Abstract
Clinical trials have demonstrated that a reduced intake of dietary sodium lowers blood pressure. However, blood pressure reduction in response to a decrease in dietary sodium intake varies considerably among different individuals–a phenomenon described as sodium-sensitivity. The Genetic Epidemiology Network of Salt Sensitivity (GenSalt) study was a large family-based dietary-feeding study conducted in rural north China. This study indicated that approximately 39% of Chinese adults were sodium-sensitive. Sodium-sensitivity was more common in women and in persons who were older and had higher usual blood pressure. Sodium-sensitivity was also more common in individuals with higher responses to cold pressor test and in individuals with the metabolic syndrome. Genetic factors might play an important role in determining sodium-sensitivity in the Chinese population. A better understanding of the genetic and environmental determinants of sodium-sensitivity has important public health and clinical implications.
Introduction
Hypertension is a major public health challenge worldwide because of its high prevalence and consequent increase in risk of vascular disease and premature death (1–3). Clinical trials have demonstrated that a reduced intake of dietary sodium lowers blood pressure (BP) in both hypertensive and normotensive persons (4,5). BP reduction in response to a decrease in dietary sodium intake, however, may vary considerably among different individuals–a phenomenon described as sodium-sensitivity (6). Identifying individuals who are more sensitive to dietary sodium intervention will enable targeted lifestyle modification to individuals who may particularly benefit from a low-sodium diet. In addition, sodium-sensitivity has been associated with increased risk of cardiovascular disease and premature death in hypertensive and normotensive participants (7,8).
Kawasaki et al. (9) and later on Weinberger et al. (6) were among the first to recognize the heterogeneity of BP response to sodium intake and to develop the concept of sodium-sensitivity in humans. The definition of sodium-sensitivity has varied between studies, mostly being defined as a proportional change (i.e., ≥3 to ≥10%) or an absolute change (i.e., ≥3 to ≥10 mm Hg) in mean arterial BP (MAP). Despite the variety of the definitions and protocols used to test for sodium-sensitivity, several findings have been consistently observed (6,9,10). First, sodium-induced changes in BP are normally distributed in populations, and there is no evidence for a bimodal distribution. Like defining hypertension, using a cut point to categorize subjects as sodium sensitive and non-sensitive is arbitrary. In addition, sodium-sensitivity is a common biological phenomenon in human populations. Depending on the definition and measurement methods, sodium sensitivity has been noted in 25–50% of normotensives and 40–75% of hypertensive patients. Furthermore, both genetic and environmental factors may determine an individual’s sodium-sensitivity (10,11).
Examining the genetic and environmental determinants of sodium-sensitivity of BP has important public health and clinical implications. Establishing a relationship between genetic variants with sodium-sensitivity will help identify individuals at high risk for hypertension who could benefit from a low sodium dietary intervention or pharmaceutical treatment using novel genotype-based drugs. In addition, identifying genes that interact significantly with dietary sodium intake on the regulation of BP will contribute significantly to knowledge about physiological pathways underlying hypertension. Identifying genetic variants associated with sodium-sensitive hypertension will also assist in the development of new antihypertensive medications targeting biological pathways related to sodium metabolism. From a public health perspective, individuals with a modifiable genetic risk of hypertension could be identified prior to disease development, and effective and appropriate lifestyle modifications or novel pharmaceutical therapies could be implemented for primary prevention. Advances in this area could significantly enhance the effectiveness of clinical patient care and population-wide prevention of hypertension.
Study Design of Genetic Epidemiology Network of Salt-Sensitivity (GenSalt)
The GenSalt study is a large family-based dietary-feeding study to examine the genetic and environmental determinants of sodium-sensitivity in rural areas of northern China (12). The study participants were recruited from rural north China because of the homogeneity of the residents regarding their ethnicity and environmental exposures, including lifestyle and nutritional factors and habitual dietary intake of high salt and low potassium. The residents as regards their ethnicity are of Han nationality, the ethnic majority in China. The GenSalt sampling design targets families at high risk for developing hypertension and may be sensitive to sodium interventions. A community-based BP screening was conducted among all residents aged 18–60 years in the study villages to identify potential probands and their families. Those with a mean systolic BP between 130–160 mm Hg and/or a diastolic BP between 85–100 mm Hg and no use of antihypertensive medication as well as their siblings, spouses, and offspring were recruited for the study. Although the relatives were recruited regardless of their BP levels, individuals who had stage-2 hypertension (BP≥160/100 mm Hg), secondary hypertension, clinical cardiovascular disease, diabetes (fasting plasma glucose ≥126 mg/dL), chronic kidney disease, current use of antihypertensive or antidiabetic medications or insulin, pregnancy, heavy alcohol consumption, or current use of a low-sodium diet were excluded from the study. A total of 1,906 participants volunteered to take part in the dietary intervention study.
