Abstract
Large-conductance Ca2+- and voltage-activated K+ (BKCa, MaxiK or Slo1) channels are expressed in almost every tissue in our body and participate in many critical functions such as neuronal excitability, vascular tone regulation and neurotransmitter release. The functional versatility of BKCa channels owes in part to the availability of a spectacularly wide array of biological modulators of the channel function. In this review, we focus on modulation of BKCa channels by small endogenous molecules, emphasizing their molecular mechanisms. The mechanistic information available from studies on the small naturally occurring modulators is expected to contribute to our understanding of the physiological and pathophysiological roles of BKCa channels.
Introduction
Large-conductance Ca2+- and voltage-activated K+ (BKCa, MaxiK, KCa1.1) channels are well known for their roles in regulation of membrane excitability partly owing to their large conductance, 250–300 pS in symmetrical 150 mM K+, and to the synergic activation mechanism encompassing membrane depolarization and intracellular Ca2+/Mg2+ (98). The inter-dependent activation mechanism enables BKCa channels to typically exert a negative feedback influence on cellular excitability (98, 118). The importance of BKCa channels in regulation of vascular tone, determination of action potential duration and frequency, and neurotransmitter release, has been well documented (98). Consistent with the functional importance, notable phenotypes, such as hypertension, erectile dysfunction, and urinary incontinence, are associated with inhibition or down regulation of the BKCa channel activity (5, 101). Conversely, enhancement or up regulation of the channel function in select cells may offer protection against some of the aforementioned disorders (20, 25, 41, 132).
Structurally, BKCa channels are composed of four pore-forming Slo1 (α) subunits, each of which contains 7 transmembrane segments (S0–S6) and a large C-terminal cytoplasmic region (98). S1–S4 form the primary voltage-sensor domain (VSD), and S5, P and S6 together form the main ion permeation domain. The cytoplasmic area is postulated to harbor two homologous structural units termed “regulators of conductance for K+” (RCK1 and RCK2) based on partial sequence similarity to the bacterial K+ channel MthK whose high-resolution structures are known (56, 57). Four sets of RCK1/RCK2 dimers are envisioned to form a moving structure termed a “gating ring”. Consistent with the gating ring hypothesis, Ca2+-dependent conformational changes in a recombinant Slo1 RCK2 protein have been detected (135). The detailed activation mechanism of the BKCa channel is yet to be elucidated but the atomic structural information available from voltage-gated Kv1.2/2.1 channels (73, 74) and Ca2+-gated MthK channels (56, 57) forms a basis of the following model of BKCa channel activation. The VSDs transduce changes in membrane potential and their movements are electromechanically coupled to the permeation gate within the pore domain. Extensive mutagenesis studies suggest that the activation by Ca2+ probably involves two distinct high-affinity Ca2+ sensors per subunit: the RCK1 sensor (130) and the Ca2+ bowl sensor (102). Activation of the Ca2+ sensors induces conformational changes in the gating ring and leads to opening of the permeation gate near/within S6 via the linkers connecting the gating ring and S6 (78, 88, 130). The interactions among the gate, VSDs and divalent cation sensors, are allosteric and reciprocal (78). Ca2+ binding facilitates opening of the gate, and, conversely, opening of the gate facilitates binding of Ca2+ to its sensors by increasing the binding affinity. However, the coupling between the gate and the voltage/intracellular ligand sensors is such that even without VSD activation and Ca2+, the gate may open once every few minutes on the average (47). Furthermore, open probability (Po) of the channel approaches unity at sufficiently depolarized voltages without Ca2+, and the channel opens frequently at negative voltages without VSD activation with high concentrations of Ca2+.
Only one gene (KCNMA1) (23) codes for the pore-forming Slo1 subunit but the functional properties of the BKCa channel are exceptionally diverse, encompassing many mechanisms, from transcriptional regulation (67), microRNA-mediated regulation (11), alternative splicing (1), to acute modulation of gating by signaling molecules. Alternative splicing of the RNA produces numerous transcripts, translating to diverse Slo1 proteins with distinct functional properties in different tissues and in different hormonal states (105, 115, 131). To further increase the diversity, native BKCa channel complexes often contain auxiliary β subunits (KCNMB) in a tissue-specific manner (66). Four β subunits, β1–β4 (10, 21, 65, 66, 89, 119), have been identified, each of which contains two transmembrane segments connected by a large extracellular loop, leaving both N and C termini in the intracellular side (89). Functionally, the presence of β subunits markedly alters the channel’s gating and pharmacological characteristics (10, 84, 89, 117). Recent evidence also shows that native BKCa channels are part of macromolecular signaling complexes that include enzymes (4, 76, 124) and/or ion channels (13, 37) to mediate local and spatially directed signaling. Once assembled, the BKCa channel activity is subject to modulation by a wide spectrum of biologically relevant factors such as serine/threonine/tyrosine phosphorylation, cysteine/methionine oxidation, steroid hormones, and gases (oxygen, nitric oxide (NO), and carbon monoxide (CO)) (52, 69, 120, 123, 124). Of the modulatory factors, phosphorylation of BKCa channels has been studied extensively and summarized (103). This review primarily focuses on examples of regulation of BKCa channels by other molecules – H+, heme, CO, reactive oxygen and nitrogen species, and lipids.
Protons, H+
H+ is a vitally important ion and its intracellular concentration is under a tight control. However, some fluctuations in pHi may occur as a consequence of normal cell function (26). Depolarization by action potentials and synaptic potentials may noticeably increase intracellular H+ concentration ([H+]i) (26). Additionally, a large fall in pHi, as much as one unit, is possible under pathological conditions, such as cerebral ischemia (111). Not surprisingly, numerous types of ion channels are sensitive to intracellular H+; an increase in [H+]i typically decreases ionic currents. The current inhibition is most frequently attributed to a rapid permeation-pore blocking action of H+ (108).
By contrast, BKCa channels are robustly activated by intracellular H+, a characteristic shared only by a few others among the K+ channel family (80). Early studies, often using native BKCa channels, reported that intracellular H+ decreased ionic currents through BKCa channels (27). Recent studies utilizing heterologously expressed Slo1 channels as well as native BKCa channels now show that H+ stimulates opening of the channel (8, 44, 51, 92). It is uncertain what accounts for the seemingly contradictory results.
Intracellular H+ increases ionic currents through BKCa channels in the absence of Ca2+ without altering the single-channel current size (i) (8, 44, 51, 92). The increase in open probability (Po) caused by H+ is accompanied by a 40~50 mV shift in the macroscopic conductance-voltage curve (GV) to the negative direction with an EC50 value of pHi 6.5 and a Hill coefficient of >2 such that the nearly full effect is observed between pHi = 6.0 and 7.2 (8), a physiologically feasible range. A mutagenesis study identified His365 and His394 (using NP 002238 numbering) in the RCK1 sensor domain, which is important in the Ca2+-dependent activation (130), as the necessary residues, with His365 and His394 accounting for 2/3 and 1/3 of the shift in GV, respectively. Mutation of His365/His394 to neutral Ala approximated the voltage dependence at pHi7.2 whereas that to positively-charged Arg produced a voltage dependence similar to that at pHi6.2. Combined with the finding that the stimulatory effect of H+ diminishes with increasing ionic strength, it has been suggested that the protonated imidazole side chains of His365 and His394 electrostatically interact with nearby electronegative elements. One of the electrostatic interaction partners appears to be Asp367, which is a critical component in high-affinity Ca2+ sensing (130). In summary, it is envisioned that intracellular H+ protonates the side chains of His365 and His394 located within the high-affinity Ca2+-sensor site in the RCK1 domain and then the positively charged side chains interact with Asp367. This interaction in part mimics the action of Ca2+, expanding the gating ring and promoting opening of the gate.
The RCK1 sensor in Slo1 responds to both Ca2+ and H+. This multi-ligand nature of the RCK1 sensor with regards to H+ and Ca2+ is probably physiologically significant as the intracellular concentrations of these two ions are also reciprocally regulated (7, 134). The H+ sensitivity of the BKCa channel may also play an important role in pathophysiological conditions, such as in cerebral ischemia during which significant increases in both intracellular H+ and Ca2+ concentrations are observed (71).
