Abstract
We report a full account of our work towards the total synthesis of (−)-terpestacin (1), a sesterterpene originally isolated from fungal strain Arthrinium sp. FA1744. Its promising anti-HIV/anti-cancer activity as well as its novel structure make terpestacin an attractive synthetic target. A strategy based on the unique reactivity of cyclic 1,2-diketones (diosphenol) was developed, and total synthesis of 1 was achieved in 20 steps in the longest linear sequence from commercially available 3-methyl-1,2-cyclopentanedione (19). The key feature of our synthesis is represented by double usage of a “Pd AAA-Claisen” protocol, first in the early stage to generate the C1 quaternary center and second in the late stage to install the side chain. In addition, a rather unusual ene-1,2-dione moiety was synthesized and utilized as an excellent Michael acceptor to attach the C15 substituent. Several possible routes towards the total synthesis have been examined and carefully evaluated. During our exploration, many interesting chemoselectivity issues have also been addressed, such as a highly selective ring-closing metathesis (RCM) and a challenging oxidation of a disubstituted olefin in the presence of three trisubstiuted ones.
Keywords: terpene, asymmetric catalysis, alkylation, Claisen Rearrangement, total synthesi
Introduction
Isolation and Biology
In 2007, the World Health Organization estimated that 30.6–36.1 million people worldwide were living with HIV, and 2.1 million people had died of AIDS that year.[1] Over the past two decades, significant effort has been made in identifying HIV pathological targets and exploring new drug candidates for chemotherapy. One important target for finding treatment of HIV infection is syncytium formation, which constitutes a major cause for the death of human T4 cells.[2] Throughout the screening for syncytium formation inhibitors, in 1993, an attractive natural product named terpestacin (1) was ultimately found from fungal strain Arthrinium sp. FA1744 by a collaboration between Oka and Bristol-Myers Squibb (BMS).[3] Terpestacin was shown to effectively inhibit the formation of syncytia (giant-multinucleated cells that are caused by expression of gp120 on cell surfaces during HIV infection[3a]), and its IC50 value is as low as 0.46 µg/ml, suggesting that it could be a promising drug lead for anti-HIV chemotherapeutics.[3a] Recently, terpestacin has also been isolated from other fungal sources such as Ulocladium[4] and Bipolaris sorokiniana.[5]
A recent oncological study shows that terpestacin is also able to inhibit angiogenesis without affecting endothelial cell viability both in vitro and in vivo, and it inhibits extracellular signal-regulated kinase (ERK) activity in the cells.[6] This result implies that terpestacin could potentially be employed for the treatment of cancer.
Structure
Besides these stimulating biological properties, the structure of terpestacin has also been attractive to the synthetic community. Terpestacin contains a trans-fused [3.0.13] bicyclic skeleton, including a 15-membered macrocycle with three geometrically defined trisubstituted olefins. It also contains a less common diosphenol functionality (cyclic 1,2-diketone with one ketone existing as an enol) within a heavily substituted 5-membered ring. Interestingly, the structure of terpestacin contains a 4+4+4 combination, which includes four oxygen atoms, four carbon-carbon double bonds, as well as four stereogenic centers with one of them being quaternary. All of these features have posed significant challenges for the total synthesis of terpestacin.
Previous Efforts
Given its promising anti-HIV and anti-cancer activities along with its novel architecture, several elegant total syntheses of terpestacin have been reported to date. In 1998, Tatsuta et al. described the first racemic synthesis of 1[7] and later that year, they also reported the first enantioselective synthesis starting from tri-O-acetyl-d-galactal 3 and using a selective Horner–Wadsworth–Emmons (HWE) reaction to close the macrocycle (Scheme 1).[8] The Myers group in 2002 completed the second enantioselective synthesis of terpestacin as well as a closely related natural product fusaproliferin (2).[9] They initiated their synthesis with pseudoephedrine derived chiral amide 5 and constructed the macrocycle via a stereoselective alkylation at the C15 position. Moreover, this synthesis unambiguously established the absolute configuration of this natural product. In 2003, Jamison reported the third enantioselective synthesis starting from chiral dihydrofuran 7, in which they discovered that siccanol is not 11-epi-terpestacin, but terpestacin itself. Jamison’s synthesis features a highly selective Ni-catalyzed reductive coupling to afford the chiral allylic alcohol motif at the C11 and a subsequent alkylation at the C1 position to provide the desired macrocycle.[10] Very recently, another racemic synthesis of 1 was completed by the Tius group, which employed an allene ether Nazarov reaction as a key step to construct the 5-membered ring core and a HWE reaction to close the macrocycle.[11] In this article, we describe a full account of our work towards the enantioselective total synthesis of (−)-terpestacin, including a journey for the development of a unique strategy to stereoselectively and programmatically alkylate 3-substituted-cyclopenta-1,2-diketones (Scheme 2).[12]
Scheme 1.
Previous enantioselective total syntheses of terpestacin (1)
Scheme 2.
Programmatic alkylation of cyclic 1,2-diketones
Diosphenols
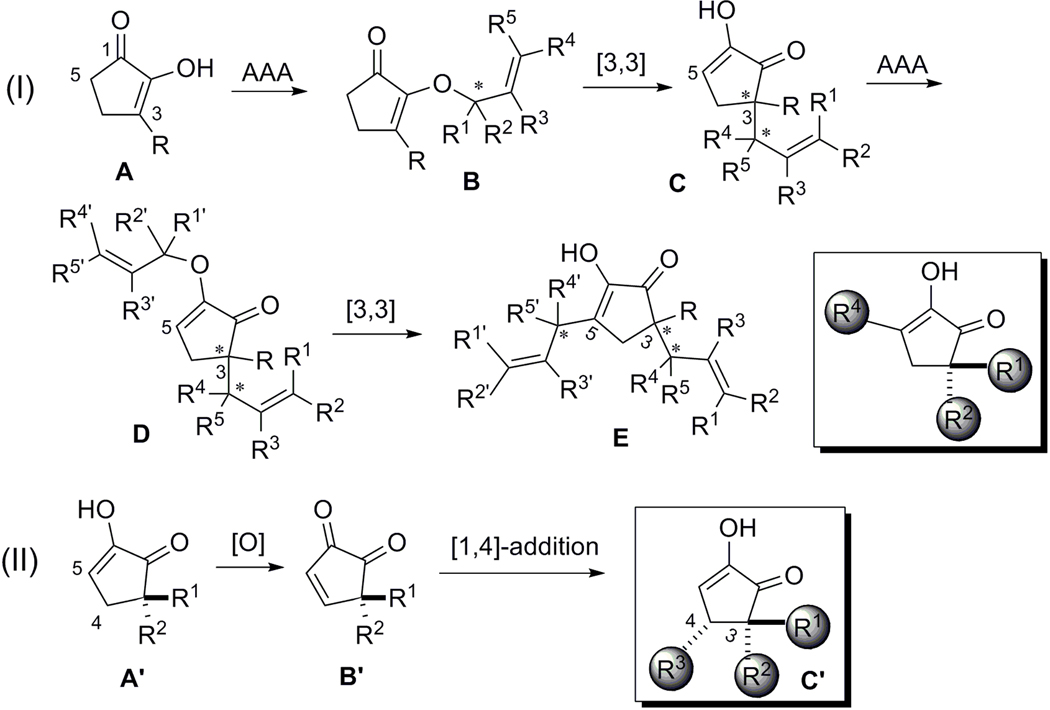
Cyclic 1,2-diketones with one of the ketones in an enol form have been named as diosphenols due to their similar reactivity with phenols.[13] Notably, 3-substituted cyclic 1,2-diketones exist as a single tautomeric species, which raises an attractive prospect of serving as a pivotal core onto which several carbon chain substituents can be installed in a stereoselective fashion. As phenols, the enol OH of cyclic 1,2-diketones exhibits substantial nucleophilicity, and generally, only O-alkylation occurred when treated with alkylating reagents.[14] In 2000, Trost and Schroeder developed an asymmetric allylic alkylation (AAA) method using diosphenols as nucleophiles, and O-allylated products were obtained with high enantioselectivity (Scheme 3).[15] Subsequent Claisen-rearrangement of these AAA adducts then provided the C-alkylation products with excellent chirality transfer.
Scheme 3.
Pd-catalyzed AAA-Claisen rearrangement sequence
Basic Principle
This O-allylation-Claisen rearrangement sequence should provide a chemo- and regioselective enolate allylation, which can be performed asymmetrically with respect to the enolate or allyl fragment or both (Figure 2, I). During this transformation, up to two stereogenic centers can be created, including all-carbon quaternary ones. Moreover, the Claisen rearrangement product (C) regains diosphenol functionality, which can be used as a substrate in the same sequence again to introduce another alkyl substituent at the C5 position. On the other hand, oxidation of 3,3-disubstituted diosphenol A´ could potentially provide a relatively unknown but highly electrophilic cyclopentenedione species B´. Conjugate addition of alkyl nucleophiles across B´ should install a third carbon chain at the C4 position (Figure 2, II). Therefore, we envision that all the carbon substituents on the 5-membered ring core of terpestacin could be programmatically introduced in a chemoselective, regioselective and stereoselective manner.
