6 months or standard docetaxel chemotherapy. The primary endpoint of the trial was improvement in OS. In 2008, Cell Genesys terminated the trial based on the results of a futility analysis conducted by the study's Independent Data Monitoring Committee (IDMC), which indicated a <30% chance of meeting the primary endpoint. VITAL-2 was a phase III trial designed to compare GVAX plus docetaxel versus docetaxel alone in mHRPC. The primary endpoint of VITAL-2 was also improved in OS. The trial was initiated in 2005 and enrolled 408 patients. In 2008, Cell Genesys announced its decision to terminate VITAL-2, as recommended by a safety review in which the IDMC reported an imbalance in deaths between the 2 treatment arms (67/114 deaths in the GVAX plus docetaxel arm and 47/114 deaths in the docetaxel-alone arm). In this case, despite encouraging preclinical and immunological data, GVAX failed to meet the defined endpoints of both phase III clinical trials [6].
Further clinical trials, employing GVAX cancer immunotherapies, are underway and include pancreatic and breast cancers. A randomized three-arm clinical trial is currently evaluating the efficacy and toxicity of GVAX for pancreatic cancer (GM-CSF secreting allogeneic pancreatic cancer vaccine) administered either alone or in combination with either a single intravenous dose or daily metronomic oral doses of cyclophosphamide for the treatment of patients undergoing chemotherapy and radiation therapy for stage I or II disease, surgically resectable.
Recently, studies of combination therapies of GVAX vaccine and CTLA-4-blocking antibodies have shown activity in melanoma and ovarian carcinoma, representing a potential new strategy to enhance vaccine-mediated antitumor effects [71].
5. DNA and RNA Vaccines
DNA-based vaccines are a recently developed strategy that has proven capable of activating strong immunity against weak TAAs. Several approaches have been developed and evaluated for enhancing the potency of DNA-based vaccines, including improved delivery systems (Gene Gun, cationic liposomes) [72, 73], simultaneous administration of cytokines (GM-CSF or IL2) [74], and the use of separate plasmids encoding nonself-antigens (i.e., hepatitis B surface antigen) [75]. The immunogenicity of DNA-based vaccines can also be enhanced by various modifications of plasmid-encoded antigens [76, 77].
Recently, several phase I/II clinical trials employing DNA-based vaccines targeting different TAAs (i.e., PSA, PAP, gp100, CEA, hsp65) have been conducted in patients with prostate cancer [78, 79], melanoma [80, 81], colorectal cancer [75], and head and neck carcinomas [82]. In all these trials, DNA-based vaccines were administered either as monotherapy or in association with different delivery systems and adjuvants. In terms of immune response, most of these trials showed a low immunogenicity of TAAs. The small sample size of these phase I/II studies precludes achieving a statistical correlation between development of an immune response and clinical outcomes in vaccinated patients. Evidence of clinical benefit must be evaluated in larger studies.
mRNA-based gene transfer vaccines are another attractive immunotherapeutic approach to cancer treatment [83, 84]. This method, based primarily on transient transfection of nondividing cells, is regarded as pharmaceutically safe because the transfected mRNA does not integrate into the host genome [85]. In addition, high transfection efficiency can be achieved by electroporation [86, 87]. mRNA, which can be effectively overexpressed in target cells, is generated by in vitro transcription from a bacteriophage promoter-equipped plasmid DNA. It is composed of a cap structure at the 5′ end, the coding RNA for target antigen, and a tail of poly-adenosine (polyA tail) [88]. The target antigen used can be a single peptide PSA [89] or CEA [90], allogeneic cancer cell lines [18, 91, 92], or autologous tumor mRNA [93]. The mRNA-based vaccine containing the mRNA-coding TAA is transfected into DCs and translated into proteins. After protein processing, the antigen can be loaded on MHC molecules for antigen presentation, thus activating an antigen-specific CTL response [94].
