Summary
To gain insight into the mechanism by which angiotensin II type 2 receptor (AT2) regulates carcinogen-induced lung tumorigenesis, we have newly developed anti-AT2 single chain variable fragment (ScFv) antibodies using a rodent phage-displayed recombinant antibody library with various peptide fragments of the receptor protein, and investigated the expression of the AT2 receptor protein. The specificity of the antibodies was verified using AT2 over-expressing COS-7 cells and AT2 naturally expressing PC12W cells. In control wild type mouse lung, a stronger immunoreactivity was observed in bronchial epithelial cells. A moderate immunoreactivity was detected in pulmonary vascular walls and vascular endothelial cells. In the lungs possessing tobacco-specific nitrosamine (NNK)-induced tumors, significantly increased AT2 and AT1 immunostaining was observed in adenomatous lesions. These data suggest that the increase in both receptors' expression in the alveolar epithelial cells may be accompanied with the onset of NNK-induced tumorigenesis and hence play important roles in lung tumorigenesis.
Keywords: Single Chain antibodies, angiotensin II, AT2, immunohistochemistry, alveolar epithelial cells, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
Introduction
Two subtypes of receptors for angiotensin II, termed type 1 (AT1) and type 2 (AT2), have been identified and cloned (Csikos et al. 1997; Inagami et al. 1999; Timmermans et al. 1993). Both subtypes belong to the G protein-coupled seven transmembrane receptor super family (Inagami et al. 1999; Timmermans et al. 1993). In contrast to the well-established physiological roles of AT1, the significance of AT2 remains largely undefined. In vitro studies showed that AT2 played an important role in the inhibition of cell proliferation and stimulation of apoptosis in various cultured cells (Berry et al. 2001; Chung et al. 1998; Horiuchi et al. 1999), at least in part through phospho-tyrosine phosphatase activation (Cui et al. 2001; Inagami et al. 1999; Tsuzuki et al. 1996). AT2 null-hemizygous (AT2-KO) mice generated by our colleagues (Ichiki et al. 1995) and others (Hein et al. 1995) show a hypertensive phenotype that indicates its importance for the regulation of blood pressure. Recently we showed that AT2 expressed in vascular endothelial cells and muscular media in resistant arteries may play a pivotal role in systemic blood pressure regulation (Utsunomiya et al 2005). On the other hand, we also demonstrated that the AT2-KO mouse has an attenuated susceptibility in tobacco-specific nitrosoamine-induced lung tumorigenesis implying that AT2 plays an important role in lung tumorigenesis (Kanehira et al. 2005). There are only a few studies elucidating the expression of AT2 in the lung tissues (Bullock et al. 2001; Chassagne et al. 2000). Although an earlier study has demonstrated that the AT2 protein is abundantly expressed in bronchial epithelium brush border and mucus glands in human lung tissues (Bullock et al. 2001), this expression pattern appears to not be associated with 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced adenoma development since NNK-induced adenocarcinoma appears to originate in type II pneumocytes (Hecht 1998).
We have recently developed anti-AT2 single chain variable fragment (ScFv) antibodies using a rodent phage-displayed recombinant antibody library with various peptide fragments of the AT2 receptor protein. These antibodies are only 28 kDa and contain variable regions of both heavy and light chains. They are currently the smallest labeling system available (Malecki et al. 2002). The benefit of ScFv antibodies is, unlike the parental antibody, they are very small allowing them to penetrate the cell membrane more efficiently (Shi et al. 2006). The objective of the present study was to gain insight into the mechanism by which AT2 regulates carcinogen-induced lung tumorigenesis. We have investigated the expression of AT2 in normal and tumor-bearing mouse lung tissue using our anti-AT2 ScFv and commercially available anti-AT2 antibodies. Our results clearly indicate that the primary AT2 expression site is alveolar epithelial cells and arterial walls. The AT2 expression level was significantly increased in the NNK-induced tumor nodule. To the best of our knowledge this is the first comprehensive immunohistochemical analysis of angiotensin II AT2 expression in mouse lung with the use of ScFv antibodies.
Materials and Methods
Materials and animals
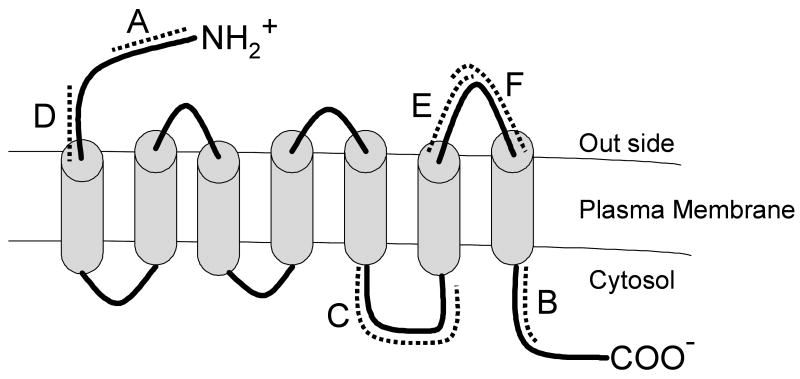
The original AT2-null mutant (Agtr2-/y) mice were produced by homologous recombination in embryonic stem cells derived from strain 129/Ola (Ichiki et al., 1995). Chimeric males were mated with C57BL/6J females such that the genetic background of the mutants consisted of 129/Ola and C57BL/6J. Wild type mice were purchased from Jackson Laboratory (Bar Harbor, MA). The N-terminus peptide (Peptide A, 15 mers; 1-15, MKDNFSFAATSRNIT), C-terminus peptide (Peptide B, 15 mers, 326-340; QQKLRSVSRVPITWL), and intracellular third loop peptide containing cysteine at its C-terminus (Peptide C, 25 mers, 233-256; GIRKHLLKTNSYGKNRITRDQVLKC) were synthesized by Research Genetics (Huntsville AL). The N-terminus peptide (Peptide D, 11 mers, 35-45; CSHKPSDKHLE), the extracellular third loop first half peptide (Peptide E, 10 mers, 281-290; LTWMGIINSC) and the extracellular third loop second half peptide (Peptide F, 10 mers, 290-299; CEVIAVIDLA) were synthesized by Tokai University Peptide Synthesis Core Laboratory (Sagamihara, Japan). Structural location and orientation of these peptides in the AT2 receptor protein is illustrated in figure 1. Culture media were obtained from the DNA Synthesis & Reagent Supply Core facility in the Vanderbilt University Diabetes Center. All other chemicals were of analytical grade. All animals were maintained in a humidity- and temperature-controlled room on 12h light/dark cycles. All procedures for handling animals were approved by the Institutional Committee for Animal Care and Use of Vanderbilt University and Kansas State University.