Heme
Like H+, heme is a fundamentally important molecule, typically as a stable protein prosthetic group. Emerging evidence suggests that free intracellular heme may function as a non-genomic signaling molecule, acutely modulating BKCa channels (50, 114). Bioinformatic inspection of the Slo1 primary sequence suggested that the sequence CKACH (114, 125) in the linker region between the cytoplasmic RCK1 and RCK2 segments might be capable of coordinating heme. Indeed, heme applied to the cytoplasmic side decreased Po in cell-free membrane patches with a high affinity (IC50 = ~70 nM) without altering i (114). The modulatory effect of heme is independent of the redox status of iron center but substitution of the iron with other metals generally interferes with its modulatory ability. Mutations in the sequence CKACH disrupted the sensitivity of the channel to heme (55, 114, 123), suggesting that the sequence may be part of the heme binding site. This idea was further corroborated by UV-vis/electron paramagnetic resonance (EPR) spectroscopic measurements (114) and thin-layer chromatography/mass spectroscopy assays (55) performed on a model peptide whose sequence corresponds to the putative heme binding segment. The detailed mechanism of the heme action was addressed by Horrigan et al. (48), who isolated the contribution of each allosteric gating component to the overall gating. The measurements all together showed that heme is a modulator, not a simple inhibitor, of the Slo1 channel function such that it increases Po at negative voltages and decreases Po at more positive voltages. Interaction of heme with the RCK1-RCK2 linker segment may expand the gating ring and impede the gating ring-VSD interaction that normally accompanies activation of the channel (48).
While the molecular mechanism of the heme action of the BKCa channel is relatively clear, its physiological significance is less certain. If heme is in fact a signaling molecule, a mechanism to activate the signaling cascade and a mechanism to terminate the signal should exist. One way to activate the heme signaling pathway may be influx of heme across the plasma membrane using heme transporters (95, 104). The heme signal can be terminated by the action of heme oxygenase (HMOX)(79), thus removing any direct influence of heme on BKCa channels. HMOX, however, produces a number of heme degradation products, including CO, a putative gaseous messenger, which may exert distinct effects on the channels.
Carbon monoxide, CO
One of the well-known effects of CO is to relax blood vessels (70). CO, like another gaseous messenger NO, binds to the heme iron center in soluble guanylyl cyclase (sGC) and increases its activity, leading to an increased level of cGMP and of phosphorylation by cGMP-dependent protein kinase (PKG) (96). Experimental phosphorylation of PKG-consensus Ser residues in Slo1 located near the Ca2+ bowl sensor (87) and at the distal C-terminus (38) increases Po by a few folds. The underlying mechanism of the PKG-mediated regulation is not yet known and neither is whether the aforementioned residues are dynamically phosphorylated in vivo in response to CO.
In addition to the PKG-dependent mechanism, electrophysiological results suggest CO directly stimulates BKCa channels, implicating that the channels themselves are gas sensors. CO, applied as a gas or using CO-releasing molecules (CORMs) (58), increases Po (54, 122, 129) even in cell-free membrane patches (120, 123, 124, 126), suggestive of the possibility that CO modulates the channel directly or indirectly through those entities intimately associated with the channel proteins, possibly in the same macromolecular complex. Typically, CO-sensitive proteins, such as sGC, are heme proteins in which the reduced iron center (Fe2+) interacts with CO (16). Thus, Jaggar et al. postulated that the heme bound to the BKCa channel acts as a sensor for CO; enhancement of the channel activity by CO reflects the ability of CO to remove the inhibitory influence of heme. However, this hypothesis is not consistent with more recent results. For example, BKCa channels treated with the oxidant H2O2, which is expected to oxidize the heme iron and disrupt the CO-protein interaction (16), remain sensitive to CO (52). Furthermore, mutations that render the BKCa channel insensitive to heme fails to disrupt the CO sensitivity (52, 123); the effects of heme and CO are mediated by different molecular loci. A recent study has shown that the RCK1 Ca2+ sensor in Slo1 is required for the stimulatory action of CO (52); mutation of His365, His394 or Asp367, the residues in the RCK1 sensor involved in the H+ and Ca2+ sensitivity, also eliminates the CO sensitivity (52). This finding suggests that both CO and H+ increase Po by mimicking the action of Ca2+ on the RCK1 sensor (52). The essential roles of His365 and His394 in the CO sensitivity are consistent with the earlier observations that low pHi and the histidine modifier DEPC antagonize the CO action (52, 120).
The results summarized above collectively show that the RCK1 sensor encompassing His365, His394 and Asp367 is essential for the high sensitivity of the BKCa channel to multiple ligands: Ca2+, H+ and CO. This finding has been interpreted to indicate that the RCK1 sensor is multi-ligand in nature and accommodates Ca2+, H+ or CO (51, 52). Alternatively, it may be postulated that a separate sensor for each ligand exists and that the binding information converges on the aforementioned His and Asp residues and transmitted to the channel’s gate. Several lines of results, including that the effects of mutations affecting the Ca2+/H+ sensitivity are generally additive (51, 94), favor the former multi-ligand postulate.
The physicochemical mechanism by which CO interacts with the RCK1 sensor remains elusive. It is conventionally believed that CO-sensing proteins requires a metal or heme cofactor (16). The available structures of the bacterial channel MthK, which shows a reasonable degree of primary sequence similarity to the BKCa channel, do not suggest that the Slo1 RCK1 sensor harbors any metal or heme cofactor (56, 57). As an alternative idea, an electrostatic interaction between the weak dipole moment of CO and the RCK1 ligand sensor pocket comprised of His365, His394, and Asp367 has been suggested to contribute (52). In addition to the RCK sensor, an additional CO interaction site may exist in the Slo1 channel (123). CO may increase Po even at saturating concentrations of Ca2+ (123), which cannot be easily explained by the effect of CO as a Ca2+ mimetic for the RCK1 sensor (52).
In many cell types, hypoxia inhibits BKCa channels and the direct stimulation of the BKCa channel by CO represents one of the cellular mechanisms of oxygen sensing (124). In oxygen-sensing carotid body glomus cells, Slo1 proteins are found closely associated with HMOX2 (124), which catalyzes heme to produce CO in an O2-dependent manner (61). It has been postulated that under normoxia, CO generated by HMOX2 continuously stimulates the BKCa channel and that hypoxia inhibits HMOX2 and removes the stimulatory influence of CO on the BKCa channel (124). The overall scheme is supported by several lines of evidence, including the results of HMOX2 gene-knockdown experiments (124). However, the HMOX2-mediated oxygen sensing mechanism may not the dominant one in vivo because mice with the HMOX2 gene constitutively disrupted show relatively normal hypoxic responses (82, 90).
Reactive oxygen/nitrogen species (ROS/RNS)
The aerobic existence inevitably creates reactive molecules capable of oxidizing cellular constituents including proteins (45). While excess concentrations of the reactive molecules are clearly deleterious, causing oxidative stress, cells utilize some of the reactive molecules at low concentrations as vital signaling molecules (53).