Figure 2.
Basic principle: controlled substitution on cyclic 1,2-diketones towards the synthesis of terpestacin.
Results and Discussion
Synthesis Plan
From a retrosynthetic viewpoint (Scheme 4), we envisaged that the side chain along with the C23 stereocenter would be accessed via the “Pd AAA-Claisen” protocol followed by oxidative alkene cleavage; while the 15-membered macrocycle could be constructed via a highly selective ring-closing metathesis (RCM) to form the C12–C13 olefin. The RCM precursor 13 or 14 could be formed by alkylation of sulfone 15 with the corresponding allyl bromides (16 or 17) that could ultimately come from diosphenol 18. The C15 stereocenter of 18 could potentially be generated either by a vinylogous enolate alkylation or through a stereoseletive Sakurai allylation of the corresponding enone, and the C1 quaternary center would arise from another “Pd AAA-Claisen” of inexpensive and commercially available 3-methyl-1,2-cyclopentanedione (19) and isoprene monoepoxide (20).
Scheme 4.
Retrosynthetic analysis.
Pd-Catalyzed AAA Reactions between Diosphenol 19 and Isoprene Monoepoxide 20 (Eq 1)
Initial attempts to effect the AAA between 19 and 20 by using the original reaction conditions[15] only gave moderate enantioselectivity (entries 1 and 2). Note that tetrabutylammonium chloride was employed as an additive to promote the π-σ-π equilibration required for resolution of vinyl epoxide 20 (Figure 3). In this case, the naphtho-Trost ligand (LNA) gave slightly higher ee (entry 2, 57% ee) than standard ligand (LST) (entry 1, 48% ee). Lowering the catalyst loading from 5 mol% to 1 mol% did not hamper the yield; on the contrary, the enantioselectivity increased from 57% ee to 67% ee (entries 3 and 4). Raising the loading of chloride salt had a slight but noticeable increase on the enantioselectivity (entry 5). Further study suggested that temperature did not play an important role on either the yield or the enantioselectivity (entries 6 and 7). Under all the previous test conditions, the reactions were observed to proceed at an extremely high rate. A high reaction rate with low ee value suggests that the π-σ-π interconversion between two diastereomeric palladium-π-allyl species X and Y (see Figure 3) is relatively slow compared with the nucleophilic attack. Thus, by decreasing the nucleophile concentration, the rate of nucleophilic addition should be lowered and the palladium-π-allyl intermediates (X and Y) would have enough time to equilibrate, which should provide a high facial selectivity. Indeed, slow addition of 19 via a syringe pump dramatically improved the enantioselectivity to 90% ee (entry 8). From these studies emerged the most practical set of conditions, and as shown in entry 9, up to 96% ee could be achieved with the standard Trost ligand. Pd-AAA adduct 21 was subsequently protected in the same pot with a bulky TIPS group, and silylated product 22 was isolated in 93–95% yield.
Figure 3.
π-σ-π Equilibration
 |
(Eq 1) |
Claisen Rearrangement Leading to C-Alkylated Product 23
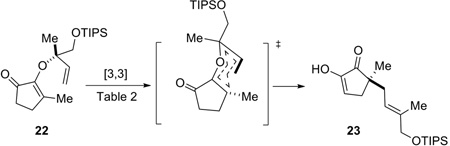
With AAA adduct 22 in hand, the stage was set for a Claisen rearrangement to transfer the chirality from the side chain to the ring (Eq 2). Ho(fod)3 has been established as an excellent catalyst for such rearrangements.[15] Indeed, treatment of TIPS ether 22 with 10 mol% Ho(fod)3 in chloroform at 35–45 °C for 5 days did provide the rearranged product (23) in 20% yield (Table 2, entry 1). The E/Z selectivity for the newly formed alkene geometry in 23 was 4.1:1 (determined by 1H-NMR), favoring the E isomer. Use of related Eu(fod)3 as the catalyst, however, gave incomplete conversion with formation of byproducts (entry 2). When warming at 55 °C for 40 h, diosphenol 23 was afforded in 33% yield and 5.8:1 E/Z selectivity (entry 3). Surprisingly, in the absence of the catalyst, this sigmatropic arrangement proceeded equally well or even slightly better by simply heating 22 in a minimal amount of chloroform (entry 4). An increased E/Z ratio (8.3:1) was observed when heating 22 at decreased reaction temperature (40 °C), however, the reaction rate was significantly diminished (entry 5). In the absence of solvent, this Claisen rearrangement occurred with increased yield (89%) and reasonably good E/Z selectivity (4.8:1), although a long period of heating (70 °C, 20 h) was still required to allow the reaction to go to completion (entry 6). A more practical protocol was then developed as shown in entry 7. Replacement of conventional heating with microwave irradiation (100 °C for 15 min, then 120 °C for 15 min) significantly increased the reaction rate, and the product (23) was isolated in 93% yield. Attempts to enhance the E/Z selectivity by employing Lewis acid catalysts proved to be unfruitful (entries 8 and 9).
Table 2.
Selected optimization of aziridination reaction.
| Entry | Additives (10 mol %) |
Solvent | Temp-Time | Yield (%) [a] |
E:Z[b] |
|---|---|---|---|---|---|
| 1 | Ho(fod)3 | CHCl3 | 35–45 °C-5d | 20 | 4.1:`1 |
| 2 | Eu(fod)3 | CHCl3 | 35 °C-15d | N/A[c] | N/A |
| 3 | Ho(fod)3 | CHCl3 | 55 °C-40h | 33 | 5.8:1 |
| 4 | none | CHCl3 | 55 °C-40h | 50 | 6.3:1 |
| 5 | none | CHCl3 | 45 °C-120h | 37 | 8.3:1 |
| 6 | none | none | 70 °C-20h | 89 | 4.8:1 |
| 7 | none | CHCl3 | 100 °C[d]-15min; 120 °C[d]-15min |
82–93 | 4–5:1 |
| 8 | Ho(fod)3 | CHCl3 | 100 °C[d]-15min; 120 °C[d]-15min |
74 | 5.0:1 |
| 9 | HoTMHD[e] | CHCl3 | 100 °C[d]
-15min; 120 °C[d]-15min |
34 | 4.0:1 |
| 10 | none | Purified CHCl3 |
100 °C[d]
-15min; 120°C[d]-15min |
N/A[c] | N/A |
| 11 | none | DME | 160 °C[d]-3h | 100[f] | 2.7:1 |
Isolated yield.
Determined by H-NMR.
Incomplete conversion with byproduct formation.
Microwave heating.
TMHD= 2,2,6,6-tetramethyl-3,5-heptanedionate.
Conversion.
 |
(Eq 2) |
Of note, choice of chloroform as solvent is not arbitrary. When DME was used as solvent, a 3 h microwave heating at 160 °C was required, and the E/Z selectivity for the product was lower (2.7:1, entry 11). Interestingly, under the same conditions as in entry 7 but using chloroform freshly distilled from K2CO3, the Claisen rearrangement failed to give full conversion and the product was contaminated with unidentified byproducts (entry 10). It was hypothesized that a trace amount of water and HCl present in “unpurified” chloroform may help to catalyze this [3,3]-sigmatropic rearrangement. In the case of solvent-free conditions, the diosphenol product (23) itself can act as the acid catalyst due to the acidity of the enol OH. Chirality transfer from 22 to 23 proved to be complete. At this point, the absolute configuration of 23 was tentatively assigned in analogy to our previous work.[15]
Installation of the Allyl Group at the C15 Position (terpestacin numbering)
Elaboration of diketone 23 to the natural product requires installation of an allyl side chain at the C15 position. One possible route is to generate a vinylogous enolate via deprotonation of a protected diketone followed by quenching with an allyl electrophile (Eq 3). Towards that end, a model system was employed to examine the feasibility of this conjecture. Model substrate (±)-24 was prepared in 88% yield over two steps from diosphenol 19.[14] Subsequent TIPS or PMB protection of the enol provided the corresponding silyl ether (±)-25 and benzyl ether (±)-26 in excellent yield (Scheme 5). However, treatment of either (±)-25 or (±)-26 with various bases and electrophiles in different solvents failed to provide any desired alkylation products. Instead, some O-alkylation byproducts and decomposition of the starting material were observed, likely attributed to a certain fragileness of the vinylogous enolate intermediate.
Scheme 5.