Fig. 1.
Schematic illustration of antigen peptides derived from the AT2 receptor protein.
Characterization of recombinant ScFv AT2 antibodies
Selection of AT2 peptide-specific ScFv antibodies from a phage-displayed recombinant antibody library
A rodent phage-displayed recombinant antibody library (∼2.9 × 109 members), generated by the Vanderbilt University Molecular Recognition Unit core facility, was used to obtain ScFv recombinant antibodies specific to AT2 receptor peptide fragments as described previously (Hennig et al. 2004). All ScFv stemming from the recombinant antibody library had been cloned into E. coli TG1 cells using the pCANTAB5E phagemid vector (Amersham Pharmacia Biotech, Inc., Piscataway, NJ). Expressed ScFv displays a tag recognized by the Pharmacia Anti-E tag HRP monoclonal antibodies. The Anti-E tag antibody can be used to detect ScFv bound to antigens in assays and can also be used to affinity-purify ScFv from bacterial extracts. Three rounds of phage antibody selection were performed using 1 ml of immobilized on Nunc Maxisorb tubes at 100 μg of each peptide/ml PBS for the first round, 10 μg/ml for the second round, and 1 μg/ml for the third round of selection. Tubes and phage antibodies were blocked in 0.1 % Tween 20 in PBS prior to selections. Phage antibodies were eluted from coated tubes with 1 ml of 100 mM triethanolamine for the first two rounds of selection and with 10 μg/ml PBS for the third round. Eluted phage antibodies were used to infect TG1 E. coli cells, which served as bacterial source for phage-displayed soluble recombinant antibody production.
Preparation of ScFv from bacterial periplasmic extract
Bacteria were grown overnight at 30° C in 250 ml of 2×YT medium with 100 μg/ml ampicillin and 2% glucose with shaking at 100 rpm. Bacteria were centrifuged to pellet cells, resuspended in 2×YT medium with 100 μg/ml ampicillin and 1 mM isopropyl-β-D-thiogalacto-pyranoside, incubated with shaking and then centrifuged as before. To prepare periplasmic extracts, bacterial pellets were resuspended sequentially in 10 ml of TES [0.2 M Tris-HCl (pH 8.0), 0.5 mM EDTA, and 0.5 M sucrose] and 15 ml of one-fifth TES [0.04 M Tris-HCl (pH 8.0), 0.1 mM EDTA, and 0.1 M sucrose] and placed on ice for 30 min or at -70° C until needed.
ICELISA to determine ScFv antigen specificity
The indirect competitive enzyme-linked immunosorbent assays (ICELISA) protocol, which accompanies Amersham Pharmacia's HRP/Anti-E tag conjugate, was employed to detect and determine antigen specificity of ScFv produced by bacterial colonies. All assays were carried out in 384-well microtiter plates with individual wells either left uncoated or coated with 50 μl of antigen AT2 peptides at 5 μg/ml PBS.
Cell Culture
COS-7 cells (ATCC, Manassas, VA) untransfected or stably transfected with plasmid containing the rat AT2 sequence (AT2/COS-7) were prepared(Kambayashi et al. 1993) and cultured in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) at 37 °C in a humidified atmosphere. PC12W pheochromocytoma cells naturally expressing AT2 were cultured in Ham's F12/DMEM (1:1) medium containing 10% FBS. The COS-7 cells lack AT2 expression, AT2/COS-7 cells stably express AT2 and PC12W cells naturally express AT2. The sub-confluent cells were utilized for immunocytochemical study in order to evaluate the validity of the recombinant antibodies.
Immunocytochemistry
COS-7 cells, AT2/COS-7 cells and PC12W cells grown in the culture dishes were individually fixed in acetone for 10 min at 4° C, detached by scraper and paraffin-embedded. Some acetone fixed cells were washed with PBS and incubated with the primary antibodies at 4° C for 16 hours. After washing with PBS, the positive signals were visualized using the indirect peroxidase-labeled antibody method; however, only paraffin-embedded and thin-sectioned cells exhibited clear immunostaining.