Redox modulation of cell function has been difficult to study in part because the experimental tools available to manipulate the levels of reactive species often lack desired specificity and because reactive species are capable of readily modifying multiple amino-acid residues, including cysteine, methionine, histidine, tryptophan, and tyrosine. Furthermore, some oxidation reactions are critically dependent on multivalent cations, such as Fe2+/Fe3+, which may be present as contaminating species. It is not surprising then that treatment of cells/membrane patches containing BKCa channels with reactive species such as H2O2 has been reported to produce a myriad of effects (9, 19, 24, 68, 121, 128, 136). A consensus effect of H2O2, a physiological oxidant produced during normal oxygen metabolism, applied to heterologously-expressed BKCa channels is inhibitory, attributed largely to a decrease in Po (19, 33, 77, 106, 112, 113) and, to a lesser extent, a decrease in the number of channels available to open (N) (106, 137). Typically, the diminished Po by H2O2 persists after wash, indicative of amino-acid modification, but the gating change is reversed by reducing agents such as the physiological reducing agent glutathione (GSH) (19, 33) as well as DTT (33, 77, 106, 113), all of which are capable of regenerating a free sulfhydryl group (-SH) in cysteine from the oxidized side chain sulfenic acid (-SOH). A variety of thiol modifying agents, such as NEM, DTNB and MTSEA (33, 113, 121), also decrease Po, thus implicating that the absence of free sulfhydryl groups in BKCa channel is critical. A systematic Cys-to-Ala mutagenesis of Slo1 showed that oxidation of Cys911 near the Ca2+ bowl sensor in the distal C-terminus decreased the energetic contribution of the Ca2+ bowl sensor to the channel activation (113). The proximity of Cys911 to the Ca2+ bowl sensor is in line with the Ca2+ dependence of the inhibitory effect; in the absence of Ca2+, the redox status of Cys911 plays little role but the effect becomes greater with increasing concentrations of Ca2+ while leaving that Ca2+ dependence mediated by the RCK1 sensor intact (113). In addition to Cys911 at the distal C-terminus, Cys430 in the RCK1 domain also contributes to the oxidation sensitivity of the Ca2+ dependence of the BKCa channel (137). Both Cys430 and Cys911 are susceptible to air oxidation and account for the rundown phenomenon following patch excision (137). Additional Cys residues, whose oxidation alters the channel gating, are also present in the channel. One interesting example is the biologically modulated inclusion of the STREX exon in the cytoplasmic region of Slo1 (131). The STREX inclusion introduces additional Cys residues and potentiates the inhibitory effect of oxidation (36).
Reactive molecules other than H2O2 have been reported to affect BKCa channels: O2•− (72, 113), NO (2, 18, 19, 69), and peroxynitrite (ONOO−) (72, 77, 113). Many of the effects are inhibitory in nature and some of the inhibitory effects are mediated by Cys911 (77, 113). In addition, nitrothiosylation (for review, see (81)) of yet an unidentified cysteine residue induced by NO-releasing compounds may increase the channel activity (2, 18, 69). It may be noted that application of a NO-releasing compound to heterologously-expressed BKCa channels failed to increase Po (52).
Direct biochemical/proteomic evidence that any of the Cys residues in the BKCa channels are dynamically oxidized and reduced under physiological conditions is not yet available. Pathophysiologically, Cys oxidation in the BKCa channel is likely to be a contributing factor in those disease states where oxidative stress is implicated. One such condition is diabetes-induced vascular dysfunction where oxidative stress is an important contributing factor and suggested to induce oxidation of Cys911, leading to impaired vasorelaxation (77).
Methionine is another amino acid readily susceptible to oxidation. Oxidation of methionine to methionine sulfoxide by the addition of an oxygen atom to its reactive sulfur atom changes its flexible and nonpolar side chain to a rigid and polar one, roughly equivalent to the side chain of lysine (15). Methionine oxidation has marked functional effects in many proteins including calmodulin (14), calcium/calmodulin-dependent kinase (35), and ion channels (28, 49, 60) and is implicated in many phenomena including aging and neurodegenerative diseases (49, 107). In BKCa channels, oxidation of any of one of the three Met residues M536, M712 and M739 located in the RCK1 and RCK2 domains by chloramines increases Po in the absence of Ca2+ in part by shifting GV to the negative direction by ~50 mV (100). The overall shift in the voltage dependence is caused by stabilization of the activated state of VSD and of the open state of the gate (100). Interestingly, the stimulatory effect of methionine oxidation in Slo1 is drastically potentiated by coexpression of the auxiliary subunit β1 (99) but a mechanistic interpretation of the finding remains to be developed.
Lipids and metabolites
Lipids are a structurally diverse group of molecules that include fatty acids, phospholipids and steroids, and many lipids and lipid-related metabolites are recognized as cellular signaling molecules involved in regulation of a variety of physiological and pathophysiological processes including gating of BKCa channels (17). The interest in modulation of BKCa channels by lipids is further stimulated by the recent finding that voltage-dependent gating of ion channels critically depends on membrane phospholipids (133).
1. Fatty acids: arachidonic acid and its metabolites
Arachidonic acid, a fatty acid, initially synthesized from dietary sources in select cells, is stored in cell membranes and released to the cytoplasm by the action of phospholipases. Once released, arachidonic acid as well as its metabolites such as hydroxyeicosatetraenoic acids (HETEs), epoxyeicosatrienoic acid (EET), dihydroxyeicosatrienoic acids (DHETs), exert a variety of effects (93, 97), including stimulation of BKCa channels in pituitary tumor cells (32, 127), artery smooth muscle cells (3, 6, 29, 46, 63), and heterologous expression systems (39, 42). The stimulatory action of various fatty acids on the BKCa channels has been typically attributed to an increase in Po (3, 29, 32) and/or to an increase in N (3). How the changes in Po correlate with the changes in the functional domains of the channel, the gate, the VSDs and the gating ring, have not been fully explored.
Despite the large number of fatty acids capable of enhancing the BKCa channel activity, considerable structural specificity has been reported, suggesting that the fatty acid effector, presumably the channel itself, has specific interaction sites. To effectively increase Po, fatty acids should have a cis conformation (32), a relatively long tail group (C > 8) and a negatively charged head group (29). For example, oleic acid (C18), arachidonic acid (C20), and eicosapentaenoic acid (C20) meet these structural requirements and increase BKCa Po by several folds (29, 32). The location of the double bonds in fatty acids may be also important (39, 127).
The biophysical mechanism and the molecular components necessary for the stimulatory action of the fatty acids are not yet clear. Because fatty acids may “flip” across cell membranes (59), it has been difficult to determine whether the fatty acid interaction sites face the intracellular side or extracellular side. Additionally, there is no clear consensus whether the auxiliary subunits of the BKCa channel, β1–4, are required for the fatty acid action. The predominantly vascular auxiliary subunit β1 may not be required for 17,18-epoxyeicosatetraenoic acid, one metabolite of arachidonic acid, to activate the BKCa channel, suggesting that the pore-forming Slo1 subunit is sufficient (46). In contrast, arachidonic acid increases Po when Slo1 is expressed with β2 or β3 but not when expressed alone or coexpressed with β4 (110). One likely but complicating possibility is that fatty acids differ in their β subunit requirement.
2. Phospholipids
Phospholipids are an important constituent of cell membranes and have been known to alter the functions of BKCa channels in many ways (40, 85, 91). Phosphatidylinositol 4, 5-bisphosphate (PIP2) in particular has been a subject of intense investigation because this negatively charged phospholipid influences numerous ion channels and because it serves as a precursor for inositol 1, 4, 5-triphosophate (IP3) and diacylglycerol (DAG), both of which in turn modulate many ion channels. This multifunctional nature of PIP2 is physiologically noteworthy but has hindered execution of well-controlled experiments (109). Nevertheless, an emerging paradigm is that PIP2, especially when phosphorylated by lipid kinases, directly affects many ion channels (109), and BKCa channels appear to be no exception. This is illustrated in a recent study by Vaithianathan et al. (116), which showed that application of exogenous PIP2 to the cytoplasmic side at a physiological concentration (~10 μM) (83) increases currents through both native vascular and heterologously-expressed BKCa channels. The current enhancing effect of PIP2 depends on its negative phosphate group and the inositol moiety, and the washout kinetics is influenced by the acyl chain length. The importance of the negative phosphate group in PIP2 prompted Vaithianathan et al. to mutate a cluster of three positively-charged residues (RKK) in the Slo1 S6-RCK1 linker segment. Neutralization of the charged residues noticeably diminished the overall effect of PIP2, perhaps suggesting that the sequence RKK is a PIP2 interaction site. The involvement of the S6-RCK1 linker in the PIP2 action is reminiscent of the results obtained in KCNQ, another voltage-gated K+ channel (138). Biophysically, PIP2 increases Po in the BKCa channel by shifting GV to the negative direction by ~15 mV at an intermediate concentration of Ca2+. It is interesting to note that a similar shift in the voltage dependence is observed when the S6-RCK1 linker is shortened (88); binding of PIP2 to the linker region may affect the coupling process between the gate in the pore module and the cytoplasmic gating structure.
