Abstract
Background
The ratio of pulse wave amplitude (PWA) during reactive hyperemia compared to baseline as measured by peripheral arterial tonometry is as a non-invasive measure of microvascular endothelial function referred to as the pulse wave amplitude reactive hyperemia index (PWA-RHI). Whether upstream conduit vessel structure may affect downstream resistance vessel PWA has not been clearly examined. We tested the hypothesis that digital PWA is influenced by brachial artery diameter (BAD) and that this association would influence comparison of PWA-RHI between genders.
Methods
Measures of vascular structure and microvascular function were carried out in 115 patients varying in cardiovascular risk profiles (average age 57 yrs, male n = 79, CAD n = 43). PWA was assessed using modified finger photoplethysmography (peripheral arterial tonometry, PAT) at baseline and following 5 minutes of brachial artery occlusion. Brachial artery diameter (BAD) was assessed using high-resolution ultrasonography.
Results
There was a negative association between BAD and PWA-RHI and (r = −0.34, p<0.05). Women had greater PWA-RHI and smaller BAD compared with men (p<0.05). When co-varying for BAD, there were no longer gender differences in PWA-RHI. Moreover, when a subgroup of men and women without CAD (n = 40) matched for BAD were examined, there were no gender differences in PWA-RHI.
Conclusions
PWA-RHI obtained from PAT is associated with BAD. Studies examining gender differences in microvascular endothelial function with PAT may need to correct for BAD as a potential confounder.
Keywords: endothelial function, peripheral arterial tonometry, vascular structure, flow mediated dilation
The ratio of digital pulse wave amplitude (PWA) during reactive hyperemia (RH) compared to baseline as measured by PAT has gained acceptance as a non-invasive measure of microvascular endothelial function. The reactive hyperemia index (RHI) obtained from PAT is partially dependent on NO synthesis 18, is associated with conduit and coronary endothelial function 5, 11, 12, and can be improved by therapies known to improve endothelial function 1, 31. PWA-RHI is also associated with cardiovascular risk factors6, 11, 12 and is predictive of future CV events 2, 24. However, a scarcity of data exists regarding the physiology of the PAT signal.
Numerous studies acknowledge an inverse association between baseline vascular dimension and flow mediated vasodilation8–10, 16, 25, 28, 29. Relative peak arterial dilation is a function of baseline vessel diameter with dilation being greater in smaller vessels compared to larger vessels 28. Given the strong association between baseline vascular structure and hyperemic function, current guidelines for the assessment of endothelial function via brachial artery reactivity advocate that measures of flow mediated dilation be expressed in absolute terms as well as relative to baseline diameter to improve diagnostic and prognostic capability as well as risk stratification 4. Upstream conduit vessel structure may affect downstream resistance vessel function27. Smaller upstream conduit arteries (i.e. reduced brachial artery diameter) result in lower absolute distal flows and this in turn could modulate digital shear stress and PWA 3, 23. Moreover, smaller conduit vessels may increase pulse wave amplification (i.e. the pulse wave is amplified as it travels from central to peripheral arteries owing to differences in vascular stiffness, wave reflections and vascular size) and this too could influence the amplitude of the digital volume pulse 3, 14. Despite the potential inter-relation of conduit vessel geometry with resistance vessel function, no study has examined the association between brachial artery diameter and digital PWA measured by PAT.
The potential association between brachial artery geometry and PWA might have important clinical and research implications for studies that compare PWA-RHI between different populations with variable vascular geometry. For example, the examination of gender differences in cardiovascular disease prevention, detection and treatment is an ever-growing and evolving field. A substantial proportion of reported gender differences in conduit artery endothelial dependent dilation may be explained by smaller baseline vessel diameters in women9, 20. Moreover, men have higher central-to-peripheral pulse wave amplification than women26 and this too may perpetuate gender differences in PWA-RHI. Studies that examine differences in microvascular endothelial function between men and women using PAT may need to account for gender differences in BAD but this has yet to be examined.
The primary purpose of this study was to examine the association between digital PWA-RHI and brachial artery diameter (BAD). A secondary purpose was to put potential findings in context by examining gender differences in PWA-RHI as they relate to brachial artery diameter (BAD).
Methods
Subjects
One hundred and fifteen patients reporting to the outpatient Preventive Cardiology Clinic of Tufts Medical Center agreed to participate in this study. For the gender sub-group study, 34 women and 31 men without CAD and matched for other potential confounders were selected from the initial cohort and compared. Exclusion criteria included patients with LDL-C > 100 mg/dl, severe valvular heart disease, recent myocardial infarction (within three months) or unstable cardiac symptoms, congestive heart failure or left ventricular ejection fraction <40%, renal insufficiency (serum creatinine > 2 mg/dL), active liver disease or Raynaud’s disease.
