Abstract
Cancer is a disease whose progression is driven by a series of accumulating genetic and epigenetic changes influenced by hereditary factors and the somatic environment. These changes result in individual cells acquiring a phenotype that provides those cells with a survival advantage over surrounding normal cells. Our understanding of the processes that occur in malignant transformation is increasing, with many discoveries in cancer cell biology having been made through the study of childhood tumors. The processes involved in oncogenesis and cancer progression will be discussed in this review.
Keywords: childhood cancer, transformation, genetics, epigenetics, hereditary
Scope of childhood cancer
Cancer in children is uncommon—it represents only about 2% of all cancer cases. Nevertheless, after trauma, it is the second most common cause of death in children older than 1 year of age. Approximately 130 new cases of cancer are identified each year per million children younger than 15 years of age (about 1 in 7,000). Therefore, the annual incidence of cancer in the United States is about 9,000 children younger than 15 years age, with another 4,000 patients being diagnosed with cancer between the ages of 15 and 19 years.1 The incidence of cancer is greatest among children in their first three years of life and then slowly declines until age 9. After that, the incidence then steadily increases again through adolescence creating a second peak incidence.
Although each specific tumor type exhibits a different age-distribution pattern, overall, leukemia is the most common form of cancer in children; brain tumors are the most common solid malignancy of childhood (Table 1). Lymphomas are the next most common malignancy in children, followed by neuroblastoma, soft tissue sarcomas, Wilms tumor, germ cell tumors, osteosarcoma, and retinoblastoma, with each of these types of cancer contributing between 3% to 8% of the total number of cancer cases in children younger than 15 years. A slightly different distribution is seen among 15- to 19-year olds, in whom Hodgkin disease and germ cell tumors are the most frequently diagnosed malignancies, and in whom non-Hodgkin lymphoma, non-rhabdomyosarcoma soft-tissue sarcoma, osteosarcoma, Ewing sarcoma, thyroid cancer, and melanoma each occur with an increased incidence.
Table 1.
Distribution of Childhood Cancer Types
| Type of Cancer | Percentage |
|---|---|
| Leukemia | 30 |
| Brain Tumors | 25 |
| Lymphoma | 15 |
| Neuroblastoma | 8 |
| Sarcoma | 7 |
| Wilms Tumor | 6 |
| Osteosarcoma | 5 |
| Retinoblastoma | 3 |
| Liver Tumors | 1 |
The probability of survival for a child with cancer has improved greatly over the last half century. In the early 1960s, approximately 30% of children with cancer survived their disease. By the mid 1980s, about 65% of children with cancer were cured; that rate increased to nearly 75% by the mid 1990s.2 Currently, survival for children with cancer approaches 80%. Because of this, it is anticipated that by 2010, one in 250 adults will be a survivor of childhood cancer.3
Normal development and growth
During normal development, cells evolve to perform highly specialized functions to meet the physiologic needs of the organism. Development and growth involve tightly regulated processes that include continued cell proliferation, differentiation to specialized cell types, and programmed cell death (apoptosis). An intricate system of checks and balances ensures proper control over these physiologic processes. The genetic composition (genotype) of a cell determines which pathway(s) will be followed in exerting that control. The local milieu also plays a crucial role in influencing cell fate. Cells use complex signal transduction pathways to sense and respond to neighboring cells and their extracellular surroundings. In addition, environmental factors may have a direct impact on cell phenotype and fate by causing DNA damage that permanently alters the host genome.
Malignant transformation
Alteration or inactivation of any of the components of normal cell regulatory pathways may lead to the dysregulated growth that is the hallmark of neoplastic cells. Malignant transformation may be characterized by the failure of cells to differentiate or cellular dedifferentiation, increased invasiveness and metastatic capacity, and decreased drug sensitivity. Tumorigenesis reflects the accumulation of excess cells that results from increased cell proliferation and decreased apoptosis or senescence. Cancer cells do not replicate more rapidly than normal cells, but they show diminished responsiveness to regulatory signals. Positive growth signals are generated by proto-oncogenes, so named because their dysregulated expression or activation can promote malignant transformation. These proto-oncogenes may encode growth factors or their receptors, intracellular signaling molecules, and nuclear transcription factors (Table 2). Conversely, tumor suppressor genes, as their name implies, control or restrict cell growth and proliferation. Their inactivation, through various mechanisms, permits the dysregulated growth of cancer cells. Also important are the genes that regulate cell death. Their inactivation leads to resistance to apoptosis and allows accumulation of additional genetic aberrations.
Table 2.