Immunohistochemistry and immunofluorescence
Mice, 8-12 week old male wild type C57BL6, AT1a-KO (Agtr1-/-), and AT2-KO (Agtr2-/y) were sacrificed by cervical dislocation following isoflurane anesthesia. Lungs were inflated by infusion of 0.8-1.0 ml 4 % paraformaldehyde, and then fixed in 10 % formalin for 24 hours at 4 °C. Some tissue specimens were frozen in liquid nitrogen after embedding in OCT compound (Sakura Finetek USA, Torrance, CA). The fixed lungs were paraffin embedded and thin-sliced with 4-5 micron thickness. After deparaffinization, antigen retrieval was carried out by placing the slides in 1 mM EDTA (pH 7.8) containing pressure cooker for 20 min. The blocking buffer (5 mg BSA/ml PBS) was applied for two hours at room temperature. After extensive washing with 0.1% Tween 20 in PBS (PBS/T) and PBS, the sections were incubated for one hour at room temperature, either with anti-E-tag mixed (Amersham, 1: 800) recombinant ScFv (1: 8) or anti-goat anti-AT2 (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA), or anti-rabbit anti-AT1a polyclonal antibodies (1:500, Advanced Targeting Systems, San Diego, CA). Incubation with secondary fluorescence antibody (1:1000, Alexa Fluor 594 (red) and Alexa flour 488 (green), Invitrogen, Carlsbad, CA) was carried out for 1 hr at 23°C in the dark. To-pro-3 (blue, 1:1000) was used to visualize the nucleus. After extensive washing with PBS/T and PBS the slide were mounted with Prolong Kit (Molecular Probes-Invitrogen) and allowed to dry at 4°C for overnight. Digital images were acquired using a Carl Zeiss confocal microscope (Carl Zeiss Axiovert 200 with motorized stage, Germany).
Antigen antibody binding affinity with ScFv and commercially available anti-AT2 antibodies (Santa Cruz Biotechnology) were also compared; their positive signals visualized by the indirect peroxidase-labeled antibody method. In addition, negative controls without primary antibodies were always examined in all immunofluorescence applications.
Experimental protocol for NNK-induced lung adenoma
All NNK-handling procedures were approved by the office of Safety and Environmental Health of Vanderbilt University. Five-week-old male SWR/J wild type mice were reared with regular mouse chow (#5015, Purina Mills, Inc., Indianapolis, IN). Mice were treated with bolus intraperitoneal administration of NNK (100 mg/kg, I.P.). The control group for the NNK treatment received saline. Mice were sacrificed 20 weeks after the NNK treatment. After macroscopic examination the whole lung was stained by injecting India Black ink solution through the trachea and fixed with Fekete's solution (Fekete 1938). All tumor bearing lung tissues were sectioned and immunohistochemically analyzed. Their morphologies were also analyzed after H & E staining.
Results
Characterization of recombinant ScFv AT2 antibodies
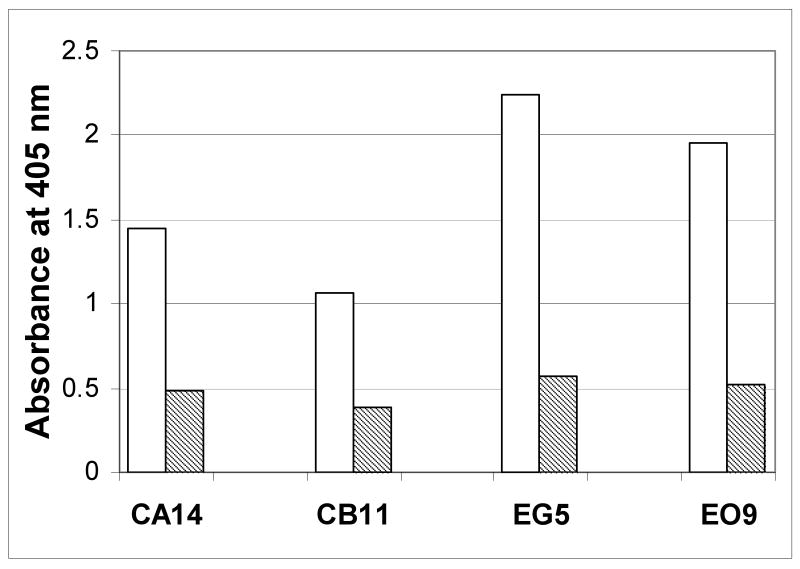
The specificity of the immunoreactivity of recombinant anti-AT2 ScFv antibodies was evaluated by ICELISA. This ICELISA screened 37 positive ScFv extracts. Among these antibodies, anti-AT2 peptide C and anti-AT2 peptide E antibodies exhibited higher titers than those from other anti-AT2 peptide fragments (Fig 2). These results indicate that our recombinant anti-AT2 antibodies specifically react with AT2 peptides. The specificity of these antibodies as examined by immunocytochemistry technique in COS-7 cells, AT2/COS-7 cells and PC12W cells showed that both anti-AT2 peptide C and anti-AT2 peptide E antibodies exhibited strong signals in both AT2/COS-7 (Fig. 3-C and -D) and PC12W cells (Fig. 3-F and -G) but not in COS-7 cells (Fig 3-A and -B). Their immunoreactivities were stronger in the plasma membrane than in the cytoplasm of PC12W cells (Fig. 3-F and -G). Pretreatment with antigen AT2 C (Fig. 3-E) and E peptides (Fig. 3-H) almost completely absorbed the immunoreactivity of these anti-AT2 antibodies. These results clearly indicate that our recombinant anti-AT2 antibodies specifically detect AT2 expressed in the cultured cells.
Fig. 2.