3. Steroid hormones
Steroid hormones are well known for their genomic effects but their acute, non-genomic mode of action involving direct binding to membrane-bound effectors, including BKCa channels, is starting to be appreciated (86). Multiple steroid hormones, including estrogen (117), testosterone (43), and dehydroepiandrosterone (62), glucocorticoids (75), have been reported to acutely affect BKCa channels. Because estrogen may offer a cardioprotective effect (30), the non-genomic action of estrogen on BKCa channels has been extensively investigated. Acute application of estradiol (17β-estradiol) relaxes vascular smooth muscle in a low μM range (64) and activates native vascular BKCa channels and heterologously-expressed Slo1-β1 channels with an EC50 of a few μM in a Ca2+-dependent manner with the current enhancing effect diminishing at higher concentrations of Ca2+ (31, 62, 117). Importantly, clear structural specificity exists because 17α-estradiol is less effective than 17β-estradiol (117). The pore-forming subunit Slo1 is not sufficient for the 17β-estradiol sensitivity but the robust 17β-estradiol sensitivity requires coexpression of a β subunit, either β1, β2 or β4 (10, 62, 117). The observation that a membrane impermeant analog of 17β-estradiol applied from the extracellular side activates the BKCa channel suggests that the estradiol interaction site may lie near the extracellular side of the Slo1-β complex (117), distinct from the PIP2 interaction site located near the cytoplasmic side of S6 (116).
At low concentrations of Ca2+, 17β-estradiol shifts GV to the negative direction without altering i (31, 62, 117). In the presence of 17β-estradiol, marked changes in the single-channel kinetics, such as an increase in the mean burst duration have been noted (31). However, how the gating changes by 17β-estradiol can be accounted for by the prevailing allosteric gating scheme (47) is unclear. In addition, a β subunit-independent inhibitory effect of estrogen mediated by the Slo1 pore probably exists (34). Other steroids such as corticosterone (62, 75), dehydroepiandrosterone (62) and lithocholate (22) also activate BKCa channels in a β subunit-dependent manner.
Concluding remarks
Acute modulation of BKCa channels greatly expands their functional repertories, allowing the channels to contribute to multitudes of physiological and pathophysiological phenomena. Important mechanistic insights into the channel regulation by small molecules such as Ca2+, Mg2+ and H+ are now available and the mechanisms of action of other modulatory agents on the BKCa channel gating should become better elucidated in the near future. The BKCa channel gating is allosterically mediated by three major domains of the channel, the pore, VSDs and the gating ring, all of which undergo rapid and marked conformational changes, and any modifications of the energetics and/kinetics of the functional domains could change the channel current size. Thus, additional modulatory phenomena of BKCa channels are certainly waiting to be discovered. Such future studies will incorporate the realization that BKCa channels form macromolecular complexes with other signaling molecules, such as voltage-dependent Ca2+ channels (12, 13), HMOX2 (124) and protein kinases/phosphatases (76). The macromolecular assembly formation itself may be dynamic and subject to modulation. The knowledge obtained from studies of the modulator action could contribute to rational design of therapeutically useful low-molecular-weight compounds targeting BKCa channels. Dysfunction of a modulatory pathway may underlie a disease state and synthetic compounds could be designed to regulate the pathway in a predictable manner. Much work and excitement lie ahead.
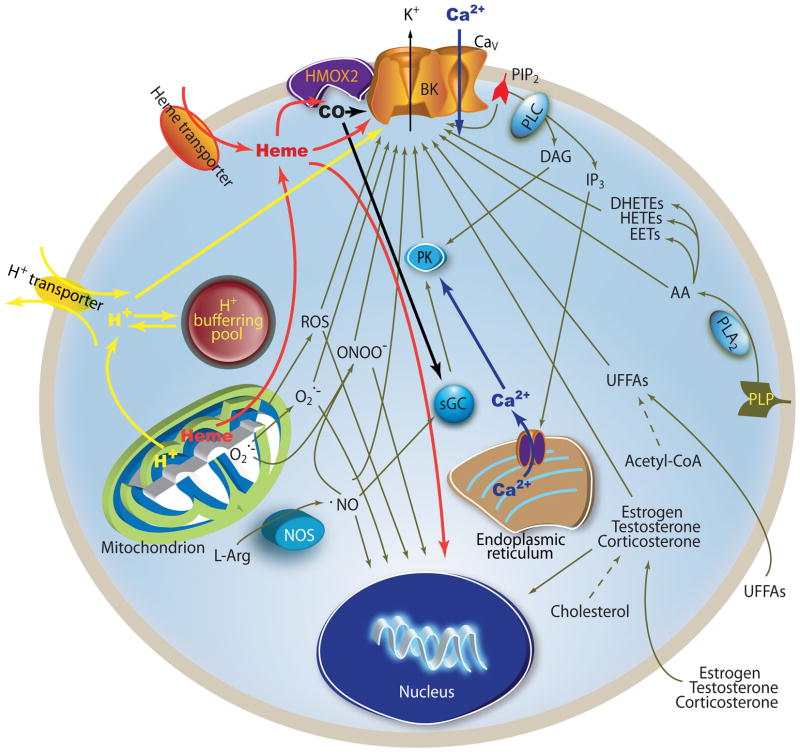
Figure 1.
Multiple intracellular signaling cascades modulate BKCa channels. PLP (phospholipids) UFFAs (unsaturated free fatty acids) NOS (nitric oxide synthase), PK (protein kinase), sGC (soluble guanylyl cyclase), PLA2 (Phospholipase A2), PLC (Phospholipase C), IP3 (Inositol triphosphate), DAG (diglyceride), L-Arg (L-arginine)
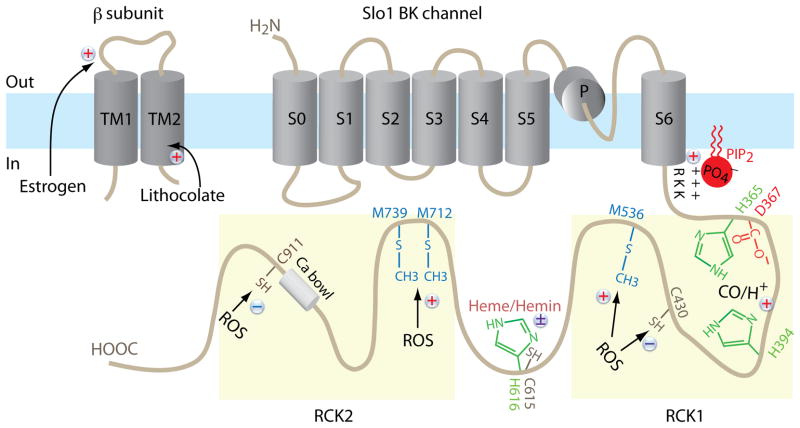
Figure 2.
The schematic diagram shows the key amino acid residues involved in modulation of BKCa channel by select intracellular messengers including H+, heme/hemin, CO, ROS, and PIP2. Stimulatory modulators are indicated by “+”, inhibitory modulators are indicated “–“and mixed-effect modulators are indicated by “±”. The residue numbers are according to NP 002238.
Acknowledgments
The authors thank Dr. F. Horrigan for discussion. The authors’ laboratories are supported by NIH and SFB 604 (TP A4).