A standard questionnaire was administered by trained staff at the baseline examination to collect information on demographic characteristics, personal and family medical history, and lifestyle risk factors. Three BP measurements were obtained each morning during the 3-day baseline observation period, and on days 2, 5, 6 and 7 of each intervention period by trained and certified observers using a random–zero sphygmomanometer according to a standard protocol. BP was measured with the participant in the sitting position after 5 minutes of rest. In addition, participants were advised to avoid consumption of alcohol, coffee, or tea, cigarette smoking, and exercise for at least 30 minutes prior to their BP measurements. BP observers were blinded to the participant’s dietary intervention. Body weight, height, and waist circumference were measured twice with the participant in light indoor clothing without shoes during their baseline examination. Waist circumference was measured one cm above the participant’s navel during minimal respiration. Overnight (≥8 hours) fasting blood specimens were obtained for measurement of glucose and lipids.
Study participants received a low-sodium diet (3 grams of sodium chloride or 51.3 mmol of sodium per day) for 7 days, followed by a high-sodium diet (18 grams of sodium chloride or 307.8 mmol of sodium per day) for an additional 7 days. During both periods of sodium intervention, dietary potassium intake remained unchanged. Dietary total energy intake was varied according to each participant’s baseline energy intake. All foods were cooked without salt, and pre-packaged salt was added to the individual study participant’s meal when it was served by the study staff. To ensure compliance to the intervention program, participants were required to eat their breakfast, lunch and dinner at the study kitchen under the supervision of study staff during the entire study. Study participants were instructed to avoid consumption of any foods that were not provided by study staff members. Three timed urinary specimens (one 24-hour and two overnights) were obtained during the 3 days of baseline examinations and the last 3 days of each intervention period (days 5, 6, and 7) to monitor each participant’s compliance with the dietary sodium intervention. The timed overnight urinary excretions of sodium and potassium were converted to 24-hour values based on a formula developed from data obtained in this study. Results from the 24-hour urinary excretion of sodium demonstrated excellent compliance: the mean (standard deviation) 24-hour urinary excretions of sodium and potassium were 242.4 (66.7) mmol and 36.9 (9.6) mmol at baseline, 47.5 (16.0) and 31.4 (7.7) at the end of the low-sodium intervention period, and 244.3 (37.7) and 35.7 (7.5) at the end of the high-sodium intervention period, respectively.
BP levels at baseline and during intervention were calculated as the mean of 9 measurements from 3 clinical visits during the 3-day baseline observation or on days 5, 6 and 7 of each intervention phase. Responses were defined as follows: BP response to low-sodium = BP on low-sodium diet – BP at baseline and BP response to high-sodium = BP on high-sodium diet – BP on low-sodium diet.
The cold pressor test (CPT) was conducted during the baseline examination. After the participant had remained sitting for 20 minutes, 3 BP measurements were obtained using a standard mercury sphygmomanometer before the ice water immersion. Then, the participant immersed his/her left hand in the ice water bath (3°C to 5°C) to just above the wrist for 1 minute. BP measurements at 0, 60, 120, and 240 seconds were obtained using a standard mercury sphygmomanometer after the left hand had been removed from the ice water bath.