Evaluation of the specificity of newly developed recombinant anti-AT2 peptide antibodies by indirect competitive enzyme-linked immunosorbent assay (ICELISA) of the AT2-C and -E peptides. ELISA end-points were determined in a 96-well microtiter plate coated with AT2-C peptide-streptavidin or AT2-E peptide-KLH at 2.5 μg/well with undiluted ScFv (open bars) and corresponding AT2 peptide-conjugate alone (hashed bars).
Fig. 3.
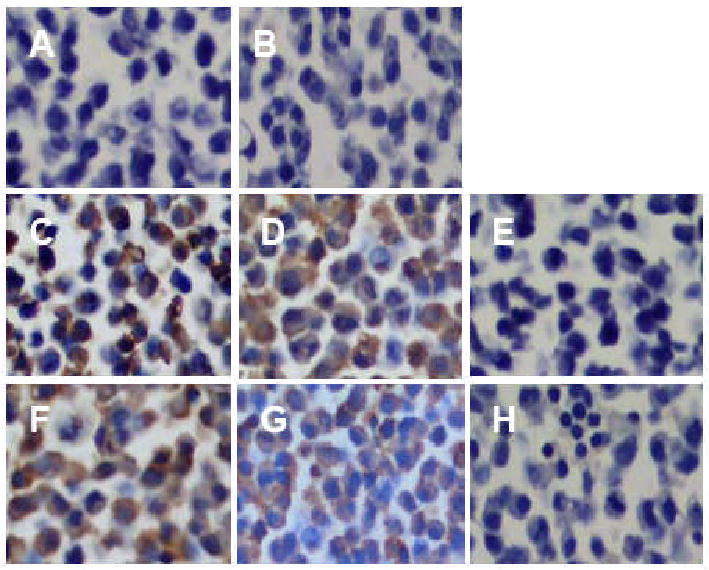
Evaluation of the specificity of recombinant anti-AT2 peptide ScFv antibodies by immunocytochemical staining of the AT2 in COS-7 (A and B), AT2-transfected COS-7 (AT2/COS-7) cells (C, D and E) and AT2 expressing PC12W cells (F, G, and H). Positive signals were detected in both the AT2/COS-7 (C and D) and PC12W cells (F and G) but not in COA-7 cells (A and B) when anti-AT2 C (A, C and F) or E peptide ScFv (B, D and G) was used. Negative control was immunostained without primary antibody (E) and with only anti-E tag secondary antibody (H). Pretreatment with antigen AT2 C and E peptides almost completely absorbed the immunoreactivity of these anti-AT2 antibodies (data not shown). Original magnifications; × 400.
Expression of AT2 in lung alveoli and blood vessels
Positive immunofluorescence staining for AT2 was observed throughout alveolar epithelial cells and bronchial epithelium (Fig. 4). A strong immunofluorescence staining was also detected in both endothelial cells and muscular media in pulmonary artery (Fig.4-A and -G). There was no clear stainability difference between recombinant and commercially available antibodies (data not shown). A very strong immunofluorescence staining for AT1a was observed in cytoplasm and plasma membrane of bronchial epithelial cells but not in their nuclei. The AT1a immunofluorescence staining was also detected in alveolar epithelium and its intensity was similar to those for AT2. As shown in panels C, F and I, AT2 and AT1a overlaid pictures clearly indicate co-localization of both receptor subtypes in much of the lung tissues. AT2 immunofluorescence staining was negative in AT2-KO mouse lung bronchial epithelium and alveoli (Fig. 4-D). However, very faint staining was detected only in apical side of the bronchial epithelial cells (Fig. 4-D). A very strong immunofluorescence staining was observed in red blood cells in wild type and two mutant mice. AT1a immunofluorescence staining was negative in AT1a-KO mouse lung (Fig. 4-H). These results indicate that AT2 nonspecific immunofluorescence staining was localized strongly in red blood cells and moderately in the apical side of bronchial epithelial cells.
Fig. 4.
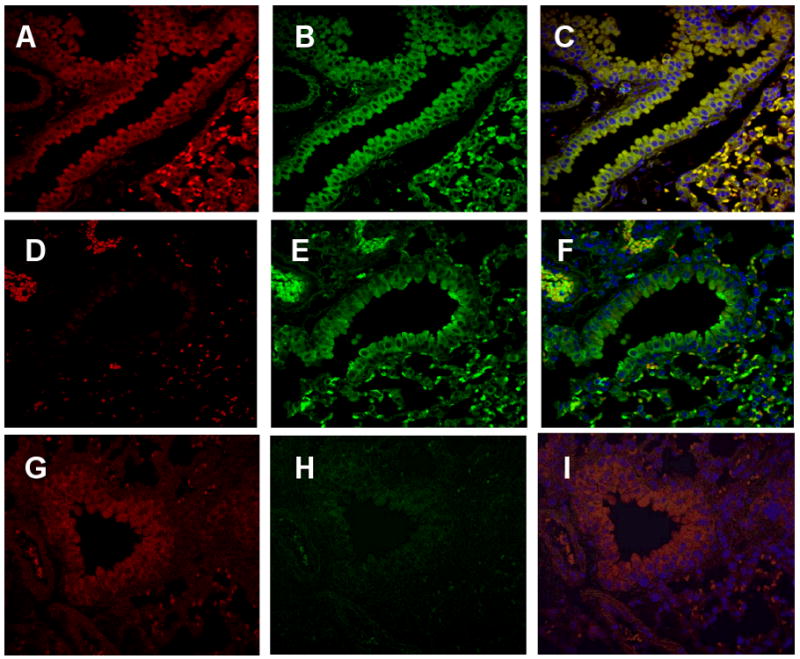
Immunohistochemical localization of the Ang II receptors in wild type (A, B and C) and Ang II AT2 (D, E and F)- or AT1a (G, H and I)-deficient mouse lungs. The lungs from 8-12-week-old wild type and mutant mice with C57BL/6 genetic back ground were paraffin-embedded, sectioned and immunostained using the anti-AT2 ScFv (A, D and G) and anti-AT1a (B, E and H) antibodies. Panels C, F and I are overlaid pictures of anti-AT2 and anti-AT1a immunofluorescence stained sections from wild type, AT2-KO and AT1a-KO mouse lung, respectively. Similarly intense immunostaining for the AT2 and AT1a were observed in the bronchial epithelium and alveoli from the wild type mouse (A, B and C, magnification × 100). However, AT2- or AT1a-deficient mouse lung tissue clearly lack corresponding staining. A faint immunostaining for AT1a was also detected in bronchial epithelial cell layer in AT1a-deficient mouse lungs implying non-specific immunostaining. Red blood cells exhibited a strong non-specific immunostaining for both anti-AT2 and -AT1a antibodies.