References
- 1.Adelman JP, Shen KZ, Kavanaugh MP, Warren RA, Wu YN, Lagrutta A, Bond CT, North RA. Calcium-activated potassium channels expressed from cloned complementary DNAs. Neuron. 1992;9:209–216. doi: 10.1016/0896-6273(92)90160-f. [DOI] [PubMed] [Google Scholar]
- 2.Ahern GP, Hsu SF, Jackson MB. Direct actions of nitric oxide on rat neurohypophysial K+ channels. J Physiol. 1999;520:165–176. doi: 10.1111/j.1469-7793.1999.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahn DS, Kim YB, Lee YH, Kang BS, Kang DH. Fatty acids directly increase the activity of Ca2+-activated K+ channels in rabbit coronary smooth muscle cells. Yonsei Med J. 1994;35:10–24. doi: 10.3349/ymj.1994.35.1.10. [DOI] [PubMed] [Google Scholar]
- 4.Alioua A, Mahajan A, Nishimaru K, Zarei MM, Stefani E, Toro L. Coupling of c-Src to large conductance voltage- and Ca2+-activated K+ channels as a new mechanism of agonist-induced vasoconstriction. Proc Natl Acad Sci U S A. 2002;99:14560–14565. doi: 10.1073/pnas.222348099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amberg GC, Bonev AD, Rossow CF, Nelson MT, Santana LF. Modulation of the molecular composition of large conductance, Ca2+ activated K+ channels in vascular smooth muscle during hypertension. J Clin Invest. 2003;112:717–724. doi: 10.1172/JCI18684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BKCa channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 7.Austin C, Wray S. Interactions between Ca2+ and H+ and functional consequences in vascular smooth muscle. Circ Res. 2000;86:355–363. doi: 10.1161/01.res.86.3.355. [DOI] [PubMed] [Google Scholar]
- 8.Avdonin V, Tang XD, Hoshi T. Stimulatory action of internal protons on Slo1 BK channels. Biophys J. 2003;84:2969–2980. doi: 10.1016/S0006-3495(03)70023-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barlow RS, White RE. Hydrogen peroxide relaxes porcine coronary arteries by stimulating BKCa channel activity. Am J Physiol. 1998;275:H1283–1289. doi: 10.1152/ajpheart.1998.275.4.H1283. [DOI] [PubMed] [Google Scholar]
- 10.Behrens R, Nolting A, Reimann F, Schwarz M, Waldschutz R, Pongs O. hKCNMB3 and hKCNMB4, cloning and characterization of two members of the large-conductance calcium-activated potassium channel β subunit family. FEBS Lett. 2000;474:99–106. doi: 10.1016/s0014-5793(00)01584-2. [DOI] [PubMed] [Google Scholar]
- 11.Bell TJ, Miyashiro KY, Sul JY, McCullough R, Buckley PT, Jochems J, Meaney DF, Haydon P, Cantor C, Parsons TD, Eberwine J. Cytoplasmic BKCa channel intron-containing mRNAs contribute to the intrinsic excitability of hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:1901–1906. doi: 10.1073/pnas.0711796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkefeld H, Fakler B. Repolarizing responses of BKCa-Cav complexes are distinctly shaped by their Cav subunits. J Neurosci. 2008;28:8238–8245. doi: 10.1523/JNEUROSCI.2274-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berkefeld H, Sailer CA, Bildl W, Rohde V, Thumfart JO, Eble S, Klugbauer N, Reisinger E, Bischofberger J, Oliver D, Knaus HG, Schulte U, Fakler B. BKCa-Cav channel complexes mediate rapid and localized Ca2+-activated K+ signaling. Science. 2006;314:615–620. doi: 10.1126/science.1132915. [DOI] [PubMed] [Google Scholar]
- 14.Bigelow DJ, Squier TC. Redox modulation of cellular signaling and metabolism through reversible oxidation of methionine sensors in calcium regulatory proteins. Biochim Biophys Acta. 2005;1703:121–134. doi: 10.1016/j.bbapap.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Black SD, Mould DR. Development of hydrophobicity parameters to analyze proteins which bear post- or cotranslational modifications. Anal Biochem. 1991;193:72–82. doi: 10.1016/0003-2697(91)90045-u. [DOI] [PubMed] [Google Scholar]
- 16.Boczkowski J, Poderoso JJ, Motterlini R. CO-metal interaction: Vital signaling from a lethal gas. Trends Biochem Sci. 2006;31:614–621. doi: 10.1016/j.tibs.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Boland LM, Drzewiecki MM. Polyunsaturated fatty acid modulation of voltage-gated ion channels. Cell Biochem Biophys. 2008;52:59–84. doi: 10.1007/s12013-008-9027-2. [DOI] [PubMed] [Google Scholar]
- 18.Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- 19.Brakemeier S, Eichler I, Knorr A, Fassheber T, Kohler R, Hoyer J. Modulation of Ca2+-activated K+ channel in renal artery endothelium in situ by nitric oxide and reactive oxygen species. Kidney Int. 2003;64:199–207. doi: 10.1046/j.1523-1755.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- 20.Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel β4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- 21.Brenner R, Jegla TJ, Wickenden A, Liu Y, Aldrich RW. Cloning and functional characterization of novel large conductance calcium-activated potassium channel β subunits, hKCNMB3 and hKCNMB4. J Biol Chem. 2000;275:6453–6461. doi: 10.1074/jbc.275.9.6453. [DOI] [PubMed] [Google Scholar]
- 22.Bukiya AN, Liu J, Toro L, Dopico AM. β1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol. 2007;72:359–369. doi: 10.1124/mol.107.034330. [DOI] [PubMed] [Google Scholar]
- 23.Butler A, Tsunoda S, McCobb DP, Wei A, Salkoff L. mSlo, a complex mouse gene encoding “maxi” calcium-activated potassium channels. Science. 1993;261:221–224. doi: 10.1126/science.7687074. [DOI] [PubMed] [Google Scholar]
- 24.Bychkov R, Pieper K, Ried C, Milosheva M, Bychkov E, Luft FC, Haller H. Hydrogen peroxide, potassium currents, and membrane potential in human endothelial cells. Circulation. 1999;99:1719–1725. doi: 10.1161/01.cir.99.13.1719. [DOI] [PubMed] [Google Scholar]
- 25.Cheney JA, Weisser JD, Bareyre FM, Laurer HL, Saatman KE, Raghupathi R, Gribkoff V, Starrett JE, Jr, McIntosh TK. The maxi-K channel opener BMS-204352 attenuates regional cerebral edema and neurologic motor impairment after experimental brain injury. J Cereb Blood Flow Metab. 2001;21:396–403. doi: 10.1097/00004647-200104000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- 27.Church J, Baxter KA, McLarnon JG. pH modulation of Ca2+ responses and a Ca2+-dependent K+ channel in cultured rat hippocampal neurones. J Physiol. 1998;511:119–132. doi: 10.1111/j.1469-7793.1998.119bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciorba MA, Heinemann SH, Weissbach H, Brot N, Hoshi T. Modulation of potassium channel function by methionine oxidation and reduction. Proc Natl Acad Sci U S A. 1997;94:9932–9937. doi: 10.1073/pnas.94.18.9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Clarke AL, Petrou S, Walsh JV, Jr, Singer JJ. Modulation of BKCa channel activity by fatty acids: structural requirements and mechanism of action. Am J Physiol Cell Physiol. 2002;283:C1441–1453. doi: 10.1152/ajpcell.00035.2002. [DOI] [PubMed] [Google Scholar]
- 30.Collins P, Rosano GM, Jiang C, Lindsay D, Sarrel PM, Poole-Wilson PA. Cardiovascular protection by oestrogen--a calcium antagonist effect? Lancet. 1993;341:1264–1265. doi: 10.1016/0140-6736(93)91158-i. [DOI] [PubMed] [Google Scholar]
- 31.De Wet H, Allen M, Holmes C, Stobbart M, Lippiat JD, Callaghan R. Modulation of the BK channel by estrogens: examination at single channel level. Mol Membr Biol. 2006;23:420–429. doi: 10.1080/09687860600802803. [DOI] [PubMed] [Google Scholar]
- 32.Denson DD, Wang X, Worrell RT, Eaton DC. Effects of fatty acids on BK channels in GH3 cells. Am J Physiol Cell Physiol. 2000;279:C1211–1219. doi: 10.1152/ajpcell.2000.279.4.C1211. [DOI] [PubMed] [Google Scholar]