Clinical trials have been performed employing mRNA-transfected DCs or injecting mRNA directly into patients with prostate cancer [18, 89, 95], RCC [96], ovarian cancer [97], lung cancer, breast cancer [90], pediatric brain cancer [98], neuroblastoma [99], and melanoma [100, 101]. A phase I clinical trial was performed using PSA-mRNA-transfected DCs in patients with metastatic prostate cancer [89]. When the effects of repeated vaccinations with PSA-mRNA-transfected DCs were examined, the results demonstrated that the vaccine was able to increase PSA-specific CTL responses.
In a phase I/II clinical trial in androgen-resistant prostate cancer, patients were vaccinated with DCs transfected with mRNA from 3 allogeneic prostate cancer cell lines (DU145, LNCaP, and PC-3) [18]. Twelve of 19 patients showed specific T-cell responses; 10 of those 12 had a positive response in IFN-γ by ELISPOT assay and 9 had a specific T-cell proliferation response. Two CD8+ CTL clones were generated from a patient who showed a positive response in both the ELISPOT and proliferation assay. The CTL clones demonstrated specific killing of tumor mRNA-transfected DCs and PC-3 cells. Of the 19 patients on-study, 11 showed stable disease and 10 developed specific T-cell responses; only 2 of 8 patients with disease progression showed T-cell responses. These results demonstrate a correlation between immune response and clinical response.
In another clinical trial, patients with metastatic RCC received a vaccine consisting of DCs transfected with total RNA extracted from clear cell carcinoma, with or without DAB389IL2 prevaccination [97]. The results showed a significant increase in the frequency of tumor-specific CD4+ and CD8+ T cells as well as a decrease in Treg frequency. This trial demonstrated that mRNA-transfected DCs can increase immune response, and that this immune response in combination with depletion of Tregs can have a synergistic effect on antitumor immunity.
6. Conclusions
The promising results of recent phase II/III clinical trials may herald a new era for cancer vaccine immunotherapy. However, in spite of exciting improvements in the activity and efficacy of various vaccine platforms, including objective response, disease-free survival, progression-free survival, and overall survival, there is still much to learn about the immunological mechanisms by which these results can be improved. Further research is required to improve our understanding of CTL antigen-specific activation, decreased Treg numbers and functionality, NK activation, antigen cascade, and the impact of tumor escape.
A paradigm shift is necessary in order to improve the design of immuno-oriented clinical trials, increase understanding of the balance between proinflammatory and immunosuppressive responses in antitumor immunity, and define new criteria for the immunological evaluation of antitumor activity and clinical outcomes. Such knowledge would not only improve the efficacy of cancer vaccines but would help to guide decisions regarding patient selection, vaccine scheduling, and the combination of vaccines and other treatment modalities such as surgery, radiotherapy, chemotherapy, and targeted therapy.