Sodium Sensitivity by Age and Gender
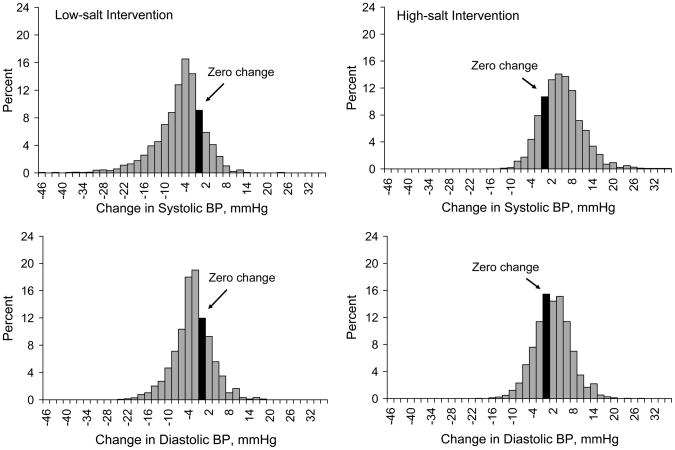
The distributions of systolic and diastolic BP responses to low-salt and high-salt interventions are shown in Figure 1. The GenSalt study indicated that BP responses to dietary sodium intake were normally distributed in populations and there was no evidence for a bimodal distribution. Both systolic and diastolic BP levels decreased during low-salt intervention and increased during high-salt intervention among the majority of study participants (13••). For example, systolic BP fell by 4, 6, and 8 mmHg among 62.4%, 45.9%, and 33.1% of study participants during the low-salt intervention and systolic BP increased by 4, 6, and 8 mmHg among 60.7%, 46.6%, and 32.9% of study participants during high-salt intervention. On the other hand, systolic BP did not decrease (≥0 mmHg) among 23.2% of study participants during the low-salt interventions and systolic BP did not increase (≤0 mmHg) among 26.1% of study participants during the high-salt intervention.
Figure 1.
Distribution of systolic (upper panels) and diastolic (low panels) BP responses to low-salt intervention (left panels) and high-salt intervention (right panels). BP response to low-sodium = BP on low-sodium diet – BP at baseline and BP response to high-sodium = BP on high-sodium diet – BP on low-sodium diet.
Because BP response to dietary sodium intervention is continuously distributed, using a cut-point to categorize subjects as sodium-sensitive and salt-resistant is arbitrary. If an absolute change of MAP ≥5 mmHg was used to define sodium-sensitivity, 33.9% of participants during low-salt intervention and 32.4% of participants during high-salt intervention would have met the criteria (13••). If a proportional change of MAP ≥5% was used to define salt-sensitivity, 38.7% of participants during low-salt intervention and 39.2% of participants during high-salt intervention would have met the criteria. Therefore, sodium-sensitivity appears to be a common biological phenomenon in human populations.
Previous studies have documented that salt-sensitivity is more common among individuals who are older, hypertensive, or of African-American descent (14–16). In the GenSalt study, both systolic and diastolic BP responses to low-salt and high-salt interventions were greater in females compared to their male counterparts (Table 1). There was a dose-response relationship between age and systolic BP responses to low-salt and high-salt interventions. In addition, there was a dose-response relationship between baseline BP categories and both systolic and diastolic BP responses to low-salt and high-salt interventions.
Table 1.
Multivariable-adjusted* mean changes (95% confidence interval) in systolic and diastolic blood pressure in response to low-salt and high-salt interventions in the GenSalt study participants
| Low-sodium intervention | High-sodium intervention | |||
|---|---|---|---|---|
| ΔSBP | ΔDBP | ΔSBP | ΔDBP | |
| Gender | ||||
| Men (n=1,010) | −7.05 (−7.50, −6.59) | −3.39 (−3.77, −3.01) | 5.25 (4.83, 5.66) | 1.74 (1.36, 2.13) |
| Women (n=896) | −8.07 (−8.57, −7.58) | −4.51 (−4.92, −4.09) | 6.35 (5.90, 6.81) | 3.08 (2.66, 3.50) |
| p-value for differences by gender | 0.0004 | <0.0001 | <0.0001 | <0.0001 |
| Age, yrs | ||||
| <35 (n=592) | −6.04 (−6.64, −5.44) | −4.05 (−4.56, −3.55) | 4.31 (3.75, 4.86) | 2.00 (1.49, 2.51) |
| 35–44 (n=783) | −7.59 (−8.08, −7.10) | −4.01 (−4.42, −3.59) | 5.73 (5.28, 6.19) | 2.57 (2.15, 2.99) |
| ≥54 (n=531) | −9.05 (−9.62, −8.47) | −3.78 (−4.27, −3.29) | 7.36 (6.83, 7.89) | 2.66 (2.17, 3.16) |
| p-value for differences by age | <0.0001 | 0.66 | <0.0001 | 0.08 |
| Baseline BP, mm Hg | ||||
| <120/80 (n=1,083) | −3.28 (−3.66, −2.90) | −1.46 (−1.78, −1.14) | 4.14 (3.79, 4.49) | 1.44 (1.12, 1.77) |
| 120-139/80–89 (n=641) | −7.57 (−8.06, −7.08) | −4.02 (−4.43, −3.61) | 5.52 (5.07, 5.98) | 2.40 (1.98, 2.82) |
| ≥140/90 (n=182) | −11.8 (−12.8, −10.9) | −6.36 (−7.15, −5.57) | 7.74 (6.88, 8.60) | 3.38 (2.58, 4.18) |
| p-value for differences by baseline BP | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
Adjusted for age, gender, baseline BP, body-mass index, education, alcohol consumption, current smoking, physical activity, and baseline 24-hour urinary excretion of sodium and potassium.