- 33.DiChiara TJ, Reinhart PH. Redox modulation of hslo Ca2+-activated K+ channels. J Neurosci. 1997;17:4942–4955. doi: 10.1523/JNEUROSCI.17-13-04942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dick GM, Sanders KM. (Xeno)estrogen sensitivity of smooth muscle BK channels conferred by the regulatory β1 subunit: a study of beta1 knockout mice. J Biol Chem. 2001;276:44835–44840. doi: 10.1074/jbc.M106851200. [DOI] [PubMed] [Google Scholar]
- 35.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erxleben C, Everhart AL, Romeo C, Florance H, Bauer MB, Alcorta DA, Rossie S, Shipston MJ, Armstrong DL. Interacting effects of N-terminal variation and strex exon splicing on slo potassium channel regulation by calcium, phosphorylation, and oxidation. J Biol Chem. 2002;277:27045–27052. doi: 10.1074/jbc.M203087200. [DOI] [PubMed] [Google Scholar]
- 37.Fakler B, Adelman JP. Control of KCa channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 38.Fukao M, Mason HS, Britton FC, Kenyon JL, Horowitz B, Keef KD. Cyclic GMP-dependent protein kinase activates cloned BKCa channels expressed in mammalian cells by direct phosphorylation at serine 1072. J Biol Chem. 1999;274:10927–10935. doi: 10.1074/jbc.274.16.10927. [DOI] [PubMed] [Google Scholar]
- 39.Fukao M, Mason HS, Kenyon JL, Horowitz B, Keef KD. Regulation of BKCa channels expressed in human embryonic kidney 293 cells by epoxyeicosatrienoic acid. Mol Pharmacol. 2001;59:16–23. doi: 10.1124/mol.59.1.16. [DOI] [PubMed] [Google Scholar]
- 40.Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol. 1991;53:341–359. doi: 10.1146/annurev.ph.53.030191.002013. [DOI] [PubMed] [Google Scholar]
- 41.Gribkoff VK, Starrett JE, Jr, Dworetzky SI, Hewawasam P, Boissard CG, Cook DA, Frantz SW, Heman K, Hibbard JR, Huston K, Johnson G, Krishnan BS, Kinney GG, Lombardo LA, Meanwell NA, Molinoff PB, Myers RA, Moon SL, Ortiz A, Pajor L, Pieschl RL, Post-Munson DJ, Signor LJ, Srinivas N, Taber MT, Thalody G, Trojnacki JT, Wiener H, Yeleswaram K, Yeola SW. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat Med. 2001;7:471–477. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- 42.Gu XQ, Siemen D, Parvez S, Cheng Y, Xue J, Zhou D, Sun X, Jonas EA, Haddad GG. Hypoxia increases BK channel activity in the inner mitochondrial membrane. Biochem Biophys Res Commun. 2007;358:311–316. doi: 10.1016/j.bbrc.2007.04.110. [DOI] [PubMed] [Google Scholar]
- 43.Han DH, Chae MR, Jung JH, So I, Park JK, Lee SW. Effect of testosterone on potassium channel opening in human corporal smooth muscle cells. J Sex Med. 2008;5:822–832. doi: 10.1111/j.1743-6109.2007.00732.x. [DOI] [PubMed] [Google Scholar]
- 44.Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Effect of acidosis on Ca2+-activated K+ channels in cultured porcine coronary artery smooth muscle cells. Pflügers Arch. 1998;436:509–514. doi: 10.1007/s004240050665. [DOI] [PubMed] [Google Scholar]
- 45.Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med. 2000;28:1456–1462. doi: 10.1016/s0891-5849(00)00252-5. [DOI] [PubMed] [Google Scholar]
- 46.Hercule HC, Salanova B, Essin K, Honeck H, Falck JR, Sausbier M, Ruth P, Schunck WH, Luft FC, Gollasch M. The vasodilator 17,18-epoxyeicosatetraenoic acid targets the pore-forming BK α channel subunit in rodents. Exp Physiol. 2007;92:1067–1076. doi: 10.1113/expphysiol.2007.038166. [DOI] [PubMed] [Google Scholar]
- 47.Horrigan FT, Aldrich RW. Coupling between voltage sensor activation, Ca2+ binding and channel opening in large conductance (BK) potassium channels. J Gen Physiol. 2002;120:267–305. doi: 10.1085/jgp.20028605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horrigan FT, Heinemann SH, Hoshi T. Heme regulates allosteric activation of the Slo1 BK channel. J Gen Physiol. 2005;126:7–21. doi: 10.1085/jgp.200509262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoshi T, Heinemann S. Regulation of cell function by methionine oxidation and reduction. J Physiol. 2001;531:1–11. doi: 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hou S, Reynolds MF, Horrigan FT, Heinemann SH, Hoshi T. Reversible binding of heme to proteins in cellular signal transduction. Acc Chem Res. 2006;39:918–924. doi: 10.1021/ar040020w. [DOI] [PubMed] [Google Scholar]
- 51.Hou S, Xu R, Heinemann SH, Hoshi T. Reciprocal regulation of the Ca2+ and H+ sensitivity in the SLO1 BK channel conferred by the RCK1 domain. Nat Struct Mol Biol. 2008;15:403–410. doi: 10.1038/nsmb.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou S, Xu R, Heinemann SH, Hoshi T. The RCK1 high-affinity Ca2+ sensor confers carbon monoxide sensitivity to Slo1 BK channels. Proc Natl Acad Sci U S A. 2008;105:4039–4043. doi: 10.1073/pnas.0800304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Irani K. Oxidant signaling in vascular cell growth, death, and survival: a review of the roles of reactive oxygen species in smooth muscle and endothelial cell mitogenic and apoptotic signaling. Circ Res. 2000;87:179–183. doi: 10.1161/01.res.87.3.179. [DOI] [PubMed] [Google Scholar]
- 54.Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, ES, Cheng X. Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels. Circ Res. 2002;91:610–617. doi: 10.1161/01.res.0000036900.76780.95. [DOI] [PubMed] [Google Scholar]
- 55.Jaggar JH, Li A, Parfenova H, Liu J, Umstot ES, Dopico AM, Leffler CW. Heme is a carbon monoxide receptor for large-conductance Ca2+-activated K+ channels. Circ Res. 2005;97:805–812. doi: 10.1161/01.RES.0000186180.47148.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang Y, Lee A, Chen J, Cadene M, Chait BT, MacKinnon R. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 2002;417:515–522. doi: 10.1038/417515a. [DOI] [PubMed] [Google Scholar]
- 57.Jiang Y, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 58.Johnson TR, Mann BE, Clark JE, Foresti R, Green CJ, Motterlini R. Metal carbonyls: a new class of pharmaceuticals? Angew Chem Int Ed Engl. 2003;42:3722–3729. doi: 10.1002/anie.200301634. [DOI] [PubMed] [Google Scholar]
- 59.Kamp F, Guo W, Souto R, Pilch PF, Corkey BE, Hamilton JA. Rapid flip-flop of oleic acid across the plasma membrane of adipocytes. J Biol Chem. 2003;278:7988–7995. doi: 10.1074/jbc.M206648200. [DOI] [PubMed] [Google Scholar]
- 60.Kassmann M, Hansel A, Leipold E, Birkenbeil J, Lu SQ, Hoshi T, Heinemann SH. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflügers Arch. 2008;456:1085–1095. doi: 10.1007/s00424-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim HP, Ryter SW, Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 62.King JT, Lovell PV, Rishniw M, Kotlikoff MI, Zeeman ML, McCobb DP. β2 and β4 subunits of BK channels confer differential sensitivity to acute modulation by steroid hormones. J Neurophysiol. 2006;95:2878–2888. doi: 10.1152/jn.01352.2005. [DOI] [PubMed] [Google Scholar]
- 63.Kirber MT, Ordway RW, Clapp LH, Walsh JV, Jr, Singer JJ. Both membrane stretch and fatty acids directly activate large conductance Ca2+-activated K+ channels in vascular smooth muscle cells. FEBS Lett. 1992;297:24–28. doi: 10.1016/0014-5793(92)80319-c. [DOI] [PubMed] [Google Scholar]
- 64.Kitazawa T, Hamada E, Kitazawa K, Gaznabi AK. Non-genomic mechanism of 17 β-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J Physiol. 1997;499:497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Knaus HG, Eberhart A, Koch RO, Munujos P, Schmalhofer WA, Warmke JW, Kaczorowski GJ, Garcia ML. Characterization of tissue-expressed α subunits of the high conductance Ca2+-activated K+ channel. J Biol Chem. 1995;270:22434–22439. doi: 10.1074/jbc.270.38.22434. [DOI] [PubMed] [Google Scholar]