Acknowledgment
The authors thank Bonnie Casey and Debra Weingarten for their editorial assistance in preparation of this manuscript.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer Journal for Clinicians. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Kantoff P, Glode L, Tannenbaum S, Bilhartz D, Pittman W, Schuetz T. Randomized, double-blind, vector-controlled study of targeted immunotherapy in patients (pts) with hormone-refractory prostate cancer (HRPC) [abstract] . Journal of Clinical Oncology. 2006;24(18S, abstract 2501) [Google Scholar]
- 3.Kantoff P, Schuetz T, Blumenstein B, et al. Overall survival (OS) analysis of a phase II randomized controlled trial (RCT) of a poxviral-based PSA targeted immunotherapy in metastatic castration-resistant prostate cancer (mCRPC) [abstract] Journal of Clinical Oncology. 2009;27(15S, abstract 5013) doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kantoff P, Schuetz T, Blumenstein B, et al. Overall survival (OS) analysis of a phase II randomized controlled trial (RCT) of a poxviral-based PSA targeted immunotherapy in metastatic castration-resistant prostate cancer (mCRPC) Journal of Clinical Oncology. 2010;28(7):1099–1105. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. Journal of Clinical Oncology. 2003;21(7):1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 6.Goldman B, DeFrancesco L. The cancer vaccine roller coaster. Nature Biotechnology. 2009;27(2):129–139. doi: 10.1038/nbt0209-129. [DOI] [PubMed] [Google Scholar]
- 7.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with Sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. Journal of Clinical Oncology. 2006;24(19):3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 8.Higano CS, Schellhammer PF, Small EJ, et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 9.Testori A, Richards J, Whitman E, et al. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 study group. Journal of Clinical Oncology. 2008;26(6):955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- 10.Wood C, Srivastava P, Bukowski R, et al. An adjuvant autologous therapeutic vaccine (HSPPC-96; vitespen) versus observation alone for patients at high risk of recurrence after nephrectomy for renal cell carcinoma: a multicentre, open-label, randomised phase III trial. The Lancet. 2008;372(9633):145–154. doi: 10.1016/S0140-6736(08)60697-2. [DOI] [PubMed] [Google Scholar]
- 11.Schwartzentruber DJ, Lawson D, Richards J. A phase III multi-institutional randomized study of immunization with the gp100:209-217(210M) peptide followed by high-dose IL-2 compared with high-dose IL-2 alone in patients with metastatic melanoma. Journal of Clinical Oncology. 2009;27(18S, abstract CRA9011) [Google Scholar]
- 12.Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. Journal of Clinical Oncology. 2005;23(27):6674–6681. doi: 10.1200/JCO.2005.13.011. [DOI] [PubMed] [Google Scholar]
- 13.Vermorken JB, Claessen AME, van Tinteren H, et al. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353(9150):345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 14.Uyl-de Groot CA, Vermorken JB, Hanna MG, Jr., et al. Immunotherapy with autologous tumor cell-BCG vaccine in patients with colon cancer: a prospective study of medical and economic benefits. Vaccine. 2005;23(17-18):2379–2387. doi: 10.1016/j.vaccine.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Hoover HC, Jr., Surdyke M, Dangel RB. Delayed cutaneous hypersensitivity to autologous tumor cells in colorectal cancer patients immunized with an autologous tumor cell: Bacillus Calmette-Guérin vaccine. Cancer Research. 1984;44(4):1671–1676. [PubMed] [Google Scholar]
- 16.Jocham D, Richter A, Hoffmann L, et al. Adjuvant autologous renal tumour cell vaccine and risk of tumour progression in patients with renal-cell carcinoma after radical nephrectomy: phase III, randomised controlled trial. Lancet. 2004;363(9409):594–599. doi: 10.1016/S0140-6736(04)15590-6. [DOI] [PubMed] [Google Scholar]