The presence or absence of the following cardiovascular risk factors was assessed in each patient: male sex; hypertension (systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg); hypercholesterolemia (total serum cholesterol > 200 mg/dL or taking lipid lowering medication); diabetes mellitus (fasting glucose levels > 126 mg/dl or treatment with an oral hypoglycemic agent); and family history of cardiovascular disease. CAD was defined as the presence of ischemia or infarction on single-photon emission computed tomographic nuclear myocardial perfusion imaging or > 50% stenosis of an epicardial coronary artery by angiography. All patients with CAD were stable (defined as patients with previous myocardial infarction, stable angina, or any patient who had undergone revascularization).
Vascular measures were performed with the subject in the supine position in a dimly lit, temperature-controlled room following a ten-minute acclimatization period. Patients were instructed to refrain from taking vasoactive medications the day of testing. Patients also refrained from caffeine, smoking, exercise and were in a fasted state for vascular testing. Patients gave written informed consent and this study was approved by the institutional review board at Tufts Medical Center.
Finger pulse wave amplitude
Beat-by-beat pulse wave amplitude was captured using finger arterial tonometry (Itamar Medical Ltd., Israel) as previously described in detail 12. A plethysmographic finger cuff was placed on the index finger of both hands. The PWA-RHI index was calculated as the ratio of the average PWA over a 1-minute period starting 1-minute after 5-min of ischemia (60–120 seconds post ischemia) induced by brachial cuff inflation to a supra-systolic BP (200 mmHg), divided by the average PWA of a 3.5 minute baseline period. The PWA obtained from the finger of the non-occluded arm was also measured continuously and served as a control signal. Final values were normalized to the contra-lateral hand to account for any drift in the magnitude of the signal due to systemic factors. This was done automatically using customized computer software.
Brachial artery diameter and flow mediated dilaton
Brachial artery diameter was assessed using high resolution ultrasonography (Philips, Andover MA). Briefly, the brachial artery was longitudinally imaged 2-cm above the antecubital fossa using a high-resolution (10mHz) linear array vascular ultrasound transducer. Diameters were measured during end-diastole (gated with ECG R-waves) using ultrasonic calipers. The average of 5 evenly spaced measures (distance between the anterior and posterior intima-blood interfaces) obtained within a 5 cm segment of the vessel was used for subsequent analysis. Reactive hyperemia was then induced (see above) and sixty seconds following release of the occlusion cuff, brachial diameter was once again measured. Responses were calculated as percentage change in brachial artery diameter from baseline (flow mediated dilation, FMD). Measures of PWA-RHI and FMD were carried out at the same time. Intra-observer and inter-observer variability in our lab has previously been established to be low (1.8% and 2.8% respectively) 12.
Statistical analysis
All data are reported as means ± SEM. A priori significance was set at p < 0.05. Normality of distribution was assessed using Kolmogorov-Smirnof and Shapiro-Wilk tests. Subjects were divided into quartiles and analysis of variance (for parametric data) with Schefe post hoc testing was used to assess differences in continuous outcome variables between groups. Mann-Whitney U tests were used to assess differences in all outcome variables that were not normally distributed. Chi-square tests were used to compare categorical variables. Pearson’s (parametric) and Spearman’s rank (nonparametric) correlation coefficients were used to assess relationships between variables of interest. Stepwise multiple regression analysis was performed to examine predictors of PWA-RHI in our cohort. Variables entered into the model included traditional cardiovascular risk factors (age, gender, presence/absence of hypertension, diabetes, hyperlipidemia, family history, and BMI), presence/absence of clinically determined CAD, baseline PWA and BAD. Multivariable logistic regression was used to examine if PWA-RHI, baseline PWA and BAD were predictors of CAD. The fit of the regression models were checked by the Hosmer-Lemeshow test for goodness of fit. Area under the receiver operator characteristic (ROC) curve was also examined to determine the predictive power of BAD and baseline PWA for detecting presence of CAD.
Recent work from Mizia-Stec et al. has demonstrated that the product of FMD and BAD (termed the FMDxBAD index) may be a more parsimonious way of accounting for the influence of vessel size when examining gender differences in flow-mediated dilation 16. Therefore, PWA-RHI×BAD was calculated as a composite index to adjust PWA-RHI for BAD 16. Similarly, PWA-RHI×baseline PWA was calculated as a composite index to adjust PWA-RHI for baseline PWA 16. Data analysis was carried out using Statistical Package for the Social Sciences (SPSS, v 16.0.1, SPSS, Inc., Chicago, IL).