Proto-Oncogenes and Tumor Suppressor Genes in Pediatric Malignancies
| Oncogene Family | Proto-oncogene | Chromosome location | Tumors |
|---|---|---|---|
| Growth Factors and Receptors | |||
| erb B2 | 17q21 | Glioblastoma | |
| trk | 9q22 | Neuroblastoma | |
| Protein Kinase | |||
| src | 7p11 | Rhabdomyosarcoma, Osteosarcoma, Ewing sarcoma | |
| Signal Transducers | |||
| H-ras | 11p15.1 | Neuroblastoma | |
| Transcription Factors | |||
| c-myc | 18q24 | Burkitt lymphoma | |
| N-myc | 2p24 | Neuroblastoma | |
| Syndrome | Tumor suppressor gene | Chromosome location | Tumors |
|---|---|---|---|
| Familial polyposis coli | APC | 5q21 | Intestinal polyposis, Colorectal cancer |
| Familial retinoblastoma | RB | 13q24 | Retinoblastoma, Osteosarcoma |
| WAGR* | WT1 | 11p13 | Wilms tumor |
| Denys-Drash† | WT1 | 11p13 | Wilms tumor |
| Beckwith-Weidemann‡ | WT2 (?) | 11p15 | Wilms tumor, Hepatoblastoma, Adrenal tumors |
| Li-Fraumeni | p53 | 17q13 | Multiple (see text) |
| Neurofibromatosis type 1 | NF1 | 17q11.2 | Sarcomas, breast cancer |
| Neurofibromatosis type 2 | NF2 | 22q12 | Neurofibroma, Neurofibrosarcoma |
| Brain tumor | |||
| Von Hippel-Lindau | VHL | 3p25-26 | Renal cell cancer, Pheochromocytoma |
| Retinal angioma, Hemangioblastoma |
WAGR: Wilms tumor, aniridia, genitourinary abnormalities, and mental retardation
Denys-Drash: Wilms tumor, pseudohermaphroditism, mesangeal sclerosis, renal failure
Beckwith-Weidemann: multiple tumors, hemihypertrophy, macroglossia, hyperinsulinism
Reprinted with permission from Davidoff AM. Principles of pediatric oncology/Genetics of cancer. In: Grosfeld JL, O'Neill JA, Fonkalsrud EW, Coran AG (Eds.) Pediatric Surgery, 6th Edition, Elsevier Science, 2006
Cancer cells carry DNA that has point mutations; viral insertions; or chromosomal or gene amplifications, deletions, or rearrangements. Each of these aberrations can alter the context and process of normal cellular growth and differentiation. Although genomic instability is an inherent property of the evolutionary process and normal development, it is through genomic instability that the malignant transformation of a cell may arise. This inherent instability may be accelerated by inherited factors or exposure to destabilizing elements in the environment. Point mutations may terminate protein translation, alter protein function, or change the regulatory target sequences that control gene expression. Chromosomal alterations create new genetic contexts within the genome and lead to the formation of novel proteins or to the dysregulation of genes displaced by aberrant events.
Genetic abnormalities associated with cancer may be detected in every cell in the body or only in the tumor cells. Constitutional or germline abnormalities are either inherited or occur de novo in the germ cells (sperm or oocyte). Interestingly, despite the presence of a constitutional genetic abnormality that might affect growth regulatory pathways in all cells, people are generally predisposed to only certain tumor types. This selectivity highlights the observation that gene function contributes to growth or development only within a particular milieu or physiologic context. Specific tumors occur earlier and are more often bilateral (in paired organs) when they result from germline mutations than when they result from sporadic or somatic alterations.
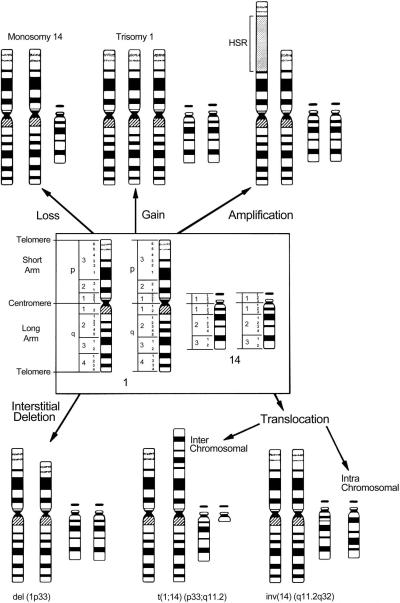
Much more common, however, are somatically acquired chromosomal aberrations, which are confined to the malignant cells. These aberrations affect growth factors and their receptors, signal transducers, and transcription factors. The general types of chromosomal alterations associated with malignant transformation are shown in Figure 1. Although a low level of chromosomal instability exists in a normal population of cells, neoplastic transformation occurs only if these alterations affect a growth-regulating pathway and confer a growth advantage.
Figure 1.