BP response to low-sodium = BP on low-sodium diet – BP at baseline; and BP response to high-sodium = BP on high-sodium diet – BP on low-sodium diet.
Cold Pressure Test and Sodium Sensitivity
The cold pressor test (CPT), which measures the response of BP to the stimulus of external cold, has long been a standard test for characterization of sympathetic function and has been documented to predict the subsequent risk of hypertension in normotensive persons (17–19). Previous studies have also suggested that sympathetic nervous system activity might play an important role in determining the salt-sensitivity of BP (20).
In the GenSalt study, a dose-response relationship between BP responses to the CPT and to dietary sodium interventions was identified (21••). After adjustment for important covariates, there were statistically significant associations between the quartiles of BP response to the CPT and BP response to the dietary sodium interventions (all p<0.0001). Compared to the lowest quartile of BP response to the CPT, systolic BP changes [95% confident interval (CI)] for the top 3 quartiles, respectively, were −2.02 (−2.87, −1.16), −3.17 (−4.05, −2.28), and −5.98 (−6.89, −5.08) mmHg during the low-sodium intervention. Corresponding systolic BP changes during the high-sodium intervention were 0.40 (−0.36, 1.16), 0.44 (−0.35, 1.22), and 2.30 (1.50, 3.10) mmHg. Similar results were found when BP response to the CPT was used as a continuous variable. After adjustment for important covariates, one standard deviation difference in the area-under-curve of systolic BP responses to the CPT was associated with −2.59 (95% CI: −2.91, −2.27; p<0.0001) mmHg reduction in systolic BP during low-sodium intervention and 0.98 (95% CI: 0.70, 1.27; p<0.0001) mmHg increase in systolic BP during high-sodium intervention. One standard deviation difference in diastolic BP responses to the CPT was associated with −1.35 (95% CI: −1.63, −1.07; p<0.0001) mmHg reduction in diastolic BP during low-sodium intervention and 0.33 (95% CI: 0.05, 0.61; p=0.02) mmHg increase in diastolic BP during high-sodium intervention. These results indicated that BP response to the CPT was associated with sodium-sensitivity. Furthermore, a low-sodium diet might be more effective to lower BP among individuals with large responses to the CPT.
Metabolic Syndrome and Sodium Sensitivity
Past small clinical studies have suggested that insulin resistance may lead to sodium retention and extracellular fluid volume expansion, thereby increasing BP responses to sodium intake (5,6). Since insulin resistance is thought to be the underlying mechanism for the metabolic syndrome, it is likely that individuals with the metabolic syndrome are more sensitive to a dietary sodium intervention. The GenSalt study identified a strong, positive, and significant association between the metabolic syndrome and salt-sensitivity of BP among persons without diabetes (24••). Multivariable-adjusted mean changes (95% CIs) in BP (mmHg) were significantly greater (all p<0.0001) among participants with compared to those without the metabolic syndrome: −7.34 (−8.21, −6.46) versus −5.17 (−5.51, −4.83) for systolic BP and −4.56 (−5.28, −3.85) versus −2.47 (−2.74, −2.19) for diastolic BP during the low-sodium intervention; and 6.51 (5.76, 7.26) versus 4.55 (4.26, 4.84) for systolic BP and 3.25 (2.56, 3.94) versus 1.69 (1.42, 1.96) for diastolic BP during the high-sodium intervention.
The sodium-sensitivity of BP increased progressively with a higher number of metabolic risk factors (Table 2). Compared to those with zero, participants with 4 or 5 risk factors for the metabolic syndrome had a 3.54-fold increased odds (95% CI: 2.05, 6.11) of high sodium-sensitivity during the low-sodium intervention and a 3.13-fold increased odds (1.80, 5.43) of high sodium-sensitivity during the high-sodium intervention. This association was independent of age, gender, body-mass index, physical inactivity, cigarette smoking, alcohol consumption, and baseline dietary intake of sodium and potassium. In addition, the association between the metabolic syndrome and salt-sensitivity remained after the participants with hypertension were excluded (24••). These findings indicate that a reduced intake of sodium may be particularly beneficial in individuals with the metabolic syndrome. Additional intervention studies are warranted to examine the effect of prevention and treatment of metabolic risk factors on sodium-sensitivity of BP.