Results
BAD, baseline PWA and PWA-RHI
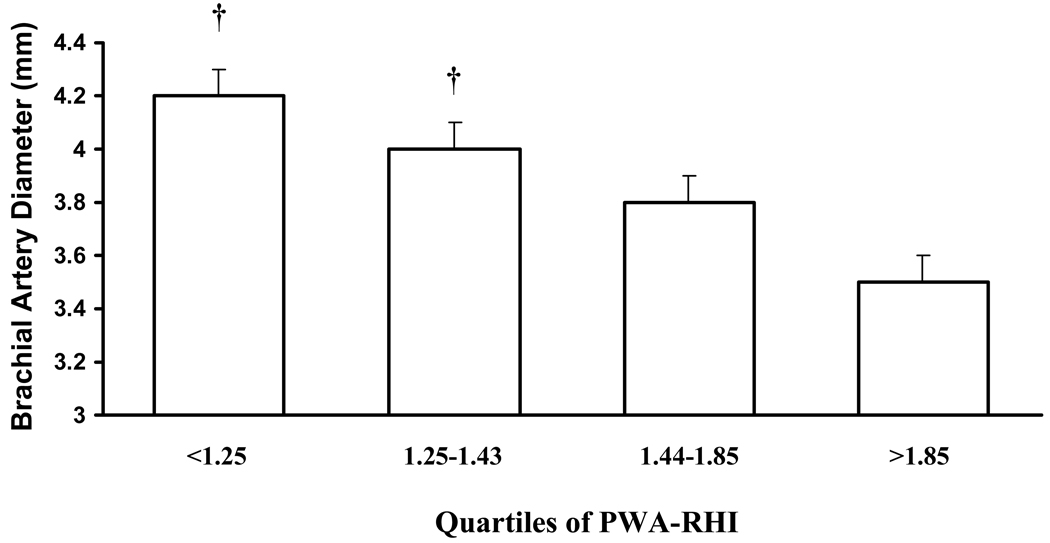
PWA-RHI and brachial artery diameter were inversely correlated (r = −0.34, p<0.05). Characteristics of the subjects according to quartile of PWA-RHI are presented in Table 1 and Table 2. Quartiles were not different in age, sex, body mass index, prevalence of hypertension or diabetes mellitus. Separating subjects according to quartile of PWA-RHI, those with the lowest PWA-RHI had the largest brachial artery diameter (Figure 1, p<0.05). This relationship remained when expressing brachial artery diameter relative to body surface area (Table 2, p<0.05). There were modest differences in use of beta-blockers and statins between quartiles (Table 1). Use of beta blockers and statins was significantly lower in quartile 1 versus quartile 2 (p<0.05). Differences in BAD across quartiles prevailed after adjusting for use of beta blockers and statins (p<0.05).
Table 1.
Patient characteristics for quartile increases in PWA-RHI.
| Variable | All n = 115 |
Quartile 1 n = 29 |
Quartile 2 n = 29 |
Quartile 3 n = 29 |
Quartile 4 n = 28 |
|---|---|---|---|---|---|
| Age, yrs | 57 ± 1 | 54 ± 3 | 59 ± 2 | 57 ± 2 | 61 ± 2 |
| Male, % | 69 | 69 | 72 | 72 | 61 |
| BMI, kg/m2 | 29 ± 1 | 28 ± 1 | 29 ± 1 | 30 ± 1 | 28 ± 1 |
| Hypertension, % | 47 | 52 | 41 | 55 | 39 |
| Diabetes mellitus, % | 14 | 3 | 21 | 17 | 14 |
| Hyperlipidemia, % | 62 | 45 | 79† | 76 | 46 |
| CAD, % | 43 | 16 | 65† | 54 | 36 |
| Family history CVD, % | 46 | 41 | 54 | 41 | 50 |
| Medications, % | |||||
| Aspirin | 45 | 43 | 62 | 39 | 36 |
| Beta-blocker | 37 | 20 | 59* | 36 | 36 |
| Ca channel blocker | 12 | 17 | 10 | 18 | 4 |
| ACE inhibitor | 35 | 20 | 41 | 43 | 36 |
| AR blocker | 7 | 7 | 7 | 11 | 4 |
| Nitrate | 10 | - | 17 | 11 | 11 |
| Diuretic | 15 | 17 | 17 | 18 | 7 |
| Digitalis | 3 | 7 | 7 | - | - |
| Oral Hypoglycemic | 9 | 3 | 10 | 11 | 11 |
| Insulin | 3 | - | 3 | 4 | 4 |
| Statin | 49 | 30 | 66* | 57 | 43 |
BMI, body mass index
Significantly different than Quartile 4 (p<0.05).
Significantly different than Quartile 1 (p<0.05).
Table 2.
Vascular characteristics for quartile increases in PWA-RHI.
| Variable | All n = 115 |
Quartile 1 n = 29 |
Quartile 2 n = 29 |
Quartile 3 n = 29 |
Quartile 4 n = 28 |
|---|---|---|---|---|---|
| BAD/BSA | 1.97 ± 0.03 | 2.15 ± 0.05† | 2.00 ± 0.06† | 1.91 ± 0.06 | 1.79 ± 0.06 |
| FMD, % | 9.6 ± 0.5 | 7.6 ± 1.0† | 7.7 ± 0.8† | 11.2 ± 1.2 | 12.0 ± 0.9 |
| PWA-baseline, AU | 497.8 ± 33.3 | 621.4 ± 73.5† | 589.6 ± 43.8† | 438.3 ± 82.8† | 269.4 ± 35.8 |
| PWA-RHI, % | 1.58 ± 0.05 | 1.07 ± 0.09† | 1.33 ± 0.01† | 1.58 ± 0.02† | 2.31 ± 0.1 |
| Range | 0.82 – 1.24 | 1.25 – 1.43 | 1.44 – 1.85 | 1.86 – 3.00 |
BAD, brachial artery diameter; BSA, body surface area; PWA, pulse wave amplitude; RHI, reactive hyperemia index; FMD, flow mediated dilation
Significantly different than Quartile 4 (p<0.05).