The spectrum of gross chromosomal aberrations with chromosomes 1 and 14 as examples. Reprinted with permission from Look AT, Kirsch IR: Molecular basis of childhood cancer. In: Pizzo PA, Poplack DG (Eds.) Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott-Raven Publishers, 1997, p 38
DNA content alterations
Normal human cells contain two copies of each of 23 chromosomes; a normal “diploid” cell therefore has 46 chromosomes. The “DNA index” is defined as the ratio of chromosomes in a cell to that in a normal cell, i.e., 46, as generally determined by flow cytometric analysis. Diploid cells, therefore, have a DNA index of 1.0, whereas near-triploid or “hyperdiploid” cells have a DNA index ranging from 1.26 to 1.76. The majority (55%) of primary neuroblastoma cells are triploid or near triploid, for example, having between 58 and 80 chromosomes; the remainder are near diploid (35–57 chromosomes) or near tetraploid (81–103 chromosomes).4 Importantly, patients with near-triploid tumors typically have favorable clinical and biologic prognostic factors and excellent survival rates compared with those who have near-diploid or near-tetraploid tumors.5
Chromosomal translocations
Many pediatric cancers, specifically hematologic malignancies and soft-tissue neoplasms, have recurrent, nonrandom abnormalities in chromosomal structure, typically chromosomal translocations (Table 3). The most common result of a nonrandom translocation is the fusion of two distinct genes from different chromosomes. The genes are typically fused within the reading frame and express a functional, chimeric protein product that has transcription factor or protein kinase activity. These fusion proteins contribute to tumorigenesis by activating genes or proteins involved in cell proliferation. For example, in Ewing sarcoma the consequence of the t(11;22)(q24;q12) translocation is a fusion of EWS, a transcription factor gene on chromosome 22, and FLI-1, a gene encoding a member of the ETS family of transcription factors on chromosome 11.6 The resultant chimeric protein, which contains the DNA-binding region of FLI-1 and the transcription activation region of EWS, has greater transcriptional activity than does EWS alone.7 The EWS:FLI-1 fusion transcript is detectable in approximately 85% of Ewing sarcomas. At least four other EWS fusions have been identified in Ewing sarcoma; fusion of EWS with ERG (another ETS family member) accounts for an additional 5% of cases.8 Alveolar rhabdomyosarcomas have characteristic translocations between the long arm of chromosome 2 (75% of cases) or the short arm of chromosome 1 (10% of cases) and the long arm of chromosome 13. These translocations result in the fusion of PAX3 (at 2q35) or PAX7 (at 1p36) with FKHR, a gene encoding a member of the forkhead family of transcription factors.9 The EWS:FLI-1 and PAX7:FKHR fusions appear to confer a better prognosis for patients with Ewing sarcoma and metastatic alveolar rhabdomyosarcoma, respectively.10,11
Table 3.
Common, recurrent translocations in soft tissue tumors
| Tumor | Genetic abnormality | Fusion transcript |
|---|---|---|
| Ewing sarcoma/Primitive neuroectodermal tumor | t(11;22)(q24;q12) t(21;22)(q22;q12) t(7;22)(p22;q12) t(17;22)(q12;q12) t(2;22)(q33;q12) |
FLI1-EWS
ERG-EWS ETV1-EWS E1AF-EWS FEV-EWS |
| Desmoplastic small round cell tumor | t(11;22)(p13;q12) t(11;22)(q24;q12) |
WT1-EWS
FLI1-EWS |
| Synovial sarcoma | t(X;18)(p11.23;q11) t(X;18)(p11.21;q11) |
SSX1-SYT
SSX2-SYT |
| Alveolar rhabdomyosarcoma | t(2;13)(q35;q14) t(1;13)(p36;q14) |
PAX3-FKHR
PAX7-FKHR |
| Malignant melanoma of soft part (clear cell sarcoma) | t(12;22)(q13;q12) | ATF1-EWS |
| Myxoid liposarcoma | t(12;16)(q13;p11) t(12;22)(q13;q12) |
CHOP-TLS(FUS)
CHOP-EWS |
| Extraskeletal myxoid chondrosarcoma | t(9;22)(q22;q12) | CHN-EWS |
| Dermatofibrosarcoma protuberans and Giant cell fibroblastoma | t(17;22)(q22;q13) | COL1A1-PDGFB |
| Congenital fibrosarcoma and Mesoblastic nephroma | t(12;15)(p13;q25) | ETV6-NTRK3 |
| Lipoblastoma | t(3;8)(q12;q11.2) t(7;8)(q31;q13) |
? ? |
Reprinted with permission from Davidoff, AM, Hill, DA. Molecular genetic aspects of solid tumors in childhood. Semin Pediatr Surg. 10: 106–118, 2001.