- 17.Doehn C, Richter A, Theodor R, Lehmacher W, Jocham D. Prolongation of progression-free and overall survival following an adjuvant vaccination with Reniale in patients with non-metastatic renal cell carcinoma: secondary analysis of a multicenter phase-III trial [abstract]. In: Proceedings of the 27th German Cancer Congress Program and Abstracts, vol. 395; 2006. [Google Scholar]
- 18.Mu LJ, Kyte JA, Kvalheim G, et al. Immunotherapy with allotumour mRNA-transfected dendritic cells in androgen-resistant prostate cancer patients. British Journal of Cancer. 2005;93(7):749–756. doi: 10.1038/sj.bjc.6602761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dougan M, Dranoff G. Immune therapy for cancer. Annual Review of Immunology. 2009;27:83–117. doi: 10.1146/annurev.immunol.021908.132544. [DOI] [PubMed] [Google Scholar]
- 20.Palena C, Abrams SI, Schlom J, Hodge JW. Cancer vaccines: preclinical studies and novel strategies. Advances in Cancer Research. 2006;95:115–145. doi: 10.1016/S0065-230X(06)95004-0. [DOI] [PubMed] [Google Scholar]
- 21.Bendle GM, Holler A, Downs A-M, Xue S-A, Stauss HJ. Broadly expressed tumour-associated proteins as targets for cytotoxic T lymphocyte-based cancer immunotherapy. Expert Opinion on Biological Therapy. 2005;5(9):1183–1192. doi: 10.1517/14712598.5.9.1183. [DOI] [PubMed] [Google Scholar]
- 22.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Seminars in Immunology. 2008;20(5):276–285. doi: 10.1016/j.smim.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emens LA, Asquith JM, Leatherman JM, et al. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. Journal of Clinical Oncology. 2009;27(35):5911–5918. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laheru D, Lutz E, Burke J, et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clinical Cancer Research. 2008;14(5):1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T-cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clinical Cancer Research. 2008;14(11):3536–3544. doi: 10.1158/1078-0432.CCR-07-4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutsiak MEC, Semnani RT, De Pascalis R, Kashmiri SVS, Schlom J, Sabzevari H. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–2868. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 27.Lechleider RJ, Arlen PM, Tsang K-Y, et al. Safety and immunologic response of a viral vaccine to prostate- specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clinical Cancer Research. 2008;14(16):5284–5291. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantor J, Abrams S, Irvine K, Snoy P, Kaufman H, Schlom J. Specific immunotherapy using a recombinant vaccinia virus expressing human carcinoembryonic antigen. Annals of the New York Academy of Sciences. 1993;690:370–373. doi: 10.1111/j.1749-6632.1993.tb44034.x. [DOI] [PubMed] [Google Scholar]
- 29.Kass E, Schlom J, Thompson J, Guadagni F, Graziano P, Greiner JW. Induction of protective host immunity to carcinoembryonic antigen (CEA), a self-antigen in CEA transgenic mice, by immunizing with a recombinant vaccinia-CEA virus. Cancer Research. 1999;59(3):676–683. [PubMed] [Google Scholar]
- 30.Acres B, Bonnefoy J-Y. Clinical development of MVA-based therapeutic cancer vaccines. Expert Review of Vaccines. 2008;7(7):889–893. doi: 10.1586/14760584.7.7.889. [DOI] [PubMed] [Google Scholar]
- 31.Taylor J, Paoletti E. Fowlpox virus as a vector in a non-avian species. Vaccine. 1988;6(6):466–468. doi: 10.1016/0264-410x(88)90091-6. [DOI] [PubMed] [Google Scholar]
- 32.Stickl H, Hochstein-Mintzel V, Mayr A, Huber HC, Schäfer H, Holzner A. MVA vaccination against smallpox: clinical tests with an attenuated live vaccinia virus strain (MVA) (author’s transl) Deutsche Medizinische Wochenschrift. 1974;99(47):2386–2392. doi: 10.1055/s-0028-1108143. [DOI] [PubMed] [Google Scholar]