Figure 1.
Brachial artery diameter for each quartile increase in PWA-RHI. Quartile 1 and Quartile 2 had significantly greater BAD than Quartile 4 (p<0.05).
According to stepwise multiple regression, baseline PWA, BAD and smoking status entered into the model as predictors of PWA-RHI (R2 = 0.47; p<0.05). Baseline PWA explained 14.7% of the variance in PWA-RHI (β = 0.00, SE = 0.00, 95% confidence interval: −0.00 – 0.00, p<0.05). BAD explained an additional/incremental 4.4% of the variance (β = −0.172, SE = 0.070, 95% confidence interval: −0.312 – −0.033, p<0.05). Smoking explained an additional/incremental 3.1% of the variance (β = 0.156, SE = 0.074, 95% confidence interval: 0.009 – 0.302, p<0.05). Presence or absence of hypertension, diabetes mellitus, coronary artery disease, hyperlipidemia, body mass index and gender did not enter into the model. Medication use did not predict PWA-RHI and PWA-RHI did not differ between patients taking a particular medication versus those not taking that medication (data not shown).
There was a significant positive correlation between BAD and baseline PWA (r = 0.48, p<0.05). There was a significant inverse association between baseline PWA and PWA-RHI (r = −0.45, p<0.05). There was a significant inverse association between BAD and FMD (r = −0.42, p<0.05). There was a significant positive association between PWA-RHI and FMD (r = 0.31, p<0.05).
Gender Differences in BAD, PWA and PWA-RHI
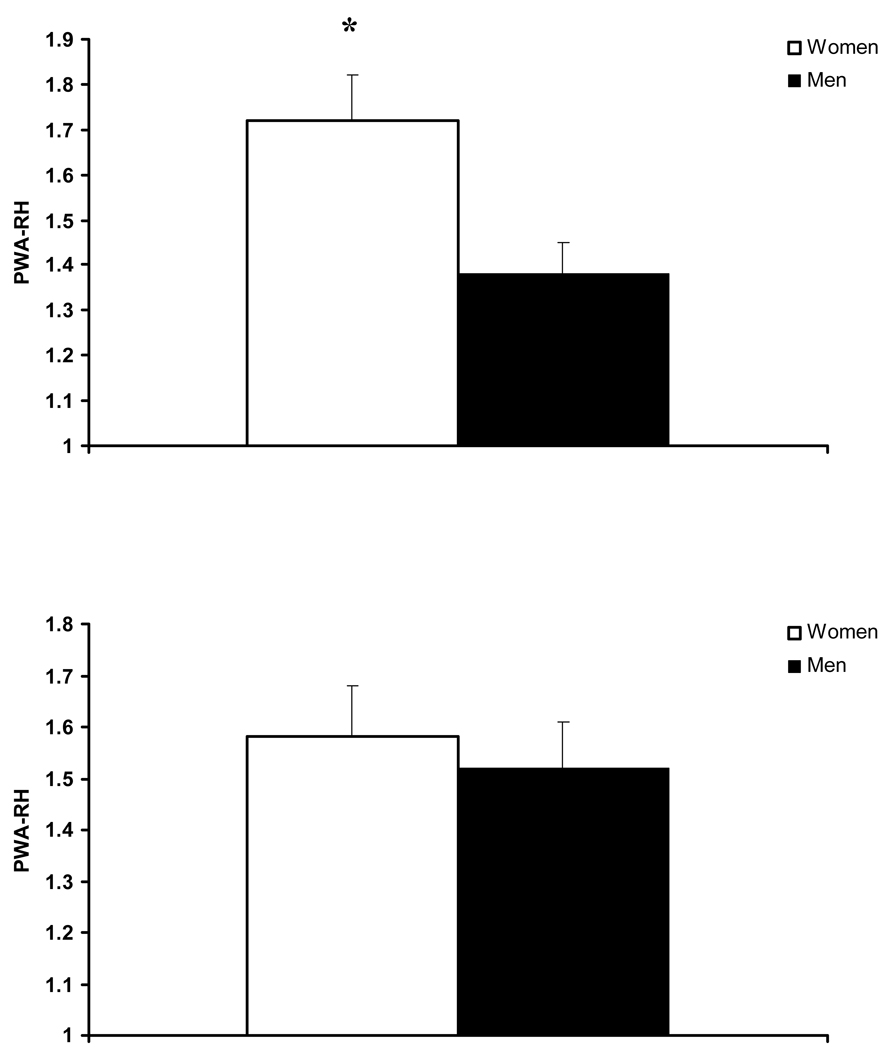
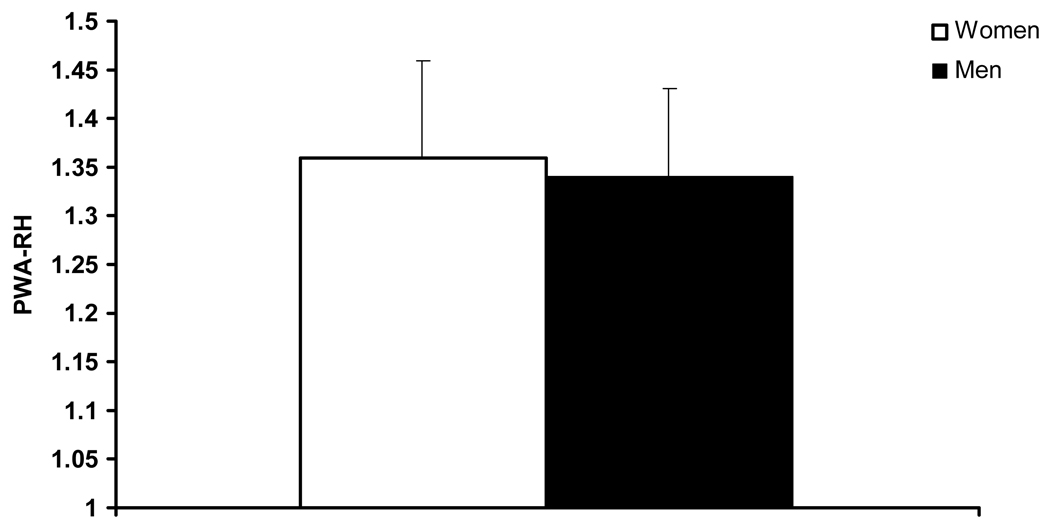
An inverse association between PWA-RHI and resting BAD in the subgroup without CAD was found (−0.47, p<0.05). As seen in Table 3, gender groups did not differ in age, body mass index, or the prevalence of hypertension, hyperlipidemia, diabetes mellitus, smoking status, or family history of CVD. Women had lower BSA, lower baseline PWA, lower resting BAD and lower hyperemic BAD (Table 2, p<0.05). Women had significantly higher PWA-RHI compared with men (Figure 2, p<0.05). However, when