- 66.Knaus HG, Folander K, Garcia-Calvo M, Garcia ML, Kaczorowski GJ, Smith M, Swanson R. Primary sequence and immunological characterization of β-subunit of high conductance Ca2+-activated K+ channel from smooth muscle. J Biol Chem. 1994;269:17274–17278. [PubMed] [Google Scholar]
- 67.Kundu P, Alioua A, Stefani E, Toro L. Regulation of mouse Slo gene expression: multiple promoters, transcription start sites, and genomic action of estrogen. J Biol Chem. 2007;282:27478–27492. doi: 10.1074/jbc.M704777200. [DOI] [PubMed] [Google Scholar]
- 68.Lang RJ, Harvey JR. Thiol reagents and nitric oxide modulate the gating of BKCa channels from the guinea-pig taenia caeci. Clin Exp Pharmacol Physiol. 2002;29:944–949. doi: 10.1046/j.1440-1681.2002.03754.x. [DOI] [PubMed] [Google Scholar]
- 69.Lang RJ, Harvey JR, McPhee GJ, Klemm MF. Nitric oxide and thiol reagent modulation of Ca2+-activated K+ (BKCa) channels in myocytes of the guinea-pig taenia caeci. J Physiol. 2000;525:363–376. doi: 10.1111/j.1469-7793.2000.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lin H, McGrath JJ. Vasodilating effects of carbon monoxide. Drug Chem Toxicol. 1988;11:371–385. doi: 10.3109/01480548809018109. [DOI] [PubMed] [Google Scholar]
- 71.Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Terata K, Chai Q, Li H, Kleinman LH, Gutterman DD. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. Circ Res. 2002;91:1070–1076. doi: 10.1161/01.res.0000046003.14031.98. [DOI] [PubMed] [Google Scholar]
- 73.Long SB, Campbell EB, Mackinnon R. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science. 2005;309:903–908. doi: 10.1126/science.1116270. [DOI] [PubMed] [Google Scholar]
- 74.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 75.Lovell PV, King JT, McCobb DP. Acute modulation of adrenal chromaffin cell BK channel gating and cell excitability by glucocorticoids. J Neurophysiol. 2004;91:561–570. doi: 10.1152/jn.01101.2002. [DOI] [PubMed] [Google Scholar]
- 76.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol. 2006;570:65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lu T, He T, Katusic ZS, Lee HC. Molecular mechanisms mediating inhibition of human large conductance Ca2+-activated K+ channels by high glucose. Circ Res. 2006;99:607–616. doi: 10.1161/01.RES.0000243147.41792.93. [DOI] [PubMed] [Google Scholar]
- 78.Magleby KL. Gating mechanism of BK (Slo1) channels: so near, yet so far. J Gen Physiol. 2003;121:81–96. doi: 10.1085/jgp.20028721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 80.Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J Biol Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 81.Matalon S, Hardiman KM, Jain L, Eaton DC, Kotlikoff M, Eu JP, Sun J, Meissner G, Stamler JS. Regulation of ion channel structure and function by reactive oxygen-nitrogen species. Am J Physiol Lung Cell Mol Physiol. 2003;285:L1184–1189. doi: 10.1152/ajplung.00281.2003. [DOI] [PubMed] [Google Scholar]
- 82.McCartney CE, McClafferty H, Huibant JM, Rowan EG, Shipston MJ, Rowe IC. A cysteine-rich motif confers hypoxia sensitivity to mammalian large conductance voltage- and Ca2+-activated K+ (BK) channel α-subunits. Proc Natl Acad Sci U S A. 2005;102:17870–17876. doi: 10.1073/pnas.0505270102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 84.Meera P, Wallner M, Toro L. A neuronal β subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moczydlowski E, Alvarez O, Vergara C, Latorre R. Effect of phospholipid surface charge on the conductance and gating of a Ca2+-activated K+ channel in planar lipid bilayers. J Membr Biol. 1985;83:273–282. doi: 10.1007/BF01868701. [DOI] [PubMed] [Google Scholar]
- 86.Nadal A, Diaz M, Valverde MA. The estrogen trinity: membrane, cytosolic, and nuclear effects. News Physiol Sci. 2001;16:251–255. doi: 10.1152/physiologyonline.2001.16.6.251. [DOI] [PubMed] [Google Scholar]
- 87.Nara M, Dhulipala PD, Ji GJ, Kamasani UR, Wang YX, Matalon S, Kotlikoff MI. Guanylyl cyclase stimulatory coupling to KCa channels. Am J Physiol Cell Physiol. 2000;279:C1938–1945. doi: 10.1152/ajpcell.2000.279.6.C1938. [DOI] [PubMed] [Google Scholar]
- 88.Niu X, Qian X, Magleby KL. Linker-gating ring complex as passive spring and Ca2+-dependent machine for a voltage- and Ca2+-activated potassium channel. Neuron. 2004;42:745–756. doi: 10.1016/j.neuron.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 89.Orio P, Rojas P, Ferreira G, Latorre R. New disguises for an old channel: MaxiK channel β-subunits. News Physiol Sci. 2002;17:156–161. doi: 10.1152/nips.01387.2002. [DOI] [PubMed] [Google Scholar]
- 90.Ortega-Saenz P, Pascual A, Gomez-Diaz R, Lopez-Barneo J. Acute oxygen sensing in heme oxygenase-2 null mice. J Gen Physiol. 2006;128:405–411. doi: 10.1085/jgp.200609591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park JB, Kim HJ, Ryu PD, Moczydlowski E. Effect of phosphatidylserine on unitary conductance and Ba2+ block of the BK Ca2+-activated K+ channel: re-examination of the surface charge hypothesis. J Gen Physiol. 2003;121:375–397. doi: 10.1085/jgp.200208746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Park JK, Kim YC, Sim JH, Choi MY, Choi W, Hwang KK, Cho MC, Kim KW, Lim SW, Lee SJ. Regulation of membrane excitability by intracellular pH (pHi) changers through Ca2+-activated K+ current (BK channel) in single smooth muscle cells from rabbit basilar artery. Pflügers Arch. 2007;454:307–319. doi: 10.1007/s00424-007-0204-8. [DOI] [PubMed] [Google Scholar]
- 93.Piomelli D. Arachidonic acid in cell signaling. Curr Opin Cell Biol. 1993;5:274–280. doi: 10.1016/0955-0674(93)90116-8. [DOI] [PubMed] [Google Scholar]
- 94.Qian X, Niu X, Magleby KL. Intra- and intersubunit cooperativity in activation of BK channels by Ca2+ J Gen Physiol. 2006;128:389–404. doi: 10.1085/jgp.200609486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rajagopal A, Rao AU, Amigo J, Tian M, Upadhyay SK, Hall C, Uhm S, Mathew MK, Fleming MD, Paw BH, Krause M, Hamza I. Haem homeostasis is regulated by the conserved and concerted functions of HRG-1 proteins. Nature. 2008;453:1127–1131. doi: 10.1038/nature06934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Roberts GP, Youn H, Kerby RL. CO-sensing mechanisms. Microbiol Mol Biol Rev. 2004;68:453–473. doi: 10.1128/MMBR.68.3.453-473.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–185. doi: 10.1152/physrev.00021.2001. [DOI] [PubMed] [Google Scholar]
- 98.Salkoff L, Butler A, Ferreira G, Santi C, Wei A. High-conductance potassium channels of the SLO family. Nat Rev Neurosci. 2006;7:921–931. doi: 10.1038/nrn1992. [DOI] [PubMed] [Google Scholar]
- 99.Santarelli LC, Chen J, Heinemann SH, Hoshi T. The β1 subunit enhances oxidative regulation of large-conductance calcium-activated K+ channels. J Gen Physiol. 2004;124:357–370. doi: 10.1085/jgp.200409144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Santarelli LC, Wassef R, Heinemann SH, Hoshi T. Three methionine residues located within the regulator of conductance for K+ (RCK) domains confer oxidative sensitivity to large-conductance Ca2+-activated K+ channels. J Physiol. 2006;571:329–348. doi: 10.1113/jphysiol.2005.101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 102.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophys J. 1997;73:1355–1363. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schubert R, Nelson MT. Protein kinases: tuners of the BKCa channel in smooth muscle. Trends Pharmacol Sci. 2001;22:505–512. doi: 10.1016/s0165-6147(00)01775-2. [DOI] [PubMed] [Google Scholar]