Table 2.
Odds ratios and 95% confidence intervals of high sodium-sensitivity during low-sodium and high-sodium intervention according to number of metabolic risk factors and metabolic syndrome status
| Variable | Age-gender-adjusted | Multivariable-adjusted* | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | P Value | Odds Ratio (95% CI) | P Value | |
| Low-sodium Intervention | ||||
| 1 risk factor (n=586) † | 1.33 (1.03,1.71) | <0.0001 | 1.33 (1.03, 1.73) | <0.0001 |
| 2 risk factors (n=382)† | 1.54 (1.16,2.04) | 1.57 (1.16, 2.12) | ||
| 3 risk factors (n=200)† | 2.32 (1.66,3.25) | 2.38 (1.62, 3.51) | ||
| 4 or 5 risk factors (n=78)† | 3.36 (2.06,5.47) | 3.53 (2.05, 6.08) | ||
| Metabolic syndrome (n=278)‡ | 2.06 (1.59, 2.68) | <0.0001 | 1.93 (1.43, 2.59) | <0.0001 |
| High-sodium Intervention | ||||
| 1 risk factor (n=584)† | 1.02 (0.79, 1.32) | <0.0001 | 1.04 (0.79, 1.35) | 0.0003 |
| 2 risk factors (n=378)† | 1.31 (0.99, 1.74) | 1.42 (1.04, 1.93) | ||
| 3 risk factors (n=199)† | 1.57 (1.12, 2.22) | 1.72 (1.16, 2.56) | ||
| 4 or 5 risk factors (n=78)† | 2.90 (1.78, 4.72) | 3.10 (1.79, 5.39) | ||
| Metabolic syndrome (n=277)‡ | 1.73 (1.33, 2.25) | <0.0001 | 1.70 (1.25, 2.30) | 0.0007 |
Adjusted for age, gender, education, physical activity, cigarette smoking, alcohol consumption, body-mass index, and 24-hour urinary excretion of sodium and potassium at baseline examination.
Compared with those with 0 metabolic risk factors
Compared with those with 2 or less metabolic risk factors
Genetic Determinants of Sodium Sensitivity
There is evidence that genetic factors might play an important role in determining an individual’s sensitivity to dietary sodium intake (11). Previous family studies conducted in various populations reported that the heritability of usual BP ranged from 20% to 50% (25–27). In the GenSalt study, heritability was computed using maximum likelihood methods under a variance components model as implemented in the computer program SOLAR. The heritabilities of baseline BP were 0.31 for systolic, 0.32 for diastolic, and 0.34 for MAP. The heritabilities increased significantly under dietary intervention and were 0.49, 0.49, and 0.51 during low-sodium and 0.47, 0.49, and 0.51 during high-sodium intervention for systolic, diastolic, and MAP, respectively. The heritabilities for percentage of BP responses to low-sodium were 0.20, 0.21, and 0.23; and to high-sodium were 0.22, 0.33, and 0.33 for systolic, diastolic, and MAP, respectively (28•). These findings suggest that BP responses to dietary sodium intervention are familial and genetic factors may play a moderate role in BP responses to dietary sodium intake in the Chinese population.
Beeks and colleagues systemically reviewed candidate gene association studies to assess the role of genetic polymorphisms in sodium-sensitivity of BP (11). Detailed investigation was conducted on the α-adducin Gly460Trp, ACE I/D, angiotensinogen M235T, G protein β 3 C825T, aldosterone synthase gene (CYP11B2) and 11 beta-hydroxysteroid dehydrogenase type 2 G534A polymorphisms. Their analysis shows that the 460Trp variant of the α-adducin polymorphism is probably associated with a sodium-sensitive form of hypertension, while the polymorphisms of the angiotensin II type 1 receptor gene and the −344C/T variant of the CYP11B2 are not associated with this phenotype (11). The findings on the associations of ACE I/D, angiotensinogen M235T, G protein β 3 C825T, and 11 beta-hydroxysteroid dehydrogenase type 2 G534A polymorphisms with sodium-sensitive of BP were inconsistent.