PWA-RHI was adjusted for resting BAD with ANCOVA, there were no longer gender differences in PWA-RHI (Figure 2). Twenty women and 20 men were matched for BAD. In this subgroup, BAD in women was 3.76 ± 0.07 mm and BAD in men was 3.77 ± 0.1 mm (p > 0.05). Groups matched for BAD did not differ in PWA-RHI (Figure 3).
Table 3.
Gender comparison characteristics.
| variable | Women n = 34 |
Men n = 31 |
|---|---|---|
| Age, yrs | 56 ± 2 | 53 ± 2 |
| Body mass index, kg/m2 | 29 ± 1 | 28 ± 1 |
| Body surface area* | 1.85 ± 0.03* | 2.06 ± 0.03 |
| Hypertension, % | 45 | 35 |
| Dyslipidemia, % | 38 | 32 |
| Diabetes mellitus, % | 3 | 16 |
| Family Hx, % | 50 | 42 |
| Smoking Hx, % | 29 | 23 |
| PWA-baseline, AU | 292.3 ± 34.1* | 593.0 ± 72.0 |
| BAD, mm | 3.4 ± 0.1* | 4.2 ± 0.1 |
| BAD/BSA | 1.87 ± 0.07* | 2.06 ± 0.06 |
| FMD, % | 11.3 ± 0.9* | 9.1 ± 0.7 |
significant group difference (p<0.05).
Figure 2.
Pulse wave amplitude during reactive hyperemia (PWA-RHI) in women and men unadjusted (top) and adjusted for brachial artery diameter (bottom).
* significant gender difference (p < 0.05).
Figure 3.
Pulse wave amplitude during reactive hyperemia (PWA-RHI) in women and men matched for brachial artery diameter.
A positive association between PWA-baseline and resting BAD in the subgroup without CAD was found (0.54, p<0.05). When PWA-RHI was adjusted for baseline PWA with ANCOVA, there were no longer gender differences in PWA-RHI (adjusted means: women 1.58 ± 0.08 vs. men 1.53 ± 0.09 %, p>0.05). Twenty women and 20 men were also matched for baseline PWA. In this sub-group, baseline PWA in women was 409.9 ± 39.4 and PWA in men was 410.6 ± 47.2 (p > 0.05). Groups matched for baseline PWA did not differ in PWA-RHI (women 1.47 ± 0.1 vs. men 1.49 ± 0.1 %, p>0.05).
There was a positive association between BAD and BSA (0.31, p<0.05). Adjusting PWA-RHI for BSA via ANCOVA had no effect on gender differences in PWA-RHI, with women maintaining higher values then men (1.72 ± 0.1 vs. 1.38 ± 1.0, p<0.05).