Synthesis of the model substrates.
 |
(Eq 3) |
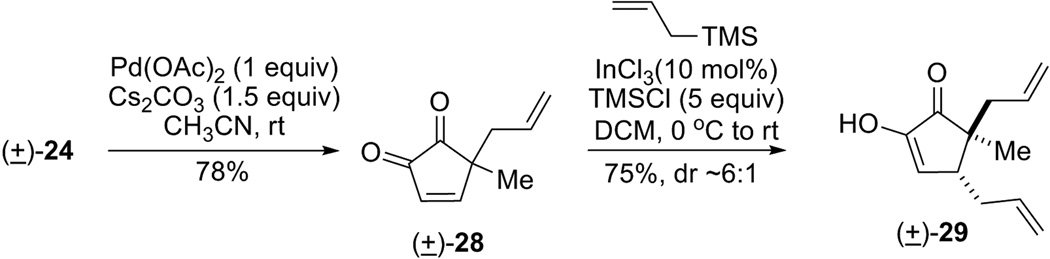
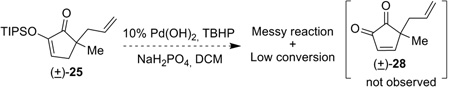
Alternatively, an umpolung strategy can be envisioned. Instead of using the diosphenol as nucleophile, oxidation of the diketone would create a “hot” electrophile, an ene-dione (27), which allows an allyl nucleophile to attack at the C15 position (Eq 4). Conjugated cyclopenten-1,2-diones containing no substituents at C3 and C4, such as 27, are rare and underutilized species,[16] about which limited chemistry is known. Reaction of TIPS enol ether (±)-25 under Corey-modified Saegusa oxidation conditions[17] resulted in a messy reaction mixture with incomplete conversion (Eq 5). IBX oxidation[18] of diketone (±)-24 gave no desired ene-dione compound but decomposition of the starting material. After extensive experimentation, we finally found that treatment of (±)-24 with 1 equiv of palladium acetate and 1.5 equiv of cesium carbonate in acetonitrile at ambient temperature cleanly provided cyclopentene-α-dione (±)-28 as a yellow oil in 78% yield (Scheme 6). To the best of our knowledge, this represents the first example of direct Saegusa oxidation of unprotected diosphenols.[19] Surprisingly, this ene-dione compound is relatively stable towards aqueous workup and silica gel chromatography. Attempts to reduce the amount of palladium were unfruitful. For example, using molecular O2 (in DMSO), benzoquinone or Cu(OAc)2 as the stoichiometric oxidant failed to turn over the Pd catalyst. A subsequent indium-catalyzed Sakurai allylation[20] was then employed, and the allyl group was installed in a diastereoselective fashion. Diosphenol (±)-29 was isolated in 75% yield as a 6:1 isomer mixture. In the major diastereomer, the two allyl groups are in a trans position relative to each other, which was determined by 1D nOe experiments. Moreover, this transformation constitutes the first example of an intermolecular C-1,4-addition into these enedione species.[21]
Scheme 6.
Model reactions for allylation at C4 position of diosphenol 24.
 |
(Eq 4) |
 |
(Eq 5) |
 |
(Eq 6) |
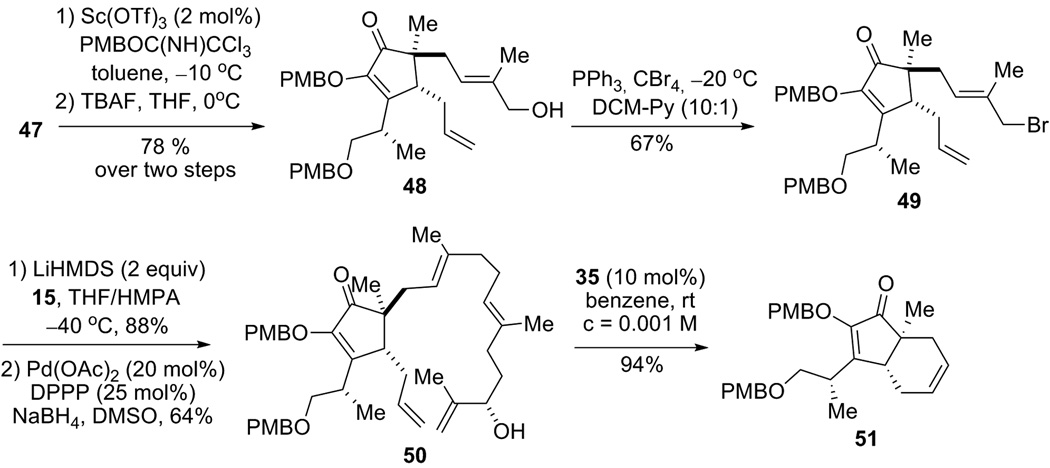
With successful installation of an allyl substituent at the C4 position in the model system, we next tested these conditions in the real system. Treatment of the Claisen rearrangement product generated from 22 under the newly developed Saegusa oxidation conditions smoothly gave cyclopentene-α-dione 27 in 78% yield over two steps (Scheme 7). For the subsequent allylation, although indium chloride was originally used as the Lewis acid in the model system, use of magnesium bromide was more effective for the real system. Allylation product 18 was isolated in 86% yield with 5.7:1 dr; the relative stereochemistry and the dr were determined by 1D nOe experiment and 1H NMR respectively.
Scheme 7.
Synthesis of diosphenol 18.
A RCM Approach to Construct the 15-Membered Carbocycle
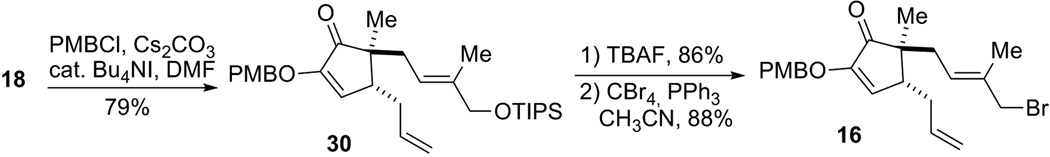
The enol OH in diosphenol 18 was next protected as a PMB ether (Scheme 8). Subsequently, the TIPS protecting group was removed by TBAF, and treatment of the resultant allyl alcohol with PPh3 and CBr4 provided allyl bromide segment 16 in high yield.
Scheme 8.
Synthesis of allyl bromide 16.
The synthesis of sulfone segment 15 is depicted in Scheme 9. Known allyl alcohol 32 was prepared in two steps from commercially available geranyl bromide.[22] Under Sharpless asymmetric epoxidation conditions, allyl alcohol 32 was converted to chiral epoxide 33 with 98% ee. A reductive epoxide rearrangement procedure was then adopted from Li[23]: the epoxy alcohol was converted in situ into the corresponding epoxy iodide with PPh3, iodine and pyridine, which subsequently underwent reductive elimination by iodide ion which forms upon addition of water to afford chiral allyl alcohol 15 in 74% yield.
Scheme 9.
Synthesis of sulfone fragment 15.