Recent publications in candidate gene studies of sodium-sensitivity have expanded the already extensive list of polymorphisms (11) and directly associated genetic variants in renal sodium excretion to sodium-sensitivity of BP (10). Polymorphisms of a number of cytochrome P450 enzymes have been associated with sodium-sensitive hypertension (29–31). These genes include CYP11B2, which encodes aldosterone synthase (29); the ATP-binding cassette, subfamily B, member 1 (ABCB1), either alone or in concert with variants of cytochrome P450 3A5 (CYP3A5) (30); and CYP4A11, which converts arachidonic acid into 20-hydroxyeicosatetraenoic acid (31). Another area of investigation involves dopamine, dopamine receptors (particularly type-1 dopamine receptor), and G-protein-coupled receptor kinase 4 (GRK4) (32–34). Dopamine-mediated activation of type-1 dopamine receptor in the proximal tubule facilitates sodium excretion by inhibiting sodium and chloride transport. GRK4γ phosphorylates ligand-bound G protein–coupled receptors, such as type-1 dopamine receptor, permitting binding to β-arrestin and subsequent G protein–coupled receptor internalization and inactivation (32). Staessen et al reported an association of renal sodium handling and BP with genetic variation in the type-1 dopamine receptor promoter, but not with the GRK4 variant (A142V), in a family-based random sampling of a white Flemish population (34). However, the phenotypic measurements were obtained without control of dietary salt intake, perhaps confounding the findings of the study (10).
In the GenSalt study, several genetic variants were associated with sodium sensitivity in the Chinese population. For example, Kelly and colleagues reported a significant association between the rare α-adducin variant rs17833172 and systolic, diastolic, and MAP responses to high-sodium (p-values <0.0001) and diastolic BP response to low-sodium (p-value=0.002) (35•). Participants homozygous for the variant A allele of this marker had systolic, diastolic and MAP responses (95% CI) to high-sodium diet of 1.6 (−1.8, 4.9), −0.8 (−5.6, 4.0), and −0.1 (−4.0, 3.9) mmHg, respectively, vs. corresponding responses of 4.6 (2.5, 6.6), 1.7 (−0.2, 3.6), and 2.7 (0.9, 4.4) mmHg, respectively, for those who were heterozygous or homozygous for the G allele. In addition, participants with at least one copy of the A allele of SNP rs1129649 of the G protein β-polypeptide 3 (GNB3) gene had significantly decreased MAP response to low-sodium diet compared to homozygotes for the C allele (P value = 0.004) with responses of −3.4 (−3.8, −3.0) vs. −4.2 (−4.6, −3.8) mmHg, respectively. These data support a role for the ADD1 and GNB3 genes in sodium-sensitivity.
Conclusions
The GenSalt study indicated that BP responses to dietary sodium intake were normally distributed in the population and there was no evidence for a bimodal distribution. If a proportional change of MAP ≥5% was used to define sodium-sensitivity, 39% of Chinese adults would have met the criteria. Therefore, sodium-sensitivity appeared to be a common biological phenomenon in this Chinese population. Sodium-sensitivity was more common in women and in persons who were older and had higher usual BP. Sodium-sensitivity was also more common in individuals with higher responses to CPT and in individuals with metabolic syndrome. Genetic factors might play an important role in determining individuals’ BP responses to dietary sodium intake and several SNPs have been associated with sodium-sensitivity of BP in the Chinese population. A better understanding of the genetic and environmental determinants of sodium-sensitivity will help identify individuals at high risk for hypertension and who should receive a low sodium dietary intervention. In addition, identifying genes that interact significantly with dietary sodium intake on the regulation of BP will contribute significantly to knowledge about the underlying physiological pathways to hypertension.
Acknowledgments
The Genetic Epidemiology Network of Salt Sensitivity is supported by research grants (U01HL072507, R01HL087263, and R01HL090682) from the National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD. Dr. Jing Chen was supported by a grant (P20-RR017659) from the National Center for Research Resources, National Institutes of Health, Bethesda, MD.