Translocations that generate chimeric proteins with increased transcriptional activity also characterize desmoplastic small round cell tumor, myxoid liposarcoma, extraskeletal myxoid chrondrosarcoma, malignant melanoma of soft parts, synovial sarcoma, congenital fibrosarcoma, cellular mesoblastic nephroma, and dermatofibrosarcoma protuberans.12
Proto-oncogene activation
Proto-oncogenes are commonly activated in transformed cells by point mutations or gene amplification. The classic example of proto-oncogene activation by a point mutation involves the cellular proto-oncogene RAS. RAS-family proteins are associated with the inner, cytoplasmic surface of the plasma membrane and function as intermediates in signal transduction pathways that regulate cell proliferation. Point mutations in RAS result in constitutive activation of the RAS protein and, therefore, the continuous activation of the RAS signal transduction pathway. Activation of RAS appears to be involved in the pathogenesis of a small percentage of pediatric malignancies, including leukemia and a variety of solid tumors.13
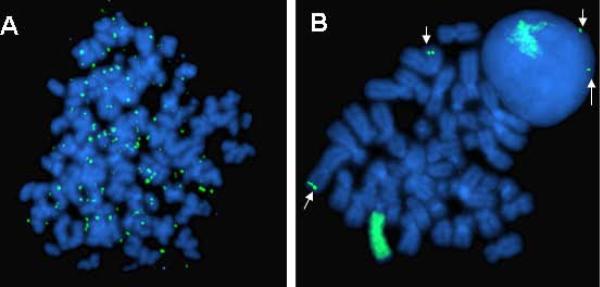
Gene amplification (i.e., selective replication of DNA sequences) enables a tumor cell to increase expression of crucial genes whose products are ordinarily tightly controlled. The amplified DNA sequences, or amplicons, may be maintained episomally (i.e., extrachromosomally) as double minutes—paired chromatin bodies lacking a centromere—or as intrachromosomal, homogeneously staining regions. In about one third of neuroblastomas, for example, the transcription factor and proto-oncogene MYCN is amplified (Figure 2). MYCN encodes a 64-kDa nuclear phosphoprotein (MycN) that forms a transcriptional complex by associating with other nuclear proteins expressed in the developing nervous system and other tissues.14 Increased expression of MycN increases the rates of DNA synthesis and cell proliferation and shortens the G1 phase of the cell cycle.15 The MYCN copy number in neuroblastoma cells can be amplified 5- and 500-fold and is usually consistent among primary and metastatic sites, and at different times during tumor evolution and treatment.16 This consistency suggests that MYCN amplification is an early event in the pathogenesis of neuroblastoma. Because, gene amplification is usually associated with advanced stages of disease, rapid tumor progression, and poor outcome, it is a powerful prognostic indicator.17
Figure 2. FISH analysis of neuroblastoma.
Shown are a tumor with (A) DMs and (B) HSRs in both metaphase chromosomes and an adjacent intact interphase nucleus. Also shown are the normal two copies of the MYCN gene (arrows). (Courtesy of Marc Valentine, St. Jude Children's Research Hospital, Memphis, TN). Reprinted with permission from Davidoff AM: Neuroblastoma. In: Holcomb GW, Murphy JP, Ostlie DJ (Eds.) Pediatric Surgery, 5th edition, Elsevier, In press, 2009.
Inactivation of tumor suppressor genes
Tumor suppressor genes, or anti-oncogenes, provide negative control of cell proliferation. Loss of function of the proteins encoded by these genes, through deletion or mutational inactivation of the gene, liberates the cell from growth constraints and contributes to malignant transformation. The cumulative effect of genetic lesions that activate proto-oncogenes or inactivate tumor suppressor genes is a breakdown in the balance between cell proliferation and cell loss due to differentiation or apoptosis. Such imbalance results in clonal overgrowth of a specific cell lineage. The first tumor suppressor gene to be recognized was the retinoblastoma susceptibility gene, RB. The nuclear phosphoprotein, p53, has also become recognized as an important tumor suppressor gene, perhaps the most commonly altered gene in all human cancers. The p53 gene is frequently inactivated in solid tumors of childhood, including osteosarcoma, rhabdomyosarcoma, brain tumors, anaplastic Wilms tumor, and a subset of chemotherapy-resistant neuroblastoma.18 In addition, heritable cancer-associated changes in the p53 tumor suppressor gene occur in families with Li-Fraumeni syndrome, an autosomal dominant predisposition for rhabdomyosarcoma, other soft tissue and bone sarcomas, premenopausal breast cancer, brain tumors, and adrenocortical carcinomas.19 Other tumor suppressor genes inactivated in pediatric malignancies include Wilms tumor 1 (WT1), neurofibromatosis 1 (NF1), and von Hippel-Lindau (VHL).
Other tumor suppressor genes are presumed to exist but have not been definitively identified. For example, early karyotype analyses of neuroblastoma-derived cell lines found frequent deletion of the short arm of chromosome 1.20 Subsequently it was found that approximately 20% to 35% of primary neuroblastomas exhibit 1p deletion, as determined by fluorescent in situ hybridization (FISH), and the smallest common region of loss is located within region 1p36.21 Deletion of genetic material in tumors suggests the presence (and subsequent loss) of a tumor suppressor gene, but a specific tumor suppressor gene has yet to be identified on chromosome 1p, although CHD5 has recently emerged as a strong candidate in neuroblastoma. In a number of neuroblastoma cell lines with 1p deletion, expression of the remaining allele appears to be suppressed by promoter methylation.22 Loss of heterozygosity (LOH – loss of one of two normally paired chromosomal regions) is also commonly found at chromosome 11q23, occurring in about 17% of neuroblastoma cases (excluding those in which there is whole loss of chromosome 11).23 Because of the favorable outcome for patients with low or intermediate risk neuroblastoma, dose reduction for these patients is planned. However, recent data have suggested that LOH at chromosome 1p or 11q (unbalanced), is independently associated with decreased progression-free survival in patients with low-and intermediate-risk disease.23 Therefore, these patients will not be eligible for dose-reduction.