Adjusted PWA-RHI for the prediction of CAD
According to binary logistic regression, PWA-RHI was not a significant predictor of CAD in our cohort (p>0.05). Moreover, the area under the ROC curve for PWA-RHI as a predictor of CAD was not significant (0.571, p = 0.19). According to binary logistic regression, PWA-RHI×BAD index was a significant predictor of CAD (β = 0.285, SE = 0.120, Wald statistic = 5.658, 95% confidence interval: 1.052 – 1.683, p<0.05). Area under the ROC curve for PWA-RHI×BAD index was significant (AUC = 0.648, p = 0.007). According to binary logistic regression, PWA-RHI×baseline PWA index was a significant predictor of CAD (β = 0.001, SE = 0.000, Wald statistic = 6.758, 95% confidence interval: 1.000 – 1.002, p<0.05). Area under the ROC curve for PWA-RHI×baseline PWA index was significant (AUC = 0.645, p = 0.008).
Discussion
In this study, there are several noteworthy findings. BAD is associated with digital PWA and PWA-RHI suggesting that measurement of peripheral microvascular function by PAT is influenced by conduit artery structure. PWA-RHI was significantly higher in women compared to men. When statistically adjusting for BAD, PWA-RHI was no longer different between genders. Moreover, when a subgroup of men and women were matched for BAD, there were no gender differences in PWA-RHI. PWA-RHI is a function of baseline PWA. When statistically adjusting for baseline PWA, PWA-RHI was no longer different between genders. Moreover, when a subgroup of men and women were matched for baseline PWA, there were no longer gender differences in PWA-RHI. Finally, adjusting PWA-RHI for BAD or baseline PWA improved the power of PWA-RHI to predict the presence of CAD in a heterogenous patient population varying in CV risk. These findings suggest that BAD and baseline PWA are significant correlates of PWA-RHI and this has both physiologic and clinical relevance.
BAD, PWA and microvascular endothelial function: Physiological implications
Previous work has shown that relative peak arterial dilation is a function of baseline arterial diameter and there is an inverse association between BAD and brachial FMD8–10, 16, 25, 28, 29. Similar to this, baseline PWA was inversely associated with PWA-RHI. Similar to what has been reported with brachial FMD, digital PWA-RHI appears to be a function of baseline PWA.
BAD was positively associated with baseline PWA and inversely associated with PWA-RHI and this may be mediated by the nature of the dilatory stimulus 19, 30. Vessels with the same blood flow may have different levels of shear stress owing to divergent diameters, providing a different stimulus to the blood vessel wall 21. There is an inverse association between artery diameter and shear such that a larger vessel experiences a much smaller shear stress 4. Thus, relative dilation in smaller vessels appears to be more than in larger vessels 28. Indeed, it has been demonstrated that noted gender differences in conduit artery flow-mediated dilation are explained by larger baseline vessel diameters in men 8, 9, 16, 25 and this may be attributable to differences in local shear stress 13, 15. Recent findings from Thijssen et al. have also suggested that lower FMD in larger vessels is due to intrinsic architectural differences in blood vessel structure 28 as smaller vessels tend to be hyper-responsive to vasoactive agents. Thus, lower shear stress coupled with altered vascular morphology (i.e. differences in wall:lumen ratio and smooth muscle/elastin concentrations) in patients with larger BAD may contribute to increased downstream digital PWA and reduced PWA-RHI. Therefore, in individuals with variable brachial geometry, adjusting PWA-RHI for BAD may be needed to accurately examine microvascular endothelial function using PAT.
Gender differences in BAD and PWA
Recently, Hamburg et al. reported gender differences in PWA-RHI in the Framingham Heart Study cohort 6. We noted similar gender differences in PWA-RHI in our patient population with women having higher values then men. Interestingly, statistically adjusting for BAD in the present study eliminated gender differences in PWA-RHI. Moreover when matched for BAD, PWA-RHI was similar in men and women. This would suggest that BAD is a significant determinant of gender-mediated differences PWA-RHI. Additionally, baseline PWA was also different between genders with men having larger amplitudes. Although brachial FMD is usually expressed relative to BAD, previous studies have noted that making an additional adjustment for BAD abolishes gender differences in FMD16. Our results build upon this observation and suggest that when examining gender differences in PWA-RHI, an additional adjustment for BAD or baseline PWA may be warranted.
There was an association between BAD and body surface area. However adjusting PWA-RHI for BSA did not have an effect on gender differences in PWA-RHI. Large BAD is not simply a manifestation of larger body size. And although a static measure, BAD reflects dynamic physiology (i.e. balance of hormonal, neural, metabolic factors acting on the vessel wall) with additional modulation occurring via increases in atherosclerotic cardiovascular disease burden 7.Therefore, body surface area may not be used as a surrogate measure of vascular geometry when correcting for gender differences in PWA-RHI.