Expression of AT2 in NNK-induced lung tumors
Lung from NNK-treated wild type SWR/J mice was collected 20 weeks after initial NNK administration, fixed in Fekete's solution and paraffin-embedded. AT2 immunofluorescence staining was carried out using both recombinant anti-AT2 ScFv and commercially available anti-goat anti-AT2 or anti-rabbit anti-AT1a antibodies. A significantly strong immunofluorescence staining for AT2 and AT1a was observed in adenomatous hyperplastic epithelium (Fig. 5). The AT2 immunofluorescence staining in vascular endothelial cells in small pulmonary vessels adjacent to the adenomatous lesion were also significantly intensified (Fig.5, arrows). However, similar sized vessels distant from the tumor were moderately stained. A commercially available anti-goat anti-AT2 antibodies exhibited similar results (data not shown). These results may suggest that angiotensin II receptor expression potentially plays an important role in NNK-induced lung tumorigenesis.
Fig. 5.
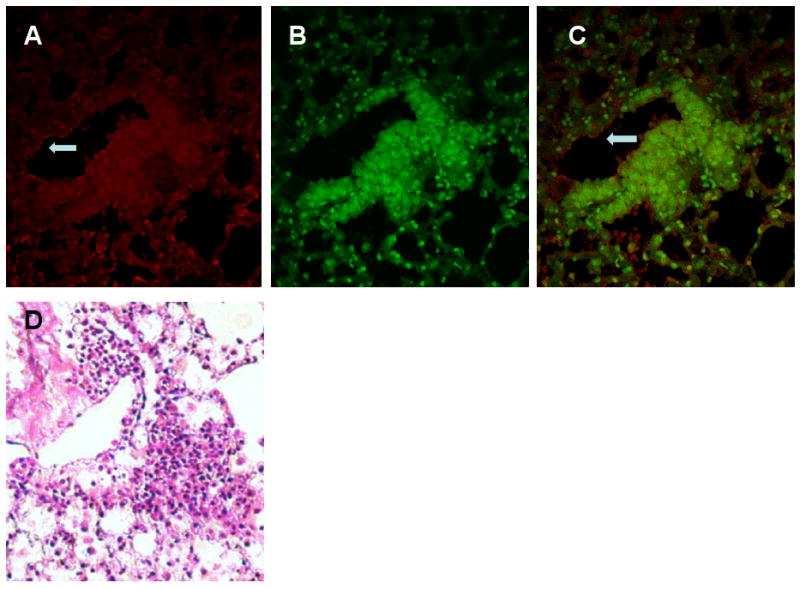
Immunohistochemical expression of Ang II receptors in adenomatous lesion in NNK-treated mouse lung. The lungs from 26-week-old mice treated with NNK were paraffin-embedded, sectioned and immunostained using the anti-AT2 ScFv and anti-AT1a antibodies as described in the methods section. Strong positive immunostainings for the AT2 and AT1a were observed in adenomatous lesion (A, anti-AT2 ScFv staining; B, anti-AT1a staining; C, merged; magnification × 100). Intensities of AT2 and AT1a immunostaining in adenomatous epithelial cells were significantly stronger than surrounding normal alveolar epithelial cells.
Discussion
Increasing evidence suggests that angiotensin II signaling plays an important role in carcinogenesis (Fujita et al. 2005; Ino et al. 2006; Kanehira et al. 2005; Suganuma et al. 2005; Takagi et al. 2002:Egami 2003). However, the specific role of AT2 in carcinogenesis has not been rigorously elucidated. We have previously demonstrated the pro-oncogenic role of AT2 in carcinogen-induced colon and lung tumorigenesis in the mouse (Kanehira et al. 2005; Takagi et al. 2002). In the present study we developed several recombinant anti-AT2 peptide ScFv antibodies by screening a rodent phage-displayed recombinant antibody library using several synthetic peptides of the AT2 protein and examined AT2 expression in normal and NNK-induced tumor bearing mouse lung. Immunohistochemical analysis provides important information for localization of AT2 protein expression and elucidation of AT2 function in carcinogen-induced lung tumorigenesis.