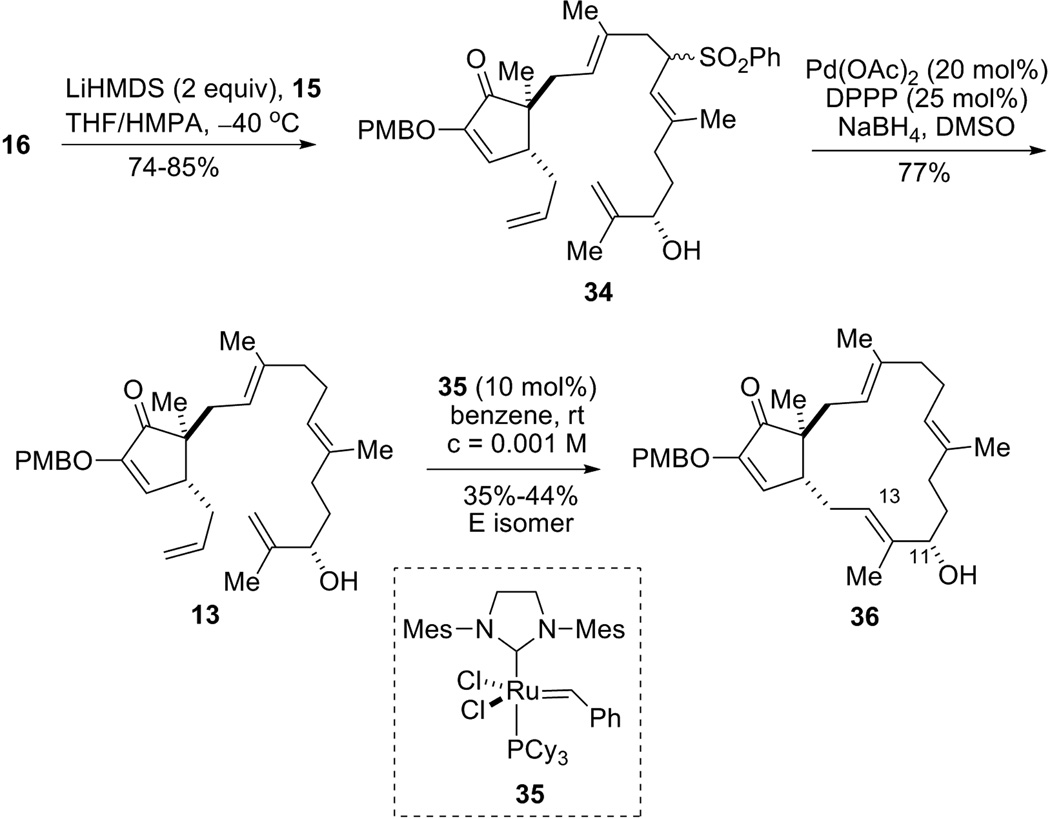
With 15 and 16 in hand, the stage was set to couple these two fragments. After careful optimization, treatment of a mixture of 15 and 16 with 2 equiv LiHMDS in THF-HMPA at −40 °C cleanly provided coupling product 34 in very good yield as a 1:1 mixture (Scheme 10). Notably, protection of the allylic alcohol is not necessary, and no O-alkylation product was isolated. The choice of base seems to be critical; for example, use of NaHMDS or KHMDS was much less effective than LiHMDS. Solvent and temperature optimization studies revealed that a THF-HMPA (3:1) mixed-solvent and −40 °C combination gave the best yield. A number of sulfone removal methods were next attempted, and a Pd-catalyzed reductive desulfonylation turned out to be most efficient for substrate 34. By using Pd(OAc)2-DPPP as catalyst and NaBH4 as stoichiometric reductant in DMSO, this desulfonation proceeded with high regioselectivity with almost no loss of olefin geometry, and RCM precursor 13 was furnished in 77% yield. It is worthy to note that carefully dried DMSO (distilled over CaH2, then stored over 4Å molecular sieves) was required for this desulfonylation reaction.
Scheme 10.
Synthesis of macrocycle 36.
Since compound 13 contains 5 olefins, many outcomes are possible for the following RCM reaction (Figure 4). For example, C13 and C3 could close to form a 6-membered ring (pathway A); C8 and C12 could close to form a 5-membered ring (pathway B), etc. After careful screening, we found that treatment of 13 with 10 mol% Grubbs 2nd generation catalyst (35)[24] in benzene at room temperature produced the desired 15-membered carbocycle 36 in a reasonably good yield[25] (35–44% of the E isomer[26]). We also found that the substrate having the C11 hydroxyl protected with a TBS group did not provide any 15-membered ring product, which indicated that the free allylic alcohol moiety could be critical for the success of this challenging RCM. We rationalize that coordination of the hydroxy group with the ruthenium catalyst or potential intramolecular hydrogen bonding between the OH and the diketone moiety might be key factors to form the macrocycle. Benzene was selected as the solvent for this RCM reaction instead of methylene chloride, because a less polar solvent would be beneficial for the formation of intramolecular hydrogen bonding. In addition, low reaction temperature also proved to be important, for various byproducts formed at elevated temperature. Moreover, using less reactive Grubbs 1st generation catalyst[27] failed to provide any macrocyclization product.
Figure 4.
Possible RCM pathways
Initial Approach to Install the Side Chain
Advancement of bicycle 36 to terpestacin (1) requires installation of the side chain along with the C23 stereogenic center. Removal of the PMB group in 36 turned out to be non-trivial. A large number of methods for PMB deprotection such as DDQ, TFA, CAN, BF3 etc, have proved unsuccessful. In the end, a relative unconventional method was tried by using MgBr2 and dimethyl sulfide.[28] We anticipated that the chelation of Mg+2 between the enol ether and ketone could facilitate the subsequent debenzylation, either by bromide or dimethyl sulfide. Indeed, under these conditions the PMB group was cleanly removed with excellent yield (Scheme 11). We envisioned that, under control by the adjacent C15 stereocenter, Claisen rearrangement of the O-crotylated diosphenol 37 should give the C-alkylation product with the desired stereochemistry. Indeed, treatment of 37 with trans-crotyl bromide and potassium carbonate followed by microwave heating in DME provided adduct 39 containing the whole carbon framework and all the stereocenters present in the natural product.[29] At this point, the stereochemistry of the newly formed C23 center in 39 was tentatively assigned as the one in Scheme 11. The enol and the allylic alcohol were subsequently protected with TES groups in one step. All efforts to selectively oxidize the terminal olefin in compound 40 without touching any of the three trisubstituted alkenes remained unfruitful. For example, under regular dihydroxylation or diboration/oxidation conditions[30], a complex reaction mixture was obtained without any desired product. This result implied that chemoselective oxidation of the terminal olefin compared to the trisubstituted olefins is very challenging considering that the latter are typically more susceptible towards oxidation.
Scheme 11.
Initial efforts to install the side chain in the presence of the macrocycle.
Alternate Route: a Second Pd AAA-Claisen Sequence to Install the Side Chain before Macrocyclization
Given the difficulties to cleave the alkene in the side chain at a very late stage, one alternate route is to furnish the side chain first and then close the macrocycle in the very end. Furthermore, instead of dealing with a less electron-rich terminal olefin, installation of a disubstituted alkene should significantly increase the chance for the desired oxidative cleavage at the side chain. Towards this end, a second Pd-catalyzed AAA reaction between diosphenol 18 and allyl carbonate 42 using (S,S) standard Trost ligand provided O-allylated product 43 in 94% yield with over 10:1 diastereoselectivity determined by 1H NMR (Scheme 12). The stereochemistry of the newly formed chiral center in 43 was assigned by analogy to other AAA reactions with allyl carbonate 42.[15] Subsequent microwave-mediated Claisen rearrangement gave diosphenol 44 that was next protected as a PMB ether. The resultant compound 45 contains various olefins: one monosubstituted-, one disubstituted- and one trisubstituted olefin, as well as one tetrasubstituted conjugated enol ether. Thus, to oxidize the disubstituted one selectively in the presence of all the others is a challenge. For example, treatment of 45 with mCPBA only afforded an epoxide at the position of the trisubstituted olefin. After extensive screening, fortunately, under Sharpless’ asymmetric dihydroxylation (SAE) conditions [31] the desired diol 46 was obtained in 40% (unoptimized) yield. It is likely that the bulky (DHQD)2PHAL ligand would have unfavorable steric interaction with the TIPS group and the C1 quaternary center, which results in slow oxidation of the trisubstituted alkene. On the other hand, the monosubstituted olefin is relatively electron-poor. Thus, those stereoelectronic biases could be the key factors for this chemoselective oxidation. Subsequent periodate cleavage of the diol and chemoselective reduction of the aldehyde in the presence of the ketone with NaBH4 at −78 °C in DCM-MeOH mixed solvent completed the construction of the side chain.
Scheme 12.
Installation of the side chain before macrocyclization.
Scandium-catalyzed PMB protection of primary alcohol 47 followed by TBAF-mediated desilylation gave allylic alcohol 48 in 78% yield over two steps (Scheme 13). Treatment of 48 with PPh3 and CBr4 in DCM-Py mixed solvent[32] at −20 °C afforded allyl bromide 49, which was then subjected to the sulfone-coupling and desulfonation conditions as described previously. Adduct 50 was isolated in 56% yield over two steps, which would serve as a precursor for the subsequent RCM reaction. Unfortunately, treatment of 50 under the exact olefin metathesis conditions as disclosed earlier for substrate 13 failed to provide any macrocycle products. Instead, trans-fused [3.0.4] bicycle 51 was obtained in 94% yield as the only product from that RCM reaction.
Scheme 13.
Unsuccessful macrocyclization.