CAD differences in BAD and PWA: Clinical implications
An interesting finding was the low prevalence of CAD in patients with very low PWA-RHI. PWA-RHI has been shown to be associated with cardiovascular risk factors, yet findings from the Framingham Heart Study have demonstrated that PWA is not a predictor of overt cardiovascular disease 6. Similar to previous findings 6, we noted that PWA-RHI was not a significant predictor of CAD in our cohort. Our findings would suggest that this may be attributable to the confounding influence of larger BAD and subsequently larger baseline PWA in select patients with low PWA-RHI 7. Individuals with CAD have larger BAD, possibly secondary to positive vascular remodeling in the presence of atherosclerosis7, 17. Indeed in our cohort, BAD was higher in CAD+ versus CAD− patients (4.03 ± 0.09 vs. 3.79 ± 0.09 mm, p<0.05). As such, baseline PWA was also higher in CAD+ versus CAD− patients (549.3 ± 49.5 vs. 430.8 ± 39.2, p<0.05). Adjusting PWA-RHI for BAD improved the predictive power of PWA-RHI for detecting CAD. Similarly, adjusting PWA-RHI for baseline PWA also improved the predictive power of PWA-RHI for detecting CAD. Therefore adjusting PWA-RHI for BAD or baseline PWA may improve the capacity of PAT for predicting the presence of CAD.
Limitations to this study should be noted. We did not quantitatively assess brachial blood flow and shear stress 19, 22. Thus, our contention that the link between BAD and digital PWA may be related to modulation of the shear stress stimulus remains speculative. Additional study is required to substantiate the association between BAD and PWA empirically.
In conclusion, BAD is associated with baseline digital PWA and this may affect the interpretation of digital PWA measured during reactive hyperemia (as an index of microvascular endothelial function). Studies that examine differences in microvascular endothelial function between individuals with variable vascular geometry (i.e. gender differences in BAD or vascular remodeling secondary to CAD) with PAT may need to account for differences in vascular size and/or baseline PWA.
References
- 1.Bonetti PO, Barsness GW, Keelan PC, et al. Enhanced external counterpulsation improves endothelial function in patients with symptomatic coronary artery disease. J Am Coll Cardiol. 2003 May 21;41(10):1761–1768. doi: 10.1016/s0735-1097(03)00329-2. [DOI] [PubMed] [Google Scholar]
- 2.Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Kuvin JT, Lerman A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004 Dec 7;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 3.Chowienczyk PJ, Kelly RP, MacCallum H, et al. Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent beta2-adrenergic vasodilation in type II diabetes mellitus. J Am Coll Cardiol. 1999 Dec;34(7):2007–2014. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- 4.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002 Jan 16;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 5.Dhindsa M, Sommerlad SM, Devan AE, et al. Interrelationships among noninvasive measures of postischemic macro- and microvascular reactivity. J Appl Physiol. 2008 Aug;105(2):427–432. doi: 10.1152/japplphysiol.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008 May 13;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holubkov R, Karas RH, Pepine CJ, et al. Large brachial artery diameter is associated with angiographic coronary artery disease in women. Am Heart J. 2002 May;143(5):802–807. doi: 10.1067/mhj.2002.121735. [DOI] [PubMed] [Google Scholar]
- 8.Jensen-Urstad K, Johansson J. Gender difference in age-related changes in vascular function. J Intern Med. 2001 Jul;250(1):29–36. doi: 10.1046/j.1365-2796.2001.00843.x. [DOI] [PubMed] [Google Scholar]
- 9.Joannides R, Costentin A, Iacob M, Compagnon P, Lahary A, Thuillez C. Influence of vascular dimension on gender difference in flow-dependent dilatation of peripheral conduit arteries. Am J Physiol Heart Circ Physiol. 2002 Apr;282(4):H1262–H1269. doi: 10.1152/ajpheart.00209.2001. [DOI] [PubMed] [Google Scholar]
- 10.Kapuku GK, Treiber FA, Hartley B, Ludwig DA. Gender influences endothelial-dependent arterial dilatation via arterial size in youth. Am J Med Sci. 2004 Jun;327(6):305–309. doi: 10.1097/00000441-200406000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kuvin JT, Mammen A, Mooney P, Alsheikh-Ali AA, Karas RH. Assessment of peripheral vascular endothelial function in the ambulatory setting. Vasc Med. 2007 Feb;12(1):13–16. doi: 10.1177/1358863X06076227. [DOI] [PubMed] [Google Scholar]
- 12.Kuvin JT, Patel AR, Sliney KA, et al. Assessment of peripheral vascular endothelial function with finger arterial pulse wave amplitude. Am Heart J. 2003 Jul;146(1):168–174. doi: 10.1016/S0002-8703(03)00094-2. [DOI] [PubMed] [Google Scholar]