In the first study, we have examined the specificity of newly developed recombinant anti-AT2 peptide antibodies by ELISA (Fig.2) and immunostaining of AT2 gene transfected and untransfected COS-7 cells and AT2 spontaneously expressing PC12W cells (Fig. 3). In the experiment using ELISA, antigen antibody-binding was specifically inhibited by pretreatment of the antiserum with excess amount of antigen peptide (Fig. 2). In the immunostaining experiment, anti-AT2 peptide antibodies (EG-5, EO-9 and CB-11) selectively stained only AT2 transfected COS-7 cells and PC12W cells. Furthermore, these antibodies immunostained adrenal medulla and glomerulosa cells and coronary artery wall (data not shown), all of which are well known expression sites of AT2 (Ozono et al. 1997; Utsunomiya et al. 2005). Accordingly, our recombinant anti-AT2 peptide antibodies appeared to be selective for the AT2 protein. The anti-AT2E-peptide antibody (see Fig. 1) may be useful for neutralizing the AT2 function since this is an important site for ligand binding. However, this possibility must be clarified by an additional study. To the best of our knowledge, the present study is the first to demonstrate that recombinant single chain antibody against specific peptide sequences derived from angiotensin II receptor protein was successfully screened from a rodent phage-displayed recombinant antibody library.
In a previous in situ hybridization study analysis in rats showed that AT2 mRNA was detected in bronchi and trachea but very little in stroma in the E19 fetal and new born pups, whereas the expression levels of AT2 mRNA in the adult rat lung is very low or negligible (Shanmugam et al. 1996). In adult human lung tissues, AT2 protein expression was detected in brush borders of bronchial epithelium by immunohistochemical analysis with anti-rabbit anti-AT2 antiserum (Bullock et al. 2001). On the contrary, our newly developed recombinant antibodies detected weak but clear signals of AT2 protein expression in the alveolar epithelial cells and bronchial epithelium of adult mouse lung. These results were also confirmed by commercially available anti-AT2 peptide antisera. Comparably abundant immunoreactivity for the AT2 protein was also observed in both vascular endothelium and muscular media of pulmonary arteries. These results are similar to those reported in immunohistochemical studies of hearts (Utsunomiya et al. 2005; Wang et al. 1998) and skeletal muscle (Nora et al. 1998). The difference between the AT2 expression in human and mouse lung may be explained by the species and antibodies differences (antibodies used for the present study are highly specific recombinant ScFv antibodies). Taken together, these results suggest that the AT2 function in the regulation of tumorigenesis may be associated with the function of alveoli epithelial cells more than bronchial epithelial cells, since NNK-induced lung adenoma appears to be initiated from the transformation of alveolar epithelial cells (Hecht 1998). Our previous study indicates that AT2 appears to be involved in carcinogen-induced lung tumorigenesis (Kanehira et al. 2005) and the resistant phenotype of AT2-KO mice in NNK-induced lung carcinogenesis may be due to the lack of AT2 expression in alveoli epithelium (Fig. 4-D). This is consistent with our earlier study in which angiotensin II-AT2 signaling is also associated with carcinogen-induced colon tumorigenesis (Takagi et al. 2002). Although expression level of the AT2 in the adult lung alveolar epithelium is low, the function of AT2 in alveolar epithelium may be important. In support of this speculation the present study clearly indicated that induction of adenomatous transformation was associated with significantly increased expression of the Ang II receptor protein (Fig. 5). Consistent with the present results we have previously demonstrated that AT2 expression is inducible in vitro (Ichiki et al. 1996) and in vivo (Tamura et al. 2000). Although it is difficult to speculate a specific function of AT2 in carcinogen-induced tumorigenesis, AT2 likely play a role in lung carcinogenesis.
With regard to overall Ang II signaling and lung cancer, AT1-mediated Ang II signaling should be taken into a consideration since the AT1 is the major Ang II receptor and its expression in the lung tissue is relatively abundant (Bullock et al. 2001). In our study, a small amount of the AT1 expression was detected in normal alveolar epithelium and this expression was markedly increased in NNK-induced adenomatous epithelial cells (Fig. 4 and 5). Since AT1-mediated Ang II signaling has been shown to be associated with the growth of various type of tumors such as melanoma (Egami et al. 2003), ovarian cancer, (Ino et al. 2006), and lung metastasis of renal cell carcinoma (Miyajima et al. 2002), AT1 over-expression in adenomatous epithelial cells may be associated with cell growth in adenoma. In support of this speculation, AT1 signaling is shown to stimulate production of vascular endothelial cell growth factor (Fujiyama et al. 2001; Richard et al. 2000) and stimulate cell growth in multiple cell types (Berry et al. 2001; Chung et al. 1998). Contrarily to AT1-mediated cell growth, AT2-mediated Ang II signaling is shown to be associated with an inhibition of cell proliferation (Stoll et al. 1995) and stimulation of apoptosis in cultured vascular endothelial cells (Dimmeler et al. 1997) and smooth muscle cells (Cui et al. 2001). Accordingly, Ang II appears to play reciprocal functions in cell growth (AT1 is growth stimulative and the AT2 is anti-stimulative). However, whether overall Ang II signaling plays a pro-oncogenic role or an anti-tumorigenic role in lung carcinogenesis, or the overall role of the Ang II in lung carcinogenesis is stage-dependent, etc. requires further study.
The benefit of ScFv antibodies is, unlike the parental antibody, they are very small allowing them to penetrate the cell membrane more efficiently (Shi et al. 2006). ScFv antibodies can also be genetically produced using cDNA of the parental antibody. Despite their smaller size the ScFv antibody retains target specificity and antigen binding affinity of a parental antibody. Therefore, the ScFv antibody specific to various regions of the AT2 can be used as a potential tool to evaluate ligand-receptor interaction and post-receptor signaling. However, these possibilities must be evaluated by future studies.