Chromosomal deletion also occurs commonly in Wilms tumor. Deletion of 1p occurs in approximately 11% of cases while 16q LOH occurs in about 20% of cases.24 The recently concluded NWTS-5 trial, a single-arm therapeutic trial designed to evaluate the prognostic value of certain biologic markers in Wilms tumor, demonstrated that LOH for genetic material on chromosome 1p and 16q in stage I and II favorable histology Wilms tumor was associated with a poorer prognosis.25 This information, loss of heterozygosity of 1p and 16q, is now being used to further stratify patients in the current Children's Oncology Group (COG) trial for Wilms tumor. Thus, in addition to improving our understanding of the pathogenesis of cancer and the identification of tumor suppressor genes, the study of these chromosomal abnormalities has also led to the generation of prognostic information that has been incorporated into current risk stratification schemas for neuroblastoma and Wilms tumor.
Epigenetic alterations
As stated previously, the hallmark of cancer is dysregulated gene expression. However, not only do genetic factors influence gene expression but epigenetic factors do as well, with these factors being at least as important as genetic changes in their contribution to the pathogenesis of cancer. Epigenetic alterations are defined as those heritable changes in gene expression that do not result from direct changes in DNA sequence. Mechanisms of epigenetic regulation most commonly include DNA methylation and modification of histones, although the contribution of microRNAs (miRNA), a class of noncoding RNAs, is becoming increasingly recognized.
DNA methylation
DNA methylation is a reversible process that involves methylation of the fifth position of cytosine within CpG dinucleotides present in DNA. These dinucleotides are usually in the promoter regions of genes; methylation of these sites typically causes gene silencing, thereby preventing expression of the encoded proteins. This process is part of the normal mechanism for imprinting, X-chromosome inactivation and generally keeping large areas of genomic DNA silent, but may also contribute to the pathogenesis of cancer by silencing tumor suppressor genes. However, both abnormal hypo- and hypermethylation states exist in human tumors, resulting in both dysregulated expression and silencing, respectively, of affected genes. These modifications of the nucleotide backbone of human DNA are becoming increasingly recognized in human cancer both for their frequency and importance. For example, promoter methylation resulting in silencing of caspase 8, a protein involved in apoptosis, likely contributes to the pathogenesis of MYCN-amplified neuroblastoma,26 as well as Ewing sarcoma.27
Histone modification
Histones are the proteins that give structure to DNA, and together with the DNA form the major components of chromatin. The functions of histones are to package DNA into a smaller volume to fit in the cell, to strengthen the DNA to allow replication, and to serve as a mechanism to control gene expression. Alterations in histones can mediate changes in chromatin structure. The compacted form of DNA, termed heterochromatin, is largely inaccessible to transcription factors and, therefore, genes in the affected regions are silent. Other modifications of histones can cause DNA to take a more open or extended configuration (euchromatin), allowing for gene transcription. The N-terminal tails of histones can be modified by a number of different processes including methylation and acetylation, mediated by histone acetyl transferases (HAT) and deacetylases (HDAC), and histone methyltransferases (HMT). Each of these processes alters histone function, which, in turn alters the structure of chromatin and, therefore, the accessibility of DNA to transcription factors. Methylation of the DNA itself can also effect changes in chromatin structure.
MicroRNA
As stated above, miRNAs are a group of small, regulatory noncoding RNAs that appear to function in gene regulation. These miRNAs are single–stranded RNA fragments of 21–23 nucleotides that are complementary to encoding mRNAs.28 Their function is to down-regulate expression of target mRNAs; it is estimated that miRNAs regulate the expression of about 30% of all human genes.29 These miRNAs regulate gene expression primarily by incorporating into silencing machinery called RNA-induced silencing complexes (RISC). MiRNAs are involved in a number of fundamental biologic processes, including development, differentiation, cell cycle regulation and senescence. However, broad analyses of miRNA expression levels has demonstrated that many miRNAs are dysregulated in a variety of different cancer types, including neuroblastoma and other pediatric tumors,30 frequently losing their function as gene silencers/tumor suppressors. The activity of miRNAs, like gene expression, is also under epigenetic regulation.
Heredity and childhood cancer
Advances in molecular genetic techniques have improved our understanding of cancer predisposition syndromes. Constitutional genetic abnormalities present in all cells of the body that are hereditary (i.e., passed from parent to child) or nonhereditary (i.e., de novo mutations in the sperm or oocyte before fertilization) contribute to an estimated 10% to 15% of pediatric cancers.31 Constitutional chromosomal abnormalities are the result of an abnormal number or structural rearrangement of the normal 46 chromosomes and may be associated with a predisposition to cancer. Examples of chromosomal abnormalities resulting in a predisposition to certain types of childhood cancers include the predisposition to leukemia seen with trisomy 21 (Down syndrome) and to germ cell tumors with Klinefelter syndrome (47XXY).