- 33.Hodge JW, McLaughlin JP, Kantor JA, Schlom J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine. 1997;15(6-7):759–768. doi: 10.1016/s0264-410x(96)00238-1. [DOI] [PubMed] [Google Scholar]
- 34.Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen-specific immune responses and antitumor effects. Cancer Research. 2001;61(11):4497–4505. [PubMed] [Google Scholar]
- 35.Slack R, Ley L, Chang P. Association between CEA-specific T cell responses (TCR) following treatment with vaccinia-CEA (V) and Alvac-CEA (A) and survival in patients with CEA-bearing cancers [abstract] WProceedings of the American Society of Clinical Oncology. 2001;20(1086) [Google Scholar]
- 36.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. Journal of Clinical Oncology. 2004;22(11):2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 37.Hodge JW, Schlom J. Costimulatory molecules in vaccine design. Ernst Schering Research Foundation Workshop. 2000;(30):23–52. doi: 10.1007/978-3-662-04183-3_3. [DOI] [PubMed] [Google Scholar]
- 38.Terasawa H, Tsang K-Y, Gulley J, Arlen P, Schlom J. Identification and characterization of a human agonist cytotoxic T-lymphocyte epitope of human prostate-specific antigen. Clinical Cancer Research. 2002;8(1):41–53. [PubMed] [Google Scholar]
- 39.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53(2):109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 40.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunology, Immunotherapy. 2010;59(5):1–12. doi: 10.1007/s00262-009-0782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaremba S, Barzaga E, Zhu M, Soares N, Tsang K-Y, Schlom J. Identification of an enhancer agonist cytotoxic T lymphocyte peptide from human carcinoembryonic antigen. Cancer Research. 1997;57(20):4570–4577. [PubMed] [Google Scholar]
- 42.Tsang K-Y, Palena C, Gulley J, Arlen P, Schlom J. A human cytotoxic T-lymphocyte epitope and its agonist epitope from the nonvariable number of tandem repeat sequence of MUC-1. Clinical Cancer Research. 2004;10(6):2139–2149. doi: 10.1158/1078-0432.ccr-1011-03. [DOI] [PubMed] [Google Scholar]
- 43.Gulley JL, Arlen PM, Tsang K-Y, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clinical Cancer Research. 2008;14(10):3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defence. Nature Reviews Immunology. 2007;7(3):179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 45.Brockstedt DG, Dubensky TW., Jr. Promises and challenges for the development of Listeria monocytogenes-based immunotherapies. Expert Review of Vaccines. 2008;7(7):1069–1084. doi: 10.1586/14760584.7.7.1069. [DOI] [PubMed] [Google Scholar]
- 46.Franzusoff A, Duke RC, King TH, Lu Y, Rodell TC. Yeasts encoding tumour antigens in cancer immunotherapy. Expert Opinion on Biological Therapy. 2005;5(4):565–575. doi: 10.1517/14712598.5.4.565. [DOI] [PubMed] [Google Scholar]
- 47.Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert Review of Vaccines. 2008;7(8):1185–1199. doi: 10.1586/14760584.7.8.1185. [DOI] [PubMed] [Google Scholar]
- 48.Cereda V, Poole DJ, Palena C, et al. New gene expressed in prostate: a potential target for T cell-mediated prostate cancer immunotherapy. Cancer Immunology, Immunotherapy. 2010;59(1):63–71. doi: 10.1007/s00262-009-0723-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palena C, Polev DE, Tsang KY, et al. The human T-box mesodermal transcription factor Brachyury is a candidate target for T-cell–mediated cancer immunotherapy. Clinical Cancer Research. 2007;13(8):2471–2478. doi: 10.1158/1078-0432.CCR-06-2353. [DOI] [PubMed] [Google Scholar]
- 50.Yokokawa J, Bera TK, Palena C, et al. Identification of cytotoxic T-lymphocyte epitope(s) and its agonist epitope(s) of a novel target for vaccine therapy (PAGE4) International Journal of Cancer. 2007;121(3):595–605. doi: 10.1002/ijc.22698. [DOI] [PubMed] [Google Scholar]