- 13.Levenson J, Pessana F, Gariepy J, Armentano R, Simon A. Gender differences in wall shear-mediated brachial artery vasoconstriction and vasodilation. J Am Coll Cardiol. 2001 Nov 15;38(6):1668–1674. doi: 10.1016/s0735-1097(01)01604-7. [DOI] [PubMed] [Google Scholar]
- 14.Millasseau SC, Ritter JM, Takazawa K, Chowienczyk PJ. Contour analysis of the photoplethysmographic pulse measured at the finger. J Hypertens. 2006 Aug;24(8):1449–1456. doi: 10.1097/01.hjh.0000239277.05068.87. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell GF, Parise H, Vita JA, et al. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004 Aug;44(2):134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 16.Mizia-Stec K, Gasior Z, Mizia M, et al. Flow-mediated dilation and gender in patients with coronary artery disease: arterial size influences gender differences in flow-mediated dilation. Echocardiography. 2007 Nov;24(10):1051–1057. doi: 10.1111/j.1540-8175.2007.00531.x. [DOI] [PubMed] [Google Scholar]
- 17.Montalcini T, Gorgone G, Gazzaruso C, Sesti G, Perticone F, Pujia A. Large brachial and common carotid artery diameter in postmenopausal women with carotid atherosclerosis. Atherosclerosis. 2008 Jan;196(1):443–448. doi: 10.1016/j.atherosclerosis.2006.11.040. [DOI] [PubMed] [Google Scholar]
- 18.Nohria A, Gerhard-Herman M, Creager MA, Hurley S, Mitra D, Ganz P. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol. 2006 Aug;101(2):545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 19.Padilla J, Johnson BD, Newcomer SC, et al. Normalization of flow-mediated dilation to shear stress area under the curve eliminates the impact of variable hyperemic stimulus. Cardiovasc Ultrasound. 2008;6:44. doi: 10.1186/1476-7120-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel AR, Kuvin JT, Sliney KA, et al. Gender-based differences in brachial artery flow-mediated vasodilation as an indicator of significant coronary artery disease. Am J Cardiol. 2005 Nov 1;96(9):1223–1226. doi: 10.1016/j.amjcard.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 21.Pyke KE, Dwyer EM, Tschakovsky ME. Impact of controlling shear rate on flow-mediated dilation responses in the brachial artery of humans. J Appl Physiol. 2004 Aug;97(2):499–508. doi: 10.1152/japplphysiol.01245.2003. [DOI] [PubMed] [Google Scholar]
- 22.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol. 2005 Oct 15;568(Pt 2):357–369. doi: 10.1113/jphysiol.2005.089755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rozanski A, Qureshi E, Bauman M, Reed G, Pillar G, Diamond GA. Peripheral arterial responses to treadmill exercise among healthy subjects and atherosclerotic patients. Circulation. 2001 Apr 24;103(16):2084–2089. doi: 10.1161/01.cir.103.16.2084. [DOI] [PubMed] [Google Scholar]
- 24.Rubinshtein R, Kuvin JT, Soffler M, Lennon RJ, Nelson RE, Pumper GM, Lerman LO, Lerman A. Assessment of Endothelial Function by Peripheral Arterial Tonometry Predicts Cardiovascular Events Beyond the Framingham Risk Score. Journal of the American College of Cardiology. 2009;53:A457. [Google Scholar]
- 25.Schroeder S, Enderle MD, Baumbach A, et al. Influence of vessel size, age and body mass index on the flow-mediated dilatation (FMD%) of the brachial artery. Int J Cardiol. 2000 Nov–Dec;76(2–3):219–225. doi: 10.1016/s0167-5273(00)00381-8. [DOI] [PubMed] [Google Scholar]
- 26.Segers P, Mahieu D, Kips J, et al. Amplification of the pressure pulse in the upper limb in healthy, middle-aged men and women. Hypertension. 2009 Aug;54(2):414–420. doi: 10.1161/HYPERTENSIONAHA.109.133009. [DOI] [PubMed] [Google Scholar]
- 27.Sinoway LI, Hendrickson C, Davidson WR, Jr, Prophet S, Zelis R. Characteristics of flow-mediated brachial artery vasodilation in human subjects. Circ Res. 1989 Jan;64(1):32–42. doi: 10.1161/01.res.64.1.32. [DOI] [PubMed] [Google Scholar]
- 28.Thijssen DH, Dawson EA, Black MA, Hopman MT, Cable NT, Green DJ. Heterogeneity in conduit artery function in humans: impact of arterial size. Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H1927–H1934. doi: 10.1152/ajpheart.00405.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thijssen DH, van Bemmel MM, Bullens LM, et al. The impact of baseline diameter on flow-mediated dilation differs in young and older humans. Am J Physiol Heart Circ Physiol. 2008 Oct;295(4):H1594–H1598. doi: 10.1152/ajpheart.00669.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Title LM, Lonn E, Charbonneau F, et al. Relationship between brachial artery flow-mediated dilatation, hyperemic shear stress, and the metabolic syndrome. Vasc Med. 2008 Nov;13(4):263–270. doi: 10.1177/1358863X08095154. [DOI] [PubMed] [Google Scholar]
- 31.Yeo TW, Lampah DA, Gitawati R, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med. 2007 Oct 29;204(11):2693–2704. doi: 10.1084/jem.20070819. [DOI] [PMC free article] [PubMed] [Google Scholar]