Footnotes
Disclosure
The authors declared no conflict of interest.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Kearney PM, Whelton M, Reynolds K, et al. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Lawes CMM, Vander Hoorn S, Rodgers A, et al. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–18. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 3.He J, Gu D, Chen J, et al. Premature deaths attributable to blood pressure in China: a prospective cohort study. Lancet. 2009;374:1765–72. doi: 10.1016/S0140-6736(09)61199-5. [DOI] [PubMed] [Google Scholar]
- 4.Whelton PK, He J, Appel LJ, et al. Primary Prevention of Hypertension. Clinical and Public Health Advisory from the National High Blood Pressure Education Program. JAMA. 2002;288:1882–1888. doi: 10.1001/jama.288.15.1882. [DOI] [PubMed] [Google Scholar]
- 5.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6.Weinberger MH. Salt sensitivity of blood pressure in humans. Hypertension. 1996;27:481–490. doi: 10.1161/01.hyp.27.3.481. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto A, Uzu T, Fujii T, et al. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet. 1997;350:1734–1737. doi: 10.1016/S0140-6736(97)05189-1. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–432. doi: 10.1161/01.hyp.37.2.429. [DOI] [PubMed] [Google Scholar]
- 9.Kawasaki T, Delea CS, Bartter FC, Smith H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am J Med. 1978;64:193–198. doi: 10.1016/0002-9343(78)90045-1. [DOI] [PubMed] [Google Scholar]
- 10.Sanders PW. Dietary salt intake, salt sensitivity, and cardiovascular health. Hypertension. 2009;53:442–5. doi: 10.1161/HYPERTENSIONAHA.108.120303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beeks E, Kessels AG, Kroon AA, van der Klauw MM, de Leeuw PW. Genetic predisposition to salt-sensitivity: a systematic review. J Hypertens. 2004;22:1243–9. doi: 10.1097/01.hjh.0000125443.28861.0d. [DOI] [PubMed] [Google Scholar]
- 12.GenSalt Collaborative Research Group. GenSalt: rationale, design, methods and baseline characteristics of study participants. J Hum Hypertens. 2007;21:639–46. doi: 10.1038/sj.jhh.1002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13••.He J, Gu D, Chen J, et al. Gender difference in blood pressure responses to dietary sodium intervention in the GenSalt study. J Hypertens. 2009;27:48–54. doi: 10.1097/hjh.0b013e328316bb87. This large, well-controlled feeding study indicated that BP responses to dietary sodium intake were normally distributed in populations and there was no evidence for a bimodal distribution. Sodium-sensitivity appears to be a common biological phenomenon in human populations. BP responses to dietary sodium intake were greater in women, those aged 45 years or older, and those with a higher baseline BP level. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luft FC, Weinberger MH. Heterogeneous responses to changes in dietary salt intake: the salt-sensitivity paradigm. Am J Clin Nutr. 1997;65 (suppl):612S–7S. doi: 10.1093/ajcn/65.2.612S. [DOI] [PubMed] [Google Scholar]
- 15.Wright JT, Jr, Rahman M, Scarpa A, et al. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension. 2003;42:1087–92. doi: 10.1161/01.HYP.0000101687.89160.19. [DOI] [PubMed] [Google Scholar]
- 16.Vollmer WM, Sacks FM, Ard J, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH-Sodium trial. Ann Intern Med. 2001;135:1019–28. doi: 10.7326/0003-4819-135-12-200112180-00005. [DOI] [PubMed] [Google Scholar]
- 17.Wood DL, Sheps SG, El veback LR, et al. Cold pressor test as a predictor of hypertension. Hypertension. 1984;6:301–6. doi: 10.1161/01.hyp.6.3.301. [DOI] [PubMed] [Google Scholar]
- 18.Menkes MS, Matthews KA, Krantz DS, et al. Cardiovascular reactivity to the cold pressor test as a predictor of hypertension. Hypertension. 1989;14:524–30. doi: 10.1161/01.hyp.14.5.524. [DOI] [PubMed] [Google Scholar]
- 19.Kasagi F, Akahoshi M, Shimaoka K. Relation between cold pressor test and development of hypertension based on 28-year follow-up. Hypertension. 1995;25:71–6. doi: 10.1161/01.hyp.25.1.71. [DOI] [PubMed] [Google Scholar]
- 20.Strazzullo P, Barbato A, Vuotto P, Galletti F. Relationships between salt sensitivity of blood pressure and sympathetic nervous system activity: a short review of evidence. Clin Exper Hypertens. 2001;23:25–33. doi: 10.1081/ceh-100001194. [DOI] [PubMed] [Google Scholar]