Structural chromosomal abnormalities include interstitial deletions resulting in the constitutional loss of one or more genes. Wilms tumors may be sporadic, familial, or associated with specific genetic disorders or recognizable syndromes. A better understanding of the molecular basis of Wilms tumor has been achieved largely through the study of the latter two types of tumors. The WAGR syndrome (Wilms tumor, aniridia, genitourinary abnormalities, and mental retardation) provides an easily recognizable phenotype for grouping children likely to have a common genetic abnormality. Constitutional deletions from chromosome 11p13 are consistent in children with WAGR syndrome and also occur in approximately 35% of children with sporadic Wilms tumor.32 A study of a large series of patients identified the gene deleted from chromosome 11p13 as WT1.33 This gene encodes a nuclear transcription factor that is essential for normal kidney and gonadal development34 and appears to act as a tumor suppressor, but its precise role is unclear at this time. Aniridia in patients with WAGR syndrome is thought to occur after the loss of one copy of the PAX6 gene located close to WT1 on chromosome 11.35 Denys-Drash syndrome, which is characterized by a very high risk of Wilms tumor, pseudohermaphroditism, and mesangeal sclerosis leading to early renal failure, is associated with germline mutations in the DNA-binding domain of WT1.36 The mutated WT1 protein appears to function by a dominant negative effect. Only 6% to 18% of sporadic Wilms tumors have WT1 mutations.37
In another subset of patients with Wilms tumor, there is loss of genetic material in a region distal to the WT1 locus toward the telomeric end of chromosome 11 (11p15).38 It has therefore been suggested that there is a second Wilms tumor susceptibility gene, tentatively named WT2, in 11p15. Loss of heterozygosity at this locus has also been described in patients with Beckwith-Wiedemann syndrome (BWS), a congenital overgrowth syndrome characterized by numerous growth abnormalities as well as predisposition to a variety of malignancies, including Wilms tumor.39
Neurofibromatosis type 1 (NF1) is one of the most common genetic disorders. The NF1 protein normally inhibits the proto-oncogene RAS, but in patients with NF1, mutation of one copy of the gene combined with deletion of the other permits uncontrolled RAS pathway activation. These patients are then susceptible to myelogenous disorders, benign tumors, gliomas, and malignant peripheral nerve sheath tumors. An inherited predisposition to pediatric cancers is also associated with Li-Fraumeni syndrome (which results from inactivating mutations of the p53 gene and in which patients are at risk of osteosarcoma, rhabdomyosarcoma, adrenocortical carcinoma, and brain tumors, among other tumors), familial retinoblastoma (which results from inactivating mutations of the RB gene and in which patients are at risk of osteosarcoma as well as retinoblastoma), familial adenomatous polyposis, and multiple endocrine neoplasia syndromes. Understanding these complex syndromes and their pathogenesis is important in efforts to screen for early detection and, possibly, for prophylactic therapy.
Recently, the germline mutation associated with hereditary neuroblastoma has been identified – activating mutations in the tyrosine kinase domain of the anaplastic lymphoma kinase (ALK) oncogene on the long arm of chromosome 2 (2p23).40 Further molecular studies have revealed that common genetic variation at chromosome bands 6p2241 and 2q3542 are associated with susceptibility to, and likely contribute to the etiology of, high risk neuroblastoma, providing the first evidence that childhood cancers also arise owing to complex interactions of polymorphic variants. Finally, the same group has also shown that inherited copy number variation at chromosome 1q21.1 is associated with neuroblastoma, implicating a neuroblastoma breakpoint family gene in early neuroblastoma genesis.43
Conclusion
Advances in basic cancer research in the past three decades have led to an increased understanding of the genetic and epigenetic events in the pathogenesis and progression of human malignancies, including those of childhood. A number of pediatric malignancies serve as models for the investigative approach to cancer, highlighting the utility of molecular analysis for a variety of purposes. Demonstration of tumor-specific translocations by cytogenetics, FISH, and RT-PCR confirms histopathologic diagnoses. Detection of chromosomal abnormalities, gene overexpression, and gene amplification is used in risk stratification and treatment planning. Elucidation of pathways involving tumor suppressor genes has increased our understanding of syndromes associated with cancer and has led to genetic screening and counseling. Treatments are tailored such that patients with biologically high-risk tumors receive intensified regimens to achieve a cure, whereas patients with biologically low-risk tumors are still nearly uniformly