In summary, the present study demonstrates that preparation of recombinant single chain antibodies specific to the AT2 receptor peptide by screening from the recombinant antibody library is feasible. We have confirmed the specificity of the immunoreactivity to AT2 using ELISA and AT2 transfected and untransfected cells, which do not express AT2. Our findings suggest that the vascular endothelium expression of AT2 in adult mouse lung alveoli provides a potential link whereby AT2 regulates carcinogen-induced lung tumorigenesis. Our discovery of strong AT2 localization in an early adenomatous lesion suggests the possible involvement of AT2 function in tumorigenesis.
Acknowledgments
The authors thank Ms. Lara Pickel (Department of Anatomy & Physiology, Kansas State University) and Dr. Dharmendra Maurya (Department of Anatomy & Physiology, Kansas State University) for critical reading and constructive comments during the preparation of the manuscript. We thank Mr. Eric F. Howard (Department of Biochemistry, Vanderbilt University) for his technical assistance in the preparation of the AT2 peptides. This work was supported in part by a Grant-in-Aid from the American Heart Association (9750624N), National Cancer Institute grants (CA091428 and CA90949) NIH P20 RR017686 and Kansas State University College of Veterinary Medicine Dean's Fund.
References
- Berry C, Touyz R, Dominiczak AF, et al. Angiotensin receptors: signaling, vascular pathophysiology, and interactions with ceramide. Am J Physiol Heart Circ Physiol. 2001;281:H2337–65. doi: 10.1152/ajpheart.2001.281.6.H2337. [DOI] [PubMed] [Google Scholar]
- Bullock GR, Steyaert I, Bilbe G, et al. Distribution of type-1 and type-2 angiotensin receptors in the normal human lung and in lungs from patients with chronic obstructive pulmonary disease. Histochem Cell Biol. 2001;115:117–124. doi: 10.1007/s004180000235. [DOI] [PubMed] [Google Scholar]
- Chassagne C, Eddahibi S, Adamy C, et al. Modulation of angiotensin II receptor expression during development and regression of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2000;22:323–2. doi: 10.1165/ajrcmb.22.3.3701. [DOI] [PubMed] [Google Scholar]
- Chung O, Kuhl H, Stoll M, Unger T. Physiological and pharmacological implications of AT1 versus AT2 receptors. Kidney Int Suppl. 1998;67:S95–9. doi: 10.1046/j.1523-1755.1998.06719.x. [DOI] [PubMed] [Google Scholar]
- Csikos T, Gallinat S, Unger T. Extrarenal aspects of angiotensin II function. Eur J Endocrinol. 1997;136:349–8. doi: 10.1530/eje.0.1360349. [DOI] [PubMed] [Google Scholar]
- Cui T, Nakagami H, Iwai M, et al. Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc Res. 2001;49:863–871. doi: 10.1016/s0008-6363(00)00299-6. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Rippmann V, Weiland U, et al. Angiotensin II induces apoptosis of human endothelial cells. Protective effect of nitric oxide. Circ Res. 1997;81:970–6. doi: 10.1161/01.res.81.6.970. [DOI] [PubMed] [Google Scholar]
- Egami K, Murohara T, Shimada T, et al. Role of host angiotensin II type 1 receptor in tumor angiogenesis and growth. J Clin Invest. 2003;112:67–75. doi: 10.1172/JCI16645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete E. A comparative morphological study of the mammary gland in a high and low tumor strain of mice. American Journal of Pathology. 1938;14:557–583. [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Hayashi I, Yamashina S, et al. Angiotensin type 1a receptor signaling-dependent induction of vascular endothelial growth factor in stroma is relevant to tumor-associated angiogenesis and tumor growth. Carcinogenesis. 2005;26:271–279. doi: 10.1093/carcin/bgh324. [DOI] [PubMed] [Google Scholar]
- Fujiyama S, Matsubara H, Nozawa Y, et al. Angiotensin AT(1) and AT(2) receptors differentially regulate angiopoietin-2 and vascular endothelial growth factor expression and angiogenesis by modulating heparin binding-epidermal growth factor (EGF)-mediated EGF receptor transactivation. Circ Res. 2001;88:22–29. doi: 10.1161/01.res.88.1.22. [DOI] [PubMed] [Google Scholar]
- Hecht SS. Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chem Res Toxicol. 1998;11:559–603. doi: 10.1021/tx980005y. [DOI] [PubMed] [Google Scholar]
- Hein L, Barsh GS, Pratt RE, et al. Behavioural and cardiovascular effects of disrupting the angiotensin II type -2 receptor in mice. Nature. 1995;377:744–747. doi: 10.1038/377744a0. [DOI] [PubMed] [Google Scholar]
- Hennig EE, Mernaugh R, Edl J, et al. Heterogeneity among Helicobacter pylori strains in expression of the outer membrane protein BabA. Infect Immun. 2004;72:3429–3435. doi: 10.1128/IAI.72.6.3429-3435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi M, Akishita M, Dzau VJ. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33:613–621. doi: 10.1161/01.hyp.33.2.613. [DOI] [PubMed] [Google Scholar]