- 21••.Chen J, Gu D, Jaquish CE, et al. Association between blood pressure responses to the cold pressor test and dietary sodium intervention in a Chinese population. Arch Intern Med. 2008;168:1740–6. doi: 10.1001/archinte.168.16.1740. This large population-based diet-feeding study identified a dose-response relationship between BP responses to the CPT and to dietary sodium interventions. This relationship was highly statistically significant and independent of other covariates. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimamoto K, Hirata A, Fukuoka M, et al. Insulin sensitivity and the effects of insulin on renal sodium handling and pressor systems in essential hypertensive patients. Hypertension. 1994;23:129–33. doi: 10.1161/01.hyp.23.1_suppl.i29. [DOI] [PubMed] [Google Scholar]
- 23.Galletti F, Strazzullo P, Ferrara I, et al. Salt sensitivity of essential hypertensive patients is related to insulin resistance. J Hypertens. 1997;15:1485–91. doi: 10.1097/00004872-199715120-00017. [DOI] [PubMed] [Google Scholar]
- 24••.Chen J, Gu D, Huang J, et al. Metabolic syndrome and salt sensitivity of blood pressure in non-diabetic people in China: a dietary intervention study. Lancet. 2009;373:829–35. doi: 10.1016/S0140-6736(09)60144-6. This large population-based diet-feeding study identified a strong, positive, and significant association between the metabolic syndrome and salt-sensitivity of BP among persons without diabetes. The salt-sensitivity of BP increased progressively with a higher number of metabolic risk factors and this association was independent of age, gender, BMI, physical inactivity, cigarette smoking, alcohol consumption, and baseline dietary intake of sodium and potassium. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saavedra JM. Studies on genes and hypertension: a daunting task. J Hypertens. 2005;23:929–32. doi: 10.1097/01.hjh.0000166829.02323.b2. [DOI] [PubMed] [Google Scholar]
- 26.Rotimi CN, Cooper RS, Cao G, et al. Maximum-likelihood generalized heritability estimate for blood pressure in Nigerian families. Hypertension. 1999;33:874–878. doi: 10.1161/01.hyp.33.3.874. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell GF, DeStefano AL, Larson MG, et al. Heritability and a genome-wide linkage scan for arterial stiffness, wave reflection, and mean arterial pressure: the Framingham Heart Study. Circulation. 2005;112:194–9. doi: 10.1161/CIRCULATIONAHA.104.530675. [DOI] [PubMed] [Google Scholar]
- 28•.Gu D, Rice T, Wang S, et al. Heritability of blood pressure responses to dietary sodium and potassium intake in a Chinese population. Hypertension. 2007;50:116–22. doi: 10.1161/HYPERTENSIONAHA.107.088310. This study reported the moderate heritabilities of blood pressure responses to dietary sodium intake in a Chinese population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwai N, Kajimoto K, Tomoike H, Takashima N. Polymorphism of CYP11B2 determines salt sensitivity in Japanese. Hypertension. 2007;49:825–31. doi: 10.1161/01.HYP.0000258796.52134.26. [DOI] [PubMed] [Google Scholar]
- 30.Eap CB, Bochud M, Elston RC, et al. CYP3A5 and ABCB1 genes influence blood pressure and response to treatment, and their effect is modified by salt. Hypertension. 2007;49:1007–14. doi: 10.1161/HYPERTENSIONAHA.106.084236. [DOI] [PubMed] [Google Scholar]
- 31.Laffer CL, Gainer JV, Waterman MR, et al. The T8590C polymorphism of CYP4A11 and 20-hydroxyeicosatetraenoic acid in essential hypertension. Hypertension. 2008;51:767–72. doi: 10.1161/HYPERTENSIONAHA.107.102921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zeng C, Villar VA, Eisner GM, et al. G protein-coupled receptor kinase 4: role in blood pressure regulation. Hypertension. 2008;51:1449–55. doi: 10.1161/HYPERTENSIONAHA.107.096487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Felder RA, Sanada H, Xu J, et al. G protein-coupled receptor kinase 4 gene variants in human essential hypertension. Proc Natl Acad Sci U S A. 2002;99:3872–7. doi: 10.1073/pnas.062694599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staessen JA, Kuznetsova T, Zhang H, et al. Blood pressure and renal sodium handling in relation to genetic variation in the DRD1 promoter and GRK4. Hypertension. 2008;51:1643–50. doi: 10.1161/HYPERTENSIONAHA.107.109611. [DOI] [PubMed] [Google Scholar]
- 35•.Kelly TN, Rice TK, Gu D, et al. Novel genetic variants in the alpha-adducin and guanine nucleotide binding protein beta-polypeptide 3 genes and salt sensitivity of blood pressure. Am J Hypertens. 2009;22:985–92. doi: 10.1038/ajh.2009.118. This study reported a significant association between genetic variants in the α-adducin (ADD1) gene and guanine nucleotide binding protein (G protein) β-polypeptide 3 (GNB3) gene and sodium-sensitivity of BP in Chinese population. [DOI] [PMC free article] [PubMed] [Google Scholar]