- 104.Shayeghi M, Latunde-Dada GO, Oakhill JS, Laftah AH, Takeuchi K, Halliday N, Khan Y, Warley A, McCann FE, Hider RC, Frazer DM, Anderson GJ, Vulpe CD, Simpson RJ, McKie AT. Identification of an intestinal heme transporter. Cell. 2005;122:789–801. doi: 10.1016/j.cell.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 105.Soom M, Gessner G, Heuer H, Hoshi T, Heinemann SH. A mutually exclusive alternative exon of slo1 codes for a neuronal BK channel with altered function. Channels (Austin) 2008;2:278–282. doi: 10.4161/chan.2.4.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Soto MA, Gonzalez C, Lissi E, Vergara C, Latorre R. Ca2+-activated K+ channel inhibition by reactive oxygen species. Am J Physiol Cell Physiol. 2002;282:C461–471. doi: 10.1152/ajpcell.00167.2001. [DOI] [PubMed] [Google Scholar]
- 107.Stadtman ER. Protein oxidation and aging. Free Radic Res. 2006;40:1250–1258. doi: 10.1080/10715760600918142. [DOI] [PubMed] [Google Scholar]
- 108.Starkus JG, Varga Z, Schonherr R, Heinemann SH. Mechanisms of the inhibition of Shaker potassium channels by protons. Pflügers Arch. 2003;447:44–54. doi: 10.1007/s00424-003-1121-0. [DOI] [PubMed] [Google Scholar]
- 109.Suh BC, Hille B. PIP2 is a necessary cofactor for ion channel function: how and why? Annu Rev Biophys. 2008;37:175–195. doi: 10.1146/annurev.biophys.37.032807.125859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sun X, Zhou D, Zhang P, Moczydlowski EG, Haddad GG. β-subunit-dependent modulation of hSlo BK current by arachidonic acid. J Neurophysiol. 2007;97:62–69. doi: 10.1152/jn.00700.2006. [DOI] [PubMed] [Google Scholar]
- 111.Syntichaki P, Tavernarakis N. The biochemistry of neuronal necrosis: rogue biology? Nat Rev Neurosci. 2003;4:672–684. doi: 10.1038/nrn1174. [DOI] [PubMed] [Google Scholar]
- 112.Tang XD, Daggett H, Hanner M, Garcia ML, McManus OB, Brot N, Weissbach H, Heinemann SH, Hoshi T. Oxidative regulation of large conductance calcium-activated potassium channels. J Gen Physiol. 2001;117:253–274. doi: 10.1085/jgp.117.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. Nat Struct Mol Biol. 2004;11:171–178. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- 114.Tang XD, Xu R, Reynolds MF, Garcia ML, Heinemann SH, Hoshi T. Haem can bind to and inhibit mammalian calcium-dependent Slo1 BK channels. Nature. 2003;425:531–535. doi: 10.1038/nature02003. [DOI] [PubMed] [Google Scholar]
- 115.Tseng-Crank J, Foster CD, Krause JD, Mertz R, Godinot N, DiChiara TJ, Reinhart PH. Cloning, expression, and distribution of functionally distinct Ca2+-activated K+ channel isoforms from human brain. Neuron. 1994;13:1315–1330. doi: 10.1016/0896-6273(94)90418-9. [DOI] [PubMed] [Google Scholar]
- 116.Vaithianathan T, Bukiya A, Liu J, Liu P, Asuncion-Chin M, Fan Z, Dopico A. Direct regulation of BK channels by phosphatidylinositol 4,5-bisphosphate as a novel signaling pathway. J Gen Physiol. 2008;132:13–28. doi: 10.1085/jgp.200709913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Valverde MA, Rojas P, Amigo J, Cosmelli D, Orio P, Bahamonde MI, Mann GE, Vergara C, Latorre R. Acute activation of Maxi-K channels (hSlo) by estradiol binding to the β subunit. Science. 1999;285:1929–1931. doi: 10.1126/science.285.5435.1929. [DOI] [PubMed] [Google Scholar]
- 118.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 119.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: a transmembrane β-subunit homolog. Proc Natl Acad Sci U S A. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang R, Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- 121.Wang ZW, Nara M, Wang YX, Kotlikoff MI. Redox regulation of large conductance Ca2+-activated K+ channels in smooth muscle cells. J Gen Physiol. 1997;110:35–44. doi: 10.1085/jgp.110.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White RE, Kryman JP, El-Mowafy AM, Han G, Carrier GO. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BKCa channel activity in coronary artery smooth muscle cells. Circ Res. 2000;86:897–905. doi: 10.1161/01.res.86.8.897. [DOI] [PubMed] [Google Scholar]
- 123.Williams SE, Brazier SP, Baban N, Telezhkin V, Muller CT, Riccardi D, Kemp PJ. A structural motif in the C-terminal tail of slo1 confers carbon monoxide sensitivity to human BKCa channels. Pflügers Arch. 2008;456:561–572. doi: 10.1007/s00424-007-0439-4. [DOI] [PubMed] [Google Scholar]
- 124.Williams SE, Wootton P, Mason HS, Bould J, Iles DE, Riccardi D, Peers C, Kemp PJ. Hemoxygenase-2 is an oxygen sensor for a calcium-sensitive potassium channel. Science. 2004;306:2093–2097. doi: 10.1126/science.1105010. [DOI] [PubMed] [Google Scholar]
- 125.Wood LS, Vogeli G. Mutations and deletions within the S8–S9 interdomain region abolish complementation of N- and C-terminal domains of Ca2+-activated K+ (BK) channels. Biochem Biophys Res Commun. 1997;240:623–628. doi: 10.1006/bbrc.1997.7714. [DOI] [PubMed] [Google Scholar]
- 126.Wu L, Cao K, Lu Y, Wang R. Different mechanisms underlying the stimulation of KCa channels by nitric oxide and carbon monoxide. J Clin Invest. 2002;110:691–700. doi: 10.1172/JCI15316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wu SN, Li HF, Chiang HT. Actions of epoxyeicosatrienoic acid on large-conductance Ca2+-activated K+ channels in pituitary GH3 cells. Biochem Pharmacol. 2000;60:251–262. doi: 10.1016/s0006-2952(00)00317-8. [DOI] [PubMed] [Google Scholar]
- 128.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xi Q, Tcheranova D, Parfenova H, Horowitz B, Leffler CW, Jaggar JH. Carbon monoxide activates KCa channels in newborn arteriole smooth muscle cells by increasing apparent Ca2+ sensitivity of α-subunits. Am J Physiol Heart Circ Physiol. 2004;286:H610–618. doi: 10.1152/ajpheart.00782.2003. [DOI] [PubMed] [Google Scholar]
- 130.Xia XM, Zeng X, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–884. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 131.Xie J, McCobb DP. Control of alternative splicing of potassium channels by stress hormones. Science. 1998;280:443–446. doi: 10.1126/science.280.5362.443. [DOI] [PubMed] [Google Scholar]
- 132.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 133.Xu Y, Ramu Y, Lu Z. Removal of phospho-head groups of membrane lipids immobilizes voltage sensors of K+ channels. Nature. 2008;451:826–829. doi: 10.1038/nature06618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yao H, Haddad GG. Calcium and pH homeostasis in neurons during hypoxia and ischemia. Cell Calcium. 2004;36:247–255. doi: 10.1016/j.ceca.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 135.Yusifov T, Savalli N, Gandhi CS, Ottolia M, Olcese R. The RCK2 domain of the human BKCa channel is a calcium sensor. Proc Natl Acad Sci U S A. 2008;105:376–381. doi: 10.1073/pnas.0705261105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zagorac D, Yamaura K, Zhang C, Roman RJ, Harder DR. The effect of superoxide anion on autoregulation of cerebral blood flow. Stroke. 2005;36:2589–2594. doi: 10.1161/01.STR.0000189997.84161.95. [DOI] [PubMed] [Google Scholar]
- 137.Zhang G, Xu R, Heinemann SH, Hoshi T. Cysteine oxidation and rundown of large-conductance Ca2+-dependent K+ channels. Biochem Biophys Res Commun. 2006;342:1389–1395. doi: 10.1016/j.bbrc.2006.02.079. [DOI] [PubMed] [Google Scholar]
- 138.Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]