- Ichiki T, Kambayashi Y, Inagami T. Differential inducibility of angiotensin II AT2 receptor between SHR and WKY vascular smooth muscle cells. Kidney Int Suppl. 1996;55:S14–7. [PubMed] [Google Scholar]
- Ichiki T, Labosky PA, Shiota C, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–50. doi: 10.1038/377748a0. [DOI] [PubMed] [Google Scholar]
- Inagami T, Kambayashi Y, Ichiki T, et al. Angiotensin receptors: molecular biology and signalling. Clin Exp Pharmacol Physiol. 1999;26:544–9. doi: 10.1046/j.1440-1681.1999.03086.x. [DOI] [PubMed] [Google Scholar]
- Ino K, Shibata K, Kajiyama H, et al. Angiotensin II type 1 receptor expression in ovarian cancer and its correlation with tumour angiogenesis and patient survival. Br J Cancer. 2006;94:552–60. doi: 10.1038/sj.bjc.6602961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambayashi Y, Bardhan S, Takahashi K, et al. Molecular cloning of a novel angiotensin II receptor isoform involved in phosphotyrosine phosphatase inhibition. J Biol Chem. 1993;268:24543–6. [PubMed] [Google Scholar]
- Kanehira T, Tani T, Takagi T, et al. Angiotensin II type 2 receptor gene deficiency attenuates susceptibility to tobacco-specific nitrosamine-induced lung tumorigenesis: involvement of transforming growth factor-beta-dependent cell growth attenuation. Cancer Res. 2005;65:7660–5. doi: 10.1158/0008-5472.CAN-05-0275. [DOI] [PubMed] [Google Scholar]
- Malecki M, Hsu A, Truong L, Sanchez S. Molecular immunolabeling with recombinant single-chain variable fragment (scFv) antibodies designed with metal-binding domains. Proc Natl Acad Sci U S A. 2002;99:213–8. doi: 10.1073/pnas.261567298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A, Kosaka T, Asano T, et al. Angiotensin II type I antagonist prevents pulmonary metastasis of murine renal cancer by inhibiting tumor angiogenesis. Cancer Res. 2002;62:4176–4179. [PubMed] [Google Scholar]
- Nora EH, Munzenmaier DH, Hansen-Smith FM, et al. Localization of the ANG II type 2 receptor in the microcirculation of skeletal muscle. Am J Physiol. 1998;275:H1395–403. doi: 10.1152/ajpheart.1998.275.4.h1395. [DOI] [PubMed] [Google Scholar]
- Ozono R, Wang ZQ, Moore AF, et al. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension. 1997;30:1238–46. doi: 10.1161/01.hyp.30.5.1238. [DOI] [PubMed] [Google Scholar]
- Richard DE, Berra E, Pouyssegur J. Nonhypoxic pathway mediates the induction of hypoxia-inducible factor 1alpha in vascular smooth muscle cells. J Biol Chem. 2000;275:26765–26771. doi: 10.1074/jbc.M003325200. [DOI] [PubMed] [Google Scholar]
- Shanmugam S, Corvol P, Gasc JM. Angiotensin II type 2 receptor mRNA expression in the developing cardiopulmonary system of the rat. Hypertension. 1996;28:91–7. doi: 10.1161/01.hyp.28.1.91. [DOI] [PubMed] [Google Scholar]
- Shi J, Liu Y, Zheng Y, et al. Therapeutic expression of an anti-death receptor 5 single-chain fixed-variable region prevents tumor growth in mice. Cancer Res. 2006;66:11946–53. doi: 10.1158/0008-5472.CAN-06-1227. [DOI] [PubMed] [Google Scholar]
- Stoll M, Steckelings UM, Paul M, et al. The angiotensin AT2-receptor mediates inhibition of cell proliferation in coronary endothelial cells. J Clin Invest. 1995;95:651–7. doi: 10.1172/JCI117710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suganuma T, Ino K, Shibata K, et al. Functional expression of the angiotensin II type 1 receptor in human ovarian carcinoma cells and its blockade therapy resulting in suppression of tumor invasion, angiogenesis, and peritoneal dissemination. Clin Cancer Res. 2005;11:2686–94. doi: 10.1158/1078-0432.CCR-04-1946. [DOI] [PubMed] [Google Scholar]
- Takagi T, Nakano Y, Takekoshi S, Inagami T, Tamura M. Hemizygous mice for the angiotensin II type 2 receptor gene have attenuated susceptibility to azoxymethane-induced colon tumorigenesis. Carcinogenesis. 2002;23:1235–41. doi: 10.1093/carcin/23.7.1235. [DOI] [PubMed] [Google Scholar]
- Tamura M, Takagi T, Howard EF, et al. Induction of angiotensin II subtype 2 receptor-mediated blood pressure regulation in synthetic diet-fed rats. J Hypertens. 2000;18:1239–46. doi: 10.1097/00004872-200018090-00010. [DOI] [PubMed] [Google Scholar]
- Timmermans PB, Wong PC, Chiu AT, et al. Angiotensin II receptors and angiotensin II receptor antagonists. Pharmacol Rev. 1993;45:205–51. [PubMed] [Google Scholar]
- Tsuzuki S, Matoba T, Eguchi S, Inagami T. Angiotensin II type 2 receptor inhibits cell proliferation and activates tyrosine phosphatase. Hypertension. 1996;28:916–8. doi: 10.1161/01.hyp.28.5.916. [DOI] [PubMed] [Google Scholar]
- Utsunomiya H, Nakamura M, Kakudo K, Inagami T, Tamura M. Angiotensin II AT2 receptor localization in cardiovascular tissues by its antibody developed in AT2 gene-deleted mice. Regul Pept. 2005;126:155–61. doi: 10.1016/j.regpep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Wang ZQ, Moore AF, Ozono R, et al. Immunolocalization of subtype 2 angiotensin II (AT2) receptor protein in rat heart. Hypertension. 1998;32:78–83. doi: 10.1161/01.hyp.32.1.78. [DOI] [PubMed] [Google Scholar]