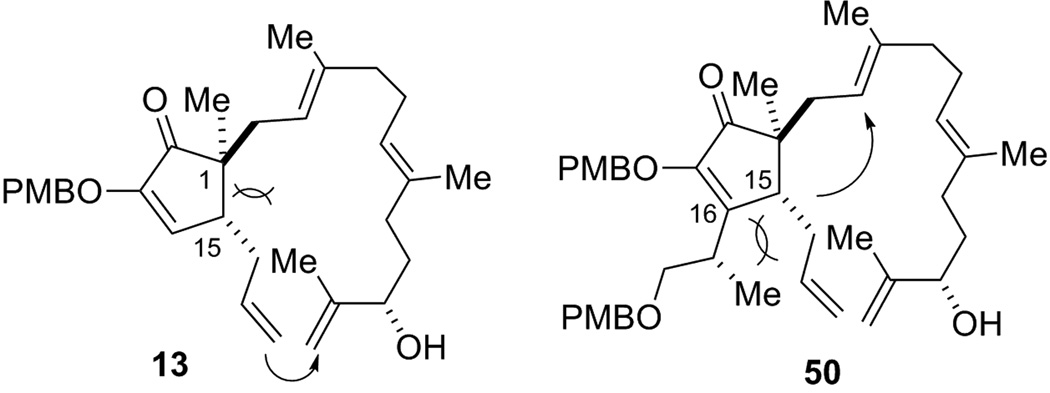
Why did substrate 13 and 50 give totally different reactivity in the RCM reaction (Figure 5)? We rationalize that the major steric interaction on the core of 13 is between the C15 allyl group and the two substituents at the C1 quaternary stereocenter. This interaction would likely push the two groups away from each other, which may ultimately result in a favorable macrocyclization. However, in compound 50, the C1–C15 interaction is largely compromised by a strong repulsion between the C15 allyl group and the C16 side chain. Moreover, formation of a 6-membered ring is also thermodynamically favored. Thus, all those factors led to a preferred cyclization of compound 50 to form the 6-membered ring in lieu of a macrocyclization.
Figure 5.
Structure comparison between RCM precursor 13 and 50.
Revised Approach Leading to the Total Synthesis of (1)
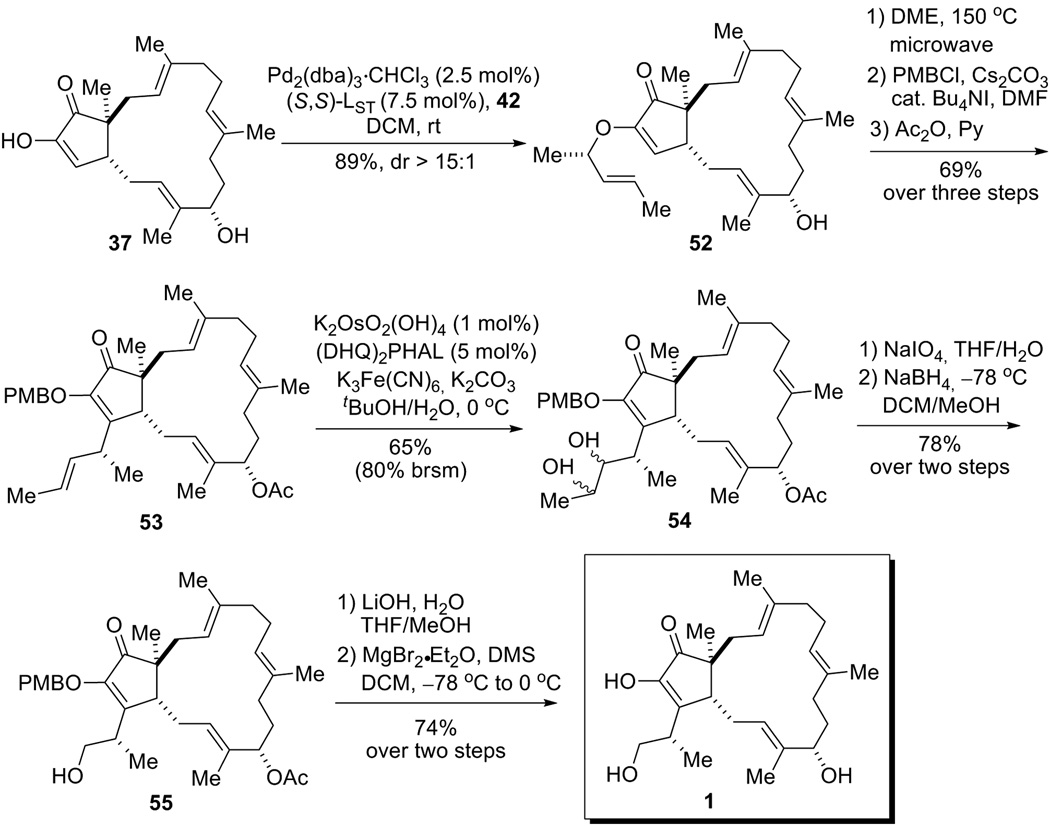
Encouraged by the success of macrocycle formation in the first route and the success of side chain installation in the second route, we decided to combine the merits of both routes and further revise the approach. We envisioned that a similar approach as that used in the second route could be applied to install the side chain on macrocycle intermediate 37 (Scheme 14). Indeed, application of AAA-Claisen rearrangement sequence on diosphenol 37 uneventfully brought in the side chain containing a trans-alkene with excellent diastereoselectivity. Note that the AAA adduct 52 was isolated as almost a single diastereomer determined by 1H NMR,[33] and the stereochemistry of the newly formed stereocenter in 52 was assigned by analogy to other similar AAA reactions with allyl carbonate 42.[15] The diosphenol OH was then protected as a PMB ether. An acetyl group was selected as the protecting group for the allyl alcohol moiety, first because it can be easily removed by hydrolysis and second, because an electron-withdrawing group like acetate should deactivate the adjacent trisubstituted olefin from oxidation. With tetra-ene 53 in hand, the remaining challenge was to selectively cleave the 1,2-disubstituted olefin in the presence of the three trisubstituted olefins. Lessons acquired from the second route led us to explore the possibility of using the Sharpless asymmetric dihydroxylation reaction to solve this chemoselectivity issue. To our delight, treatment of 53 with AD-mix α[34]/CH3SO2NH2 gave the desired diol 54 in 65% (brsm 80%) yield, while the use of AD-mix β resulted in much lower yield (24%).[35] We rationalized that the endocyclic trisubstituted olefins would have a facial bias owing to their restricted rotation, whereas the disubstituted olefin is more conformationally flexible. In addition, the crystal structure of terpestacin[3b] indicated that within the macrocycle the C3–C4 and the C7–C8 olefins are oriented in a similar fashion. Therefore, by using an asymmetric oxidation that is mismatched for the trisubstituted alkenes, oxidation of the disubstituted olefin would be kinetically favored. Advancement of diol 54 to the natural product was then achieved in a straightforward manner. Periodate cleavage of the vicinal diol followed by reduction of the resulting aldehyde furnished the side chain. Subsequent removal of the acetate and PMB protecting groups ultimately afforded (−)-terpestacin (1), which is spectroscopically identical to that previously reported.[9],[10]
Scheme 14.
Total synthesis of (−)-terpestacin (1).
Conclusion
As summarized in Scheme 15, a unique strategy has been developed for the enantioselective total synthesis of terpestacin (1) based on the unusual reactivity of diosphenols. By multiple usage of the α-diketone functionality, twice in the “Pd AAA-Claisen” protocol, and once by the employment of its oxidized form, the ene-1,2-dione, stereoselective alkylation of cyclopenta-1,2-diketones could be achieved in a programmatically controlled fashion. An unusual procedure for direct oxidization of the diosphenol moiety to the ene-1,2-dione was developed, and this enedione species proved to be an excellent Michael acceptor for a subsequent intramolecular 1,4-Sakurai allylation. The chemo- and regioselective desulfonylation of an allylic sulfone catalyzed by Pd(0) is also noteworthy. Several possible routes towards the total synthesis have been examined and carefully evaluated. During our exploration, many interesting chemoselectivity issues have been addressed and discussed in detail, including a chemoselective RCM to form the 15-membered carbocycle and a dihydroxylation reaction to oxidize a disubstituted olefin in the presence of various other electronically more activated olefins. It is envisaged that this diosphenol-based strategy along with the issues solved in this work, should have the potential to shed new light on the total syntheses of related terpenoid natural products.
Scheme 15.
Summary.
Experimental Section
Selected experimental procedures for the preparation of 22, 27, 18, 34, 13, 36, 52–55 and 1 (terpestacin) appear below. Full experimental details for all new compounds are given in the Supporting Information
Compound 22
(e.g. 3.60 mmol scale) In a flame dried, argon purged round bottom flask Pd2(dba)3·CHCl3 (71.0 mg, 0.0685 mmol), (R,R)-LST (145.4 mg, 0.210 mmol), and Bu4NCl (497.2 mg, 1.79 mmol) were combined and dissolved in dry, deoxygenated DCM (100 ml). To this solution was added isoprene monoepoxide (754 mg, 8.97 mmol). The resulting yellow solution was stirred at rt for 15 min at which time a solution of 3-methyl-1,2-cyclopentandione 19 (403.6 mg, 3.60 mmol) in dry, deoxygenated DCM (50 ml) was added over 6 h by syringe pump. After completion of the slow addition, the reaction was cooled to −78 °C. 2,6-Lutidine (0.92 g, 8.95 mmol) and then TIPSOTf (2.51 g, 8.18 mmol) were added and the solution was warmed to rt slowly over 16 h. Concentration in vacuo and purification by flash chromatography (petroleum ether/ether = 95/5, then 9/1) gave the product as a yellow oil (1.16 g, 93%, 96% ee. The ee of this sample was assumed to be the same as the ee of the acetate derivative prepared from a 1 ml aliquot of the unisolated, intermediate alcohol. The ee was determined by HPLC OC column, 1.0 ml/min, 90:10 heptane: isopropanol, tr (major): 29.32 min, tr (minor): 38.41 min).