- 51.Thomas AM, Santarsiero LM, Lutz ER, et al. Mesothelin-specific CD8+ T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients. Journal of Experimental Medicine. 2004;200(3):297–306. doi: 10.1084/jem.20031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ai WZ, Tibshirani R, Taidi B, Czerwinski D, Levy R. Anti-idiotype antibody response after vaccination correlates with better overall survival in follicular lymphoma. Blood. 2009;113(23):5743–5746. doi: 10.1182/blood-2009-01-201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernández AM, Toledo D, Martínez D, et al. Characterization of the antibody response against NeuGcGM3 ganglioside elicited in non-small cell lung cancer patients immunized with an anti-idiotype antibody. Journal of Immunology. 2008;181(9):6625–6634. doi: 10.4049/jimmunol.181.9.6625. [DOI] [PubMed] [Google Scholar]
- 54.So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert Review of Anticancer Therapy. 2006;6(9):1163–1167. doi: 10.1586/14737140.6.9.1163. [DOI] [PubMed] [Google Scholar]
- 55.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Current Opinion in Genetics and Development. 2008;18(1):11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalinski P, Urban J, Narang R, Berk E, Wieckowski E, Muthuswamy R. Dendritic cell-based therapeutic cancer vaccines: what we have and what we need. Future Oncology. 2009;5(3):379–390. doi: 10.2217/FON.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cerundolo V, Hermans IF, Salio M. Dendritic cells: a journey from laboratory to clinic. Nature Immunology. 2004;5(1):7–10. doi: 10.1038/ni0104-7. [DOI] [PubMed] [Google Scholar]
- 58.Lesterhuis WJ, de Vries IJM, Adema GJ, Punt CJA. Dendritic cell-based vaccines in cancer immunotherapy: an update on clinical and immunological results. Annals of Oncology. 2004;15(supplement 4):iv145–iv151. doi: 10.1093/annonc/mdh919. [DOI] [PubMed] [Google Scholar]
- 59.Yokokawa J, Palena C, Arlen P, et al. Identification of novel human CTL epitopes and their agonist epitopes of mesothelin. Clinical Cancer Research. 2005;11(17):6342–6351. doi: 10.1158/1078-0432.CCR-05-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254(5038):1643–1647. doi: 10.1126/science.1840703. [DOI] [PubMed] [Google Scholar]
- 61.Kessler JH, Melief CJM. Identification of T-cell epitopes for cancer immunotherapy. Leukemia. 2007;21(9):1859–1874. doi: 10.1038/sj.leu.2404787. [DOI] [PubMed] [Google Scholar]
- 62.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27(45):5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Butts CM, Maksymiuk A, Goss G. A multicentre phase IIB randomized controlled study of BLP25 liposome vaccine (L-BLP25 or Stimuvax) for active specific immunotherapy of non-small cell lung cancer (NSCLC): updated survival analysis: B1–01 [abstract] Journal of Thoracic Oncology. 2007;2:S332–S333. [Google Scholar]
- 64.Copier J, Dalgleish A. Overview of tumor cell-based vaccines. International Reviews of Immunology. 2006;25(5-6):297–319. doi: 10.1080/08830180600992472. [DOI] [PubMed] [Google Scholar]
- 65.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nature Immunology. 2006;7(5):475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 66.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD4+ T lymphocytes. Nature. 2003;421(6925):852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 67.Simmons AD, Li B, Gonzalez-Edick M, et al. GM-CSF-secreting cancer immunotherapies: preclinical analysis of the mechanism of action. Cancer Immunology, Immunotherapy. 2007;56(10):1653–1665. doi: 10.1007/s00262-007-0315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Simons JW, Carducci MA, Mikhak B, et al. Phase I/II trial of an allogeneic cellular immunotherapy in hormone-naïve prostate cancer. Clinical Cancer Research. 2006;12(11, part 1):3394–3401. doi: 10.1158/1078-0432.CCR-06-0145. [DOI] [PubMed] [Google Scholar]