cured while also benefiting from the lower toxicity of less intensive therapy. Elucidation of the complex molecular pathways involved in tumorigenesis will likely further lead to the production of targeted anticancer agents with high specificity, efficacy, and therapeutic index. In the near future, translation of the molecular profile of a specific tumor will form the basis of a unique therapeutic approach. In addition, other approaches to cancer therapy including angiogenesis inhibition, immunotherapy and radionuclide therapy will increasingly be integrated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- (1).Young JL, Jr., Ries LG, Silverberg E, et al. Cancer incidence, survival, and mortality for children younger than age 15 years. Cancer. 1986;58(2 Suppl):598–602. doi: 10.1002/1097-0142(19860715)58:2+<598::aid-cncr2820581332>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- (2).Ries LG. Childhood Cancer Mortality. In: Ries LG, Smith MA, Gurney JG, Linet M, Tamura T, Young JL Jr., et al., editors. Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program; Bethesda, MD: 1999. pp. 165–9. [Google Scholar]
- (3).Bleyer WA. The impact of childhood cancer on the United States and the world. CA Cancer J Clin. 1990;40(6):355–67. doi: 10.3322/canjclin.40.6.355. [DOI] [PubMed] [Google Scholar]
- (4).Kaneko Y, Kanda N, Maseki N, et al. Different karyotypic patterns in early and advanced stage neuroblastomas. Cancer Res. 1987;47(1):311–8. [PubMed] [Google Scholar]
- (5).Look AT, Hayes FA, Nitschke R, et al. Cellular DNA content as a predictor of response to chemotherapy in infants with unresectable neuroblastoma. N Engl J Med. 1984;311(4):231–5. doi: 10.1056/NEJM198407263110405. [DOI] [PubMed] [Google Scholar]
- (6).May WA, Gishizky ML, Lessnick SL, et al. Ewing sarcoma 11;22 translocation produces a chimeric transcription factor that requires the DNA-binding domain encoded by FLI1 for transformation. Proc Natl Acad Sci U S A. 1993;90(12):5752–6. doi: 10.1073/pnas.90.12.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).May WA, Lessnick SL, Braun BS, et al. The Ewing's sarcoma EWS/FLI-1 fusion gene encodes a more potent transcriptional activator and is a more powerful transforming gene than FLI-1. Mol Cell Biol. 1993;13(12):7393–8. doi: 10.1128/mcb.13.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Sorensen PH, Lessnick SL, Lopez-Terrada D, et al. A second Ewing's sarcoma translocation, t(21;22), fuses the EWS gene to another ETS-family transcription factor, ERG. Nat Genet. 1994;6(2):146–51. doi: 10.1038/ng0294-146. [DOI] [PubMed] [Google Scholar]
- (9).Galili N, Davis RJ, Fredericks WJ, et al. Fusion of a fork head domain gene to PAX3 in the solid tumour alveolar rhabdomyosarcoma. Nat Genet. 1993;5(3):230–5. doi: 10.1038/ng1193-230. [DOI] [PubMed] [Google Scholar]
- (10).Barr FG. The role of chimeric paired box transcription factors in the pathogenesis of pediatric rhabdomysarcoma. Cancer Res. 1999;59(7 Suppl):1711s–5s. [PubMed] [Google Scholar]
- (11).Sorensen PH, Lynch JC, Qualman SJ, et al. PAX3-FKHR and PAX7-FKHR gene fusions are prognostic indicators in alveolar rhabdomyosarcoma: a report from the children's oncology group. J Clin Oncol. 2002;20(11):2672–9. doi: 10.1200/JCO.2002.03.137. [DOI] [PubMed] [Google Scholar]
- (12).Davidoff AM, Hill DA. Molecular genetic aspects of solid tumors in childhood. Semin Pediatr Surg. 2001;10(3):106–18. doi: 10.1053/spsu.2001.24700. [DOI] [PubMed] [Google Scholar]
- (13).Chen Y, Takita J, Hiwatari M, et al. Mutations of the PTPN11 and RAS genes in rhabdomyosarcoma and pediatric hematological malignancies. Genes Chromosomes Cancer. 2006;45(6):583–91. doi: 10.1002/gcc.20322. [DOI] [PubMed] [Google Scholar]
- (14).Kohl NE, Kanda N, Schreck RR, et al. Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 1983;35(2 Pt 1):359–67. doi: 10.1016/0092-8674(83)90169-1. [DOI] [PubMed] [Google Scholar]
- (15).Lutz W, Stohr M, Schurmann J, et al. Conditional expression of N-myc in human neuroblastoma cells increases expression of alpha-prothymosin and ornithine decarboxylase and accelerates progression into S-phase early after mitogenic stimulation of quiescent cells. Oncogene. 1996;13(4):803–12. [PubMed] [Google Scholar]
- (16).Brodeur GM, Hayes FA, Green AA, et al. Consistent N-myc copy number in simultaneous or consecutive neuroblastoma samples from sixty individual patients. Cancer Res. 1987;47(16):4248–53. [PubMed] [Google Scholar]
- (17).Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313(18):1111–6. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- (18).Kusafuka T, Fukuzawa M, Oue T, et al. Mutation analysis of p53 gene in childhood malignant solid tumors. J Pediatr Surg. 1997;32(8):1175–80. doi: 10.1016/s0022-3468(97)90677-1. [DOI] [PubMed] [Google Scholar]
- (19).Malkin D, Li FP, Strong LC, et al. Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science. 1990;250(4985):1233–8. doi: 10.1126/science.1978757. [DOI] [PubMed] [Google Scholar]