For a larger scale: (e.g. 7.14 mmol scale) In a flame dried, argon purged round bottom flask Pd2(dba)3·CHCl3 (140.9 mg, 0.136 mmol), (R,R)-LST (297.7 mg, 0.431 mmol), and Bu4NCl (1.04 g, 3.73 mmol) were combined and dissolved in dry, deoxygenated DCM (100 ml). To this solution was added isoprene monoepoxide (1.50 g, 17.8 mmol). The resulting yellow solution was stirred at rt for 15 min at which time a solution of 3-methyl-1,2-cyclopentandione 19 (800.1 mg, 7.14 mmol) in dry, deoxygenated DCM (100 ml) was added over 6 h by syringe pump. After completion of the slow addition, the reaction was cooled to −78 °C. 2,6-Lutidine (2.02 g, 18.9 mmol) and TIPSOTf (4.56 g, 14.9 mmol) were added and the solution was warmed to rt slowly over 16 h. Concentration in vacuo and purification by flash chromatography (petroleum ether/ether = 95/5, then 9/1) gave the product as a yellow oil (2.39 g, 95 %, 88% ee).
Rf: 0.46 (petroleum ether/ethyl acetate = 8:1); [α]D: +7.2 (c 1.02, DCM, in this case 88% ee); 1H NMR (CDCl3, 500 MHz): δ 6.05 (dd, J= 17.6, 10.9 Hz, 1H), 5.18 (dt, J = 17.6, 0.61 Hz, 1H), 5.12 (dq, J= 10.9, 0.61 Hz, 1H), 3.79 (s, 2H), 2.43-2.40 (m, 2H), 2.33-2.28 (m, 2H), 1.98 (d, J = 0.61 Hz, 3H), 1.37 (s, 3H), 1.07-1.02 (m, 21H); 13C NMR (CDCl3, 125 MHz): δ 204.4, 161.1, 150.7, 140.3, 115.6, 83.7, 70.1, 32.5, 27.5, 20.4, 18.0, 17.7, 16.0, 11.9; IR (film): 2943, 2866, 1749, 1714, 1640, 1463, 1410, 1384, 1333, 1247, 1204, 1096, 996, 882, 810 cm−1; EA (C20H36O3Si): Calc’d. C 68.13, H 10.29, Found C 68.36, H 10.10.
Compound 27
Compound 22 (1.468 g, 4.17 mmol) was transferred into a microwave vial with 3 ml CHCl3. The microwave vial was sealed and heated to 100 °C for 15 min, then 120 °C for 15 min. The CHCl3 was removed under reduced pressure, and the crude compound was then dissolved into CH3CN (20 ml), before Cs2CO3 (2.0 g, 6.26 mmol) and Pd(OAc)2 (1.03 g, 4.59 mmol) were added. The resulting mixture was stirred at rt for 30 min. Palladium black was filtered out through a celite-silica gel cake, and compound 27 was purified via silica gel flash column chromatography (petroleum ether/ethyl acetate = 9/1) as an orange-yellow oil (1.135 g, 78%; E/Z = 4:1): Rf: 0.35 (petroleum ether/ethyl acetate = 9/1); [a]D: +68.9 (c 0.5, DCM); 1H NMR (CDCl3, 500 MHz): δ 7.81 (d, J = 7.5 Hz, 1H), 6.84 (d, J = 7.0 Hz, 1H), 5.32 (m, 1H), 4.01 (d, J = 1 Hz, 2H), 2.55 (dd, J = 14.5, 8.5 Hz, 1H), 2.33 (dd, J = 14, 7.5 Hz, 1H), 1.53 (s, 3H), 1.27 (s, 3H), 1.00–1.05 (m, 21H); 13C NMR (CDCl3, 125 MHz) δ 202.8, 189.3, 168.3, 139.3, 136.0, 115.4, 67.4, 47.9, 34.7, 21.4, 18.0, 17.7, 13.4, 12.0; IR (film): 3431, 2940, 2866, 1763, 1719, 1570, 1459, 1382, 1226, 1115, 996 cm−1; HRMS (C17H27O3Si + iPr): Calc’d. 307.172948 ([M - iPr] +), Found 307.172637.
Compound 18
Allyltrimethylsilane (0.14 ml, 0.91 mmol) was added to a suspension of 27 (32 mg, 0.091 mmol), MgBr2·Et2O (70.8 mg, 0.274 mmol) in DCM at −78 °C. The resulting suspension was stirred at −78 °C for 10 min, before it was warmed to 0 °C. The suspension was stirred at 0 °C for 10 min, and then was slowly warmed to rt. The mixture was stirred at rt for one hour, before poured into a pre-cooled NaHCO3 solution. The mixture was extracted with ethyl acetate (10 ml × 3), and the combined organic fractions were dried over Na2SO4. Compound 18 was purified via silica gel flash column chromatography (petroleum ether/ethyl acetate = 9/1) as a colorless oil (30.8 mg, 86%, dr 5.7:1): Rf: 0.35 (petroleum ether/ethyl acetate = 9/1); [α]D: −19.6 (c 1.56, DCM); 1H NMR (CDCl3, 500 MHz): δ 6.44 (d, J = 3 Hz, 1H), 5.80 (m, 1H), 5.65 (br, 1H), 5.35 (tt, J = 7.5, 1.5 Hz, 1H), 5.07 (dt, J = 15.5, 1.5 Hz, 2H), 4.04 (s, 3H), 2.61 (ddd, J = 2.5, 5, 10.5 Hz, 1H), 2.37 (td, J = 5.5, 14 Hz, 1H), 2.3-2.2 (m, 2H), 1.92 (m, 1H), 1.80 (s, 3H), 1.05-1.03 (m, 24H); 13C NMR (CDCl3, 125 MHz) δ 208.9, 150.7, 137.8, 136.3, 131.4, 117.6, 117.0, 67.8, 48.8, 43.2, 36.1, 35.3, 19.6, 18.1, 13.8, 12.0; IR (film): 3355 (br), 2943, 2867, 1694, 1654, 1464, 1394, 1214, 1115, 1066 cm−1; HRMS (C20H33O3Si + iPr): Calc’d. 349.219899 ([M - iPr] +), Found 349.217262.
Compound 34
LHMDS (0.62 ml, 0.5 M in THF) was added dropwisely into a solution of 16 (65 mg, 0.155 mmol) and 15 (45.7 mg, 0.155 mmol) in THF (0.6 ml) and HMPA (0.2 ml) at −40 °C under nitrogen. The resulting solution was stirred at −40 °C for 5 mins, before poured into an ice-cold NaH2PO4 (1M) solution. The mixture was extracted with ethyl acetate (10 ml × 3), and the combined organic fractions were dried over Na2SO4. The sulfone adducts 34 (mixture of diastereomers) was purified via silica gel flash column chromatography (petroleum ether/ethyl acetate = 7/3, then 1/1) to give a colorless oil (83.7 mg, 85%).
Compound 13
DMSO (3 ml) was added to the sulfone adducts 34 (242 mg, 0.38 mmol), Pd(OAc)2 (17.2 mg, 0.076 mmol) and DPPP (37.6 mg, 0.091 mmol) at rt under N2. The resulting solution was stirred at rt for 15 min, before NaBH4 (17.3 mg, 0.46 mmol) was added. The resulting dark mixture was stirred overnight, before poured into brine. The mixture was extracted with ethyl acetate (25 ml × 3), and the combined organic fractions were dried over Na2SO4. Compound 13 was purified via silica gel flash column chromatography (petroleum ether/ethyl acetate = 9/1, then 3/1) to give a colorless oil (163 mg, 77%): Rf: 0.35 (petroleum ether/ethyl acetate = 7/3); [α]D: −11.82 (c 0.38, DCM); 1H NMR (CDCl3, 500 MHz): δ 7.29 (d, J = 8.5 Hz, 2H), 6.87 (d, J = 8.5 Hz, 2H), 6.29 (d, J = 2.5 Hz, 1H), 5.80 (m, 1H), 5.11-5.04 (2H), 5.00 (t, J = 7.5 Hz, 1H), 4.92 (s, 1H), 4.86 (s, 2H), 4.83 (s, 1H), 4.02 (t, J = 6 Hz, 1H), 3.80 (s, 3H), 2.56 (ddd, J = 10, 5, 2.5 Hz, 1H), 2.34 (dt, J = 14, 5.5 Hz, 1H), 2.20-2.18 (2H), 2.06-1.96 (6H), 1.88 (m, 1H), 1.72(s, 3H), 1.69-1.61 (2H), 1.59 (s, 3H), 1.02 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 207.2, 159.6, 154.4, 147.5, 138.4, 136.6, 134.9, 130.2, 129.5, 127.9, 124.5, 119.5, 116.9, 113.9, 111.1, 75.7, 71.3, 55.3, 49.5, 43.0, 39.9, 36.6, 35.8, 35.6, 33.2, 26.6, 19.7, 17.7, 16.3, 16.0; IR (film): 3480 (br), 3073, 2935, 1715, 1622, 1515, 1455, 1372, 1249, 1034, 913, 824 cm−1; HRMS (C32H44O4): Calc’d. 492.323960, Found 492.322332.