- 69.Small EJ, Sacks N, Nemunaitis J, et al. Granulocyte macrophage colony-stimulating factor-secreting allogeneic cellular immunotherapy for hormone-refractory prostate cancer. Clinical Cancer Research. 2007;13(13):3883–3891. doi: 10.1158/1078-0432.CCR-06-2937. [DOI] [PubMed] [Google Scholar]
- 70.Higano CS, Corman JM, Smith DC, et al. Phase 1/2 dose-escalation study of a GM-CSF-secreting, allogeneic, cellular immunotherapy for metastatic hormone-refractory prostate cancer. Cancer. 2008;113(5):975–984. doi: 10.1002/cncr.23669. [DOI] [PubMed] [Google Scholar]
- 71.Hodi FS, Butler M, Oble DA, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):3005–3010. doi: 10.1073/pnas.0712237105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Best SR, Peng S, Juang C-M, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27(40):5450–5459. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.U’Ren L, Kedl R, Dow SW. Vaccination with liposome-DNA complexes elicits enhanced antitumor immunity. Cancer Gene Therapy. 2006;13(11):1033–1044. doi: 10.1038/sj.cgt.7700982. [DOI] [PubMed] [Google Scholar]
- 74.Pasquini S, Xiang Z, Wang Y, et al. Cytokines and costimulatory molecules as genetic adjuvants. Immunology and Cell Biology. 1997;75(4):397–401. doi: 10.1038/icb.1997.62. [DOI] [PubMed] [Google Scholar]
- 75.Conry RM, Curiel DT, Strong TV, et al. Safety and immunogenicity of a DNA vaccine encoding carcinoembryonic antigen and hepatitis B surface antigen in colorectal carcinoma patients. Clinical Cancer Research. 2002;8(9):2782–2787. [PubMed] [Google Scholar]
- 76.Binder RJ, Srivastava PK. Peptides chaperoned by heat-shock proteins are a necessary and sufficient source of antigen in the cross-priming of CD8+ T cells. Nature Immunology. 2005;6(6):593–599. doi: 10.1038/ni1201. [DOI] [PubMed] [Google Scholar]
- 77.Segal BH, Wang X-Y, Dennis CG, et al. Heat shock proteins as vaccine adjuvants in infections and cancer. Drug Discovery Today. 2006;11(11-12):534–540. doi: 10.1016/j.drudis.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 78.Pavlenko M, Roos A-K, Lundqvist A, et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. British Journal of Cancer. 2004;91(4):688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McNeel DG, Dunphy EJ, Davies JG, et al. Safety and immunological efficacy of a DNA vaccine encoding prostatic acid phosphatase in patients with stage D0 prostate cancer. Journal of Clinical Oncology. 2009;27(25):4047–4054. doi: 10.1200/JCO.2008.19.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yuan J, Ku GY, Gallardo HF, et al. Safety and immunogenicity of a human and mouse gp100 DNA vaccine in a phase I trial of patients with melanoma. Cancer Immunity. 2009;9(5) [PMC free article] [PubMed] [Google Scholar]
- 81.Cassaday RD, Sondel PM, King DM, et al. A phase I study of immunization using particle-mediated epidermal delivery of genes for gp100 and GM-CSF into uninvolved skin of melanoma patients. Clinical Cancer Research. 2007;13(2):540–549. doi: 10.1158/1078-0432.CCR-06-2039. [DOI] [PubMed] [Google Scholar]
- 82.Michaluart P, Abdallah KA, Lima FD, et al. Phase I trial of DNA-hsp65 immunotherapy for advanced squamous cell carcinoma of the head and neck. Cancer Gene Therapy. 2008;15(10):676–684. doi: 10.1038/cgt.2008.35. [DOI] [PubMed] [Google Scholar]
- 83.Kyte JA, Gaudernack G. Immuno-gene therapy of cancer with tumour-mRNA transfected dendritic cells. Cancer Immunology, Immunotherapy. 2006;55(11):1432–1442. doi: 10.1007/s00262-006-0161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weide B, Garbe C, Rammensee H-G, Pascolo S. Plasmid DNA- and messenger RNA-based anti-cancer vaccination. Immunology Letters. 2008;115(1):33–42. doi: 10.1016/j.imlet.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto A, Kormann M, Rosenecker J, Rudolph C. Current prospects for mRNA gene delivery. European Journal of Pharmaceutics and Biopharmaceutics. 2009;71(3):484–489. doi: 10.1016/j.ejpb.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 86.Van Tendeloo VFI, Ponsaerts P, Lardon F, et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 2001;98(1):49–56. doi: 10.1182/blood.v98.1.49. [DOI] [PubMed] [Google Scholar]