- (20).Brodeur GM, Sekhon G, Goldstein MN. Chromosomal aberrations in human neuroblastomas. Cancer. 1977;40(5):2256–63. doi: 10.1002/1097-0142(197711)40:5<2256::aid-cncr2820400536>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- (21).Fong CT, Dracopoli NC, White PS, et al. Loss of heterozygosity for the short arm of chromosome 1 in human neuroblastomas: correlation with N-myc amplification. Proc Natl Acad Sci U S A. 1989;86(10):3753–7. doi: 10.1073/pnas.86.10.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Fujita T, Igarashi J, Okawa ER, et al. CHD5, a tumor suppressor gene deleted from 1p36.31 in neuroblastomas. J Natl Cancer Inst. 2008;100(13):940–9. doi: 10.1093/jnci/djn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353(21):2243–53. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- (24).Grundy P, Coppes MJ, Haber D. Molecular genetics of Wilms tumor. Hematol Oncol Clin North Am. 1995;9(6):1201–15. [PubMed] [Google Scholar]
- (25).Grundy PE, Breslow NE, Li S, et al. Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol. 2005;23(29):7312–21. doi: 10.1200/JCO.2005.01.2799. [DOI] [PubMed] [Google Scholar]
- (26).Teitz T, Wei T, Valentine MB, et al. Caspase 8 is deleted or silenced preferentially in childhood neuroblastomas with amplification of MYCN. Nat Med. 2000;6(5):529–35. doi: 10.1038/75007. [DOI] [PubMed] [Google Scholar]
- (27).Fulda S, Kufer M, Meyer E, et al. Sensitization for death receptor- or drug-induced apoptosis by re-expression of caspase-8 through demethylation or gene transfer. Oncogene. 2001;20(41):5865–77. doi: 10.1038/sj.onc.1204750. [DOI] [PubMed] [Google Scholar]
- (28).Ruvkun G. Molecular biology. Glimpses of a tiny RNA world. Science. 2001;294(5543):797–9. doi: 10.1126/science.1066315. [DOI] [PubMed] [Google Scholar]
- (29).Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120(1):15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- (30).Wei JS, Johansson P, Chen QR, et al. microRNA profiling identifies cancer-specific and prognostic signatures in pediatric malignancies. Clin Cancer Res. 2009;15(17):5560–8. doi: 10.1158/1078-0432.CCR-08-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Quesnel S, Malkin D. Genetic predisposition to cancer and familial cancer syndromes. Pediatr Clin North Am. 1997;44(4):791–808. doi: 10.1016/s0031-3955(05)70530-7. [DOI] [PubMed] [Google Scholar]
- (32).Coppes MJ, Haber DA, Grundy PE. Genetic events in the development of Wilms' tumor. N Engl J Med. 1994;331(9):586–90. doi: 10.1056/NEJM199409013310906. [DOI] [PubMed] [Google Scholar]
- (33).Call KM, Glaser T, Ito CY, et al. Isolation and characterization of a zinc finger polypeptide gene at the human chromosome 11 Wilms' tumor locus. Cell. 1990;60(3):509–20. doi: 10.1016/0092-8674(90)90601-a. [DOI] [PubMed] [Google Scholar]
- (34).Pritchard-Jones K, Fleming S, Davidson D, et al. The candidate Wilms' tumour gene is involved in genitourinary development. Nature. 1990;346(6280):194–7. doi: 10.1038/346194a0. [DOI] [PubMed] [Google Scholar]
- (35).Ton CC, Hirvonen H, Miwa H, et al. Positional cloning and characterization of a paired box- and homeobox-containing gene from the aniridia region. Cell. 1991;67(6):1059–74. doi: 10.1016/0092-8674(91)90284-6. [DOI] [PubMed] [Google Scholar]
- (36).Pelletier J, Bruening W, Kashtan CE, et al. Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell. 1991;67(2):437–47. doi: 10.1016/0092-8674(91)90194-4. [DOI] [PubMed] [Google Scholar]
- (37).Varanasi R, Bardeesy N, Ghahremani M, et al. Fine structure analysis of the WT1 gene in sporadic Wilms tumors. Proc Natl Acad Sci U S A. 1994;91(9):3554–8. doi: 10.1073/pnas.91.9.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Coppes MJ, Bonetta L, Huang A, et al. Loss of heterozygosity mapping in Wilms tumor indicates the involvement of three distinct regions and a limited role for nondisjunction or mitotic recombination. Genes Chromosomes Cancer. 1992;5(4):326–34. doi: 10.1002/gcc.2870050408. [DOI] [PubMed] [Google Scholar]
- (39).Koufos A, Grundy P, Morgan K, et al. Familial Wiedemann-Beckwith syndrome and a second Wilms tumor locus both map to 11p15.5. Am J Hum Genet. 1989;44(5):711–9. [PMC free article] [PubMed] [Google Scholar]
- (40).Mosse YP, Laudenslager M, Longo L, et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature. 2008;455(7215):930–5. doi: 10.1038/nature07261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Maris JM, Mosse YP, Bradfield JP, et al. Chromosome 6p22 locus associated with clinically aggressive neuroblastoma. N Engl J Med. 2008;358(24):2585–93. doi: 10.1056/NEJMoa0708698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Capasso M, Devoto M, Hou C, et al. Common variations in BARD1 influence susceptibility to high-risk neuroblastoma. Nat Genet. 2009;41(6):718–23. doi: 10.1038/ng.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Diskin SJ, Hou C, Glessner JT, et al. Copy number variation at 1q21.1 associated with neuroblastoma. Nature. 2009;459(7249):987–91. doi: 10.1038/nature08035. [DOI] [PMC free article] [PubMed] [Google Scholar]