Compound 36
Grubbs 2nd generation catalyst (8.5 mg, 0.01 mmol) was added to a solution of 13 (50.7 mg, 0.10 mmol) in benzene (40 ml) under N2 at rt. The resulting solution was stirred at rt for 16 hr, before concentrated under vacuum. Compound 36 was directly purified via silica gel preparative TLC (petroleum ether/ethyl acetate = 1/4, then 2/3) as a colorless oil (21.1 mg, 44%): Rf: 0.30 (petroleum ether/ethyl acetate = 2/3); [α]D: −42.38 (c 0.68, DCM); 1H NMR (CDCl3, 500 MHz): δ 7.31 (d, J = 8 Hz, 2H), 6.88 (d, J = 7.5 Hz, 2H), 6.24 (d, J = 3.0Hz, 1H), 5.43 (m, 1H), 5.19 (dd, J = 10, 5 Hz, 1H), 5.08 (m, 1H), 4.90 (dd, J = 24.5, 11.5 Hz, 2H), 4.04 (dd, J = 10, 3 Hz, 1H), 3.80 (s, 3H), 2.75 (dt, J = 10.5, 3Hz, 1H), 2.40 (dd, J = 13.5, 10.5 Hz, 1H), 2.26-1.96 (8H), 1.80 (m, 3H), 1.62 (d, J = 4.0 Hz, 3H), 1.56 (s, 3H), 0.99 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 207.8, 159.7, 153.7, 138.0, 136.4, 133.2, 131.7, 129.5, 128.5, 127.9, 124.4, 121.6, 114.0, 76.7, 71.5, 55.4, 49.5, 44.8, 40.2, 38.4, 34.9, 31.0, 30.0, 23.9, 16.9, 15.6, 15.3, 10.5; IR (film):3480 (br), 2934, 1712, 1628, 1516, 1455, 1248, 1156, 1053, 1033 cm−1; HRMS (C30H40O4): Calc’d. 464.292660, Found 464.290731.
Compound 52
In a flame dried, argon purged round bottom flask Pd2dba3·CHCl3 (1.5 mg, 0.0015 mmol) and (S,S)-LST (3.0 mg, 0.0044 mmol) were combined and dissolved in dry, deoxygenated DCM (0.5 ml). To this solution was added t-butyl 1-methyl-but-2-enyl carbonate 4235 (23.2 mg, 0.118 mmol). The resulting yellow solution was stirred at rt for 15 min at which time a solution of 37 (20.3 mg, 0.059 mmol) in dry, deoxygenated DCM (0.7 ml) was added over 1 h by syringe pump. After addition, the solution was concentrated under vacuum, and compound 52 was purified via silica gel flash column chromatography (petroleum ether/ethyl acetate = 4/1, then 7/3) to give a colorless oil (19.2 mg, 89%, dr > 15:1): Rf: 0.35 (petroleum ether/ethyl acetate = 4/1); [α]D: −57.33 (c 0.85, DCM); 1H NMR (CDCl3, 500 MHz): δ 6.18 (d, J = 3H, 1H), 5.67 (m, 1H), 5.47 (m, 1H), 5.42 (dd, J = 6, 4.5 Hz, 1H), 5.20 (dd, J = 10, 5.5 Hz, 1H), 5.10 (dd, J = 7.5, 5 Hz, 1H), 4.57 (m, 1H), 4.04 (m, 1H), 2.74 (dt, J = 11, 3 Hz, 1H), 2.39 (dd, J = 14, 10.5 Hz, 1H), 2.26-1.95 (m, 7H), 1.82-1.76 (m, 3H), 1.71 (dd, J = 6.5, 1.5 Hz, 3H), 1.63 (s, 3H), 1.62 (s, 3H), 1.55 (s, 3H), 1.39 (s, 3H), 0.97 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 208.5, 152.6, 137.9, 136.3, 133.1, 132.5, 131.2, 128.7, 128.4, 124.4, 121.7, 76.70, 76.67, 49.2, 44.9, 40.3, 38.5, 34.9, 31.1, 29.9, 23.9, 21.1, 17.7, 16.8, 15.6, 15.4, 10.5; IR (film): 3448 (br), 2934, 1708, 1625, 1438, 1376, 1309, 1245, 1157, 1050 cm−1; HRMS (C27H40O3): Calc’d. 412.297746, Found 412.297427.
Compound 53
Compound 52 (9.1 mg, 0.022 mol) was dissolved with DME (2.5 ml) in a microwave vial under N2. The solution was heated at 150 °C under microwave for 1h, before the solvent was removed under vacuum. CsCO3 (14.3 mg, 0.044 mmol), Bu4NI (1.6 mg, 0.0044 mmol) and DMF (0.22 ml) were added to the above residue at rt under N2. The resulting mixture was stirred at rt for 15 min, before PMBCl (5.2 mg, 0.033 mmol) was added. The resulting suspension was then stirred at dark for 2 hr, before poured into brine. The mixture was extracted with ethyl acetate (10 ml × 3), and the combined organic fractions were dried over Na2SO4. After purified via silica gel flash column chromatography (10% v/v ether in petroleum ether, then 25% v/v ethyl acetate in petroleum ether), this PMB-ether (~11.6 mg) was dissolve with pyridine (0.1 ml) and acetic anhydride (0.1 ml) at 0 °C. The resulting solution was stirred at rt for 3h, before it was concentrated under vacuum. Compound 53 was purified via silica gel flash column chromatography (petroleum ether/ether = 9/1, then petroleum ether/ethyl acetate = 9/1) to give a light-yellow oil (8.7 mg, 69%, three steps): Rf: 0.35 (petroleum ether/ethyl acetate = 9/1); [α]D: −89.58 (c 0.26, DCM); 1H NMR (CDCl3, 500 MHz): δ 7.29 (d, J = 8.5 Hz, 2H), 6.86 (d, J = 8.5 Hz, 2H), 5.43-5.32 (m, 3H), 5.25-5.11 (m, 5H), 3.80 (s, 3H), 3.15 (m, 1H), 2.60 (dd, J = 11, 3 Hz, 1H), 2.36 (d, J = 17 Hz, 1H), 2.26 (m, 3H), 2.14-2.00 (m, 6H), 1.82 (m, 3H), 1.72-1.67 (m, 1-2H), 1.64 (s, 3H), 1.61 (s, 3H), 1.59 (d, J = 5.5Hz, 3H), 1.53 (s, 3H), 1.16 (d, J = 7Hz, 3H), 0.94 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 209.1, 170.4, 162.4, 159.5, 148.4, 137.7, 132.6, 132.4, 131.9, 131.6, 130.5, 129.6, 124.9, 124.3, 121.8, 113.7, 78.8, 71.1, 55.3, 49.7, 47.1, 40.2, 39.3, 37.1, 34.6, 29.5, 27.7, 23.9, 21.6, 17.88, 17.87, 16.2, 15.7, 15.5, 11.1; IR (film): 2920, 2851, 1732, 1698, 1634, 1613, 1514, 1463, 1370, 1247, 1019 cm−1; HRMS (C37H50O5): Calc’d. 574.365825, Found 574.367201.
Compound 55
A solution of 53 (6.5 mg, 0.011 mmol) in t-BuOH (0.05 ml) was added to a mixture of AD-α-mix (21.1 mg) and CH3SO2NH2 (1.6 mg, 0.017 mmol) in H2O (0.1 ml) and t-BuOH (0.05 ml) at 0 °C. The resulting mixture was stirred at 4 °C for 2 days, before quenched with Na2SO3 and brine. The mixture was extracted with ethyl acetate (10 ml × 3), and the combined organic fractions were dried over Na2SO4. The diol 54 (mixture of diastereomers) was purified via silica gel flash column chromatography (petroleum ether/ethyl acetate = 7/3, then 1/1) to give a colorless oil (4.5 mg, 65%; 1.2 mg 53 was recovered).