- 87.Ponsaerts P, van der Sar S, Van Tendeloo VFI, Jorens PG, Berneman ZN, Singh PB. Highly efficient mRNA-based gene transfer in feeder-free cultured H9 human embryonic stem cells. Cloning and Stem Cells. 2004;6(3):211–216. doi: 10.1089/clo.2004.6.211. [DOI] [PubMed] [Google Scholar]
- 88.Krieg PA, Melton DA. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Research. 1984;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Heiser A, Coleman D, Dannull J, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. Journal of Clinical Investigation. 2002;109(3):409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morse MA, Nair SK, Mosca PJ, et al. Immunotherapy with autologous, human dendritic cells transfected with carcinoembryonic antigen mRNA. Cancer Investigation. 2003;21(3):341–349. doi: 10.1081/cnv-120018224. [DOI] [PubMed] [Google Scholar]
- 91.Hirschowitz EA, Foody T, Kryscio R, Dickson L, Sturgill J, Yannelli J. Autologous dendritic cell vaccines for non-small-cell lung cancer. Journal of Clinical Oncology. 2004;22(14):2808–2815. doi: 10.1200/JCO.2004.01.074. [DOI] [PubMed] [Google Scholar]
- 92.Pandha HS, John RJ, Hutchinson J, et al. Dendritic cell immunotherapy for urological cancers using cryopreserved allogeneic tumour lysate-pulsed cells a phase I/II study. BJU International. 2004;94(3):412–418. doi: 10.1111/j.1464-410X.2004.04922.x. [DOI] [PubMed] [Google Scholar]
- 93.Kyte JA, Kvalheim G, Aamdal S, Sæbøe-Larssen S, Gaudernack G. Preclinical full-scale evaluation of dendritic cells transfected with autologous tumor-mRNA for melanoma vaccination. Cancer Gene Therapy. 2005;12(6):579–591. doi: 10.1038/sj.cgt.7700837. [DOI] [PubMed] [Google Scholar]
- 94.Pascolo S. Messenger RNA-based vaccines. Expert Opinion on Biological Therapy. 2004;4(8):1285–1294. doi: 10.1517/14712598.4.8.1285. [DOI] [PubMed] [Google Scholar]
- 95.Su Z, Dannull J, Yang BK, et al. Telomerase mRNA-transfected dendritic cells stimulate antigen-specific CD8+ and CD4+ T cell responses in patients with metastatic prostate cancer. Journal of Immunology. 2005;174(6):3798–3807. doi: 10.4049/jimmunol.174.6.3798. [DOI] [PubMed] [Google Scholar]
- 96.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Research. 2003;63(9):2127–2133. [PubMed] [Google Scholar]
- 97.Dannull J, Su Z, Rizzieri D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. Journal of Clinical Investigation. 2005;115(12):3623–3633. doi: 10.1172/JCI25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Caruso DA, Orme LM, Neale AM, et al. Results of a phase 1 study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children and young adults with brain cancer. Neuro-Oncology. 2004;6(3):236–246. doi: 10.1215/S1152851703000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caruso DA, Orme LM, Amor GM, et al. Results of a phase I study utilizing monocyte-derived dendritic cells pulsed with tumor RNA in children with stage 4 neuroblastoma. Cancer. 2005;103(6):1280–1291. doi: 10.1002/cncr.20911. [DOI] [PubMed] [Google Scholar]
- 100.Kyte JA, Mu L, Aamdal S, et al. Phase I/II trial of melanoma therapy with dendritic cells transfected with autologous tumor-mRNA. Cancer Gene Therapy. 2006;13(10):905–918. doi: 10.1038/sj.cgt.7700961. [DOI] [PubMed] [Google Scholar]
- 101.Weide B, Pascolo S, Scheel B, et al. Direct injection of protamine-protected mRNA: results of a phase 1/2 vaccination trial in metastatic melanoma patients. Journal of Immunotherapy. 2009;32(5):498–507. doi: 10.1097/CJI.0b013e3181a00068. [DOI] [PubMed] [Google Scholar]