To a solution of diol 54 (9.0 mg, 0.015 mmol) in THF-H2O (4:1, 0.2 ml), was added NaIO4 (19 mg, 0.089 mmol) at 0 °C. The resulting solution was stirred at rt for 1h, before quenched with brine. The mixture was then extracted with ethyl acetate (10 ml × 3), and the combined organic fractions were dried over Na2SO4. After the solvent was removed under vacuum, the crude aldehyde was dissolved with DCM (0.1 ml) and MeOH (0.1 ml). The resulting solution was cooled to −78 °C, before NaBH4 (3 mg, 0.065 mmol) was added. The mixture was stirred at −78 °C for 0.5 h, before quenched with acetone (0.05 ml) and brine (3 ml). The mixture was extracted with ethyl acetate (3 ml × 3), and the combined organic fractions were dried over Na2SO4. Compound 55 was purified via silica gel flash column chromatography (petroleum ether/ethyl acetate = 7/3, then 3/2) to give a colorless oil (6.5 mg, 78%): Rf: 0.35 (petroleum ether/ethyl acetate = 7/3); [α]D: −57.36 (c 0.60, DCM); 1H NMR (CDCl3, 500 MHz): δ 7.29 (d, J = 8 Hz, 2H), 6.87 (d, J = 8Hz, 2H), 5.44 (m, 1H), 5.35 (d, J = 11 Hz, 1H), 5.21 (dd, J = 10, 6 Hz, 1H), 5.12 (d, J = 11.5 Hz, 1H), 5.10 (m, 1H), 3.80 (s, 3H), 3.62 (m, 2H), 2.62 (m, 2H), 2.34-2.22 (5H), 2.13-2.00 (4H), 2.00 (s, 3H), 1.92-1.65 (4H), 1.63 (s, 3H), 1.54 (s, 3H), 1.43 (s, 3H), 1.11 (d, J = 7 Hz, 3H), 0.96 (s, 3H); 13C NMR (CDCl3, 125 MHz) δ 208.9, 170.5, 160.9, 159.7, 149.2, 138.0, 132.4, 131.9, 131.7, 130.6, 129.2, 124.5, 121.6, 113.8, 79.0, 66.1, 55.4, 49.8, 49.0, 40.2, 39.4, 37.7, 34.6, 28.9, 27.5, 23.9, 21.6, 16.5, 15.6, 15.5, 14.6, 11.1; IR (film): 3462 (br), 2923, 2851, 1734, 1700, 1637, 1612, 1515, 1248, 1035, 1019 cm−1; HRMS (C25H36O3 + PMB + OAc): Calc’d. 384.266445 ([M-PMB-OAc]+), Found 384.262829.
Compound 1 (terpestacin)
LiOH (0.06 ml, 1 m) was added to a solution of 55 (7.3 mg, 0.013 mmol) in THF (0.18 ml) and MeOH (0.06 ml) at rt. The resulting solution was stirred at rt for 2 h, before quenched with NaH2PO4 (1 M). The mixture was extracted with ethyl acetate (10 ml × 3), and the combined organic fractions were dried over Na2SO4. The diol was purified via silica gel column chromatography (petroleum ether/ethyl acetate = 1/1, then 3/7) to give a colorless oil (6.0 mg, 89%). Dry dimethyl sulfide (0.015 ml) was added to a mixture of the above diol (3.0 mg, 0.0057 mmol) and MgBr2x000B7;Et2O (14.5 mg, 0.056 mmol) in DCM (0.2 ml) at −78 °C. The resulting suspension was stirred at −78 °C for 10 min, before it was warmed to 0 °C. The suspension was stirred at 0 °C for 10 min, and then it was slowly warmed to rt. The mixture was stirred at rt for 40 min, before poured into a pre-cooled NaHCO3 solution. The mixture was extracted with ethyl acetate (10 ml × 3), and the combined organic fractions were dried over Na2SO4. Preparative silica gel TLC (10% MeOH in DCM) afforded (−)-terpestacin 1 (1.7 mg, 74%): Rf: 0.3 (10 % MeOH in DCM); [α]D: −17.7 (c 0.085, MeOH); 1H NMR (CDCl3, 500 MHz): 5.89 (br, 1H), 5.40 (m, 1H), 5.24 (dd, J = 10, 5.0 Hz, 1H), 5.13 (m, 1H), 4.06 (dd, J = 10, 3.5 Hz, 1H), 3.89 (dd, J = 10.5, 7.0 Hz, 1H), 3.82 (dd, J = 10, 5.5 Hz, 1H), 2.71 (dd, J = 11.5, 2.0 Hz, 1H), 2.68 (m, 1H), 2.44 (d, J = 17.0 Hz, 1H), 2.39 (dd, J = 13.5, 10.5 Hz, 1H), 2.29-2.21 (m, 2H), 2.13-2.08 (m, 2H), 2.04-1.89 (m, 2H), 1.79-1.67 (m, 3H), 1.64 (s, 3H), 1.63 (s, 3H), 1.57 (s, 3H), 1.29 (d, J = 7.0 Hz, 3H), 1.00 (s, 3H); IR (film): 3347 (br), 2923, 2853, 1694, 1645, 1455, 1029 cm−1; HRMS (C25H38O4): Calc’d. 402.277010, Found 402.275848.
Figure 1.
Terpestacin and fusaproliferin.
Experimental formula.
Table 1.
Selected optimization studies.[a]
| Entry | L* | x | y | Time (h) |
Temp. (°C) |
Conc. (M) |
Yield (%)[b] |
ee (%)[c] |
|---|---|---|---|---|---|---|---|---|
| 1 | LST | 2.5 | 30 | 1 | 40 | 0.05 | 72 | 48 |
| 2 | LNA | 2.5 | 30 | 1 | 40 | 0.05 | 85 | 57 |
| 3 | LNA | 0.8 | 35 | 1 | 40 | 0.05 | 79 | 66 |
| 4 | LNA | 0.5 | 30 | 0.3 | 40 | 0.05 | 88 | 67 |
| 5 | LNA | 0.5 | 50 | 1 | 40 | 0.05 | 83 | 74 |
| 6 | LNA | 0.5 | 30 | 0.3 | rt | 0.05 | 79 | 66 |
| 7 | LNA | 0.5 | 30 | 0.3 | 60 | 0.05 | 85 | 67 |
| 8 | LNA | 0.5 | 50 | 2.5[d] | 40 | 0.08 | 94 | 90 |
| 9 | LST | 2.0 | 50 | 6[d] | rt | 0.07 | 93–95[e] | 88–96 |
The reaction was operated with 2 equiv 20 and 1 equiv 19.
Isolated yield.
Enantioselectivities were determined on its acetate derivative by chiral HPLC.
A solution of 19 was added slowly via a syringe pump over the indicated amount of time.
Isolated yield after direct conversion to TIPS ether 22.
Acknowledgements
We thank the National Institutes of Health (GM33049) for their generous support of our programs. Mass spectra were provided by the Mass Spectrometry Regional Center of the University of California-San Francisco, supported by the NIH Division of Research Resources. GD is a Stanford Graduate Fellow. Palladium salts were generously supplied by Johnson-Matthey.
Footnotes
Supporting information for this article is available on the WWW under http://www.chemeurj.org/ or from the author.
References
- 1.UNAIDS, WHO. 2007 AIDS epidemic update. 2007 December; Retrieved on 2008-03-12.
- 2.De Clercq E. In: Design of Anti-AIDS Drugs. De Clercq E, editor. Vol. 14. Tokyo: Elsevier; 1990. pp. 1–24. [Google Scholar]
- 3.(a) Oka M, Iimura S, Tenmyo O, Sawada Y, Sugawara M, Ohkusa H, Yamamoto H, Kawano K, Hu SL, Fukagawa Y, Oki T. J. Antibiot. 1993;46:367–373. doi: 10.7164/antibiotics.46.367. [DOI] [PubMed] [Google Scholar]; (b) Iimura S, Oka M, Narita Y, Konishi M, Kakisawa H, Gao Q, Oki T. Tetrahedron Lett. 1993;34:493–496. [Google Scholar]; (c) Oka M, Iimura S, Narita Y, Furumai T, Konishi M, Oki T, Gao Q, Kakisawa H. J. Org. Chem. 1993;58:1875–1881. [Google Scholar]
- 4.Schlegel B, Schmidtke M, Dorfelt H, Kleinwachter P, Grafe U. J. Basic Microbiol. 2001;41:179–183. doi: 10.1002/1521-4028(200107)41:3/4<179::aid-jobm179>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 5.Nihashi Y, Lim C-H, Tanaka C, Miyagawa H, Ueno T. Biosci. Biotechnol. Biochem. 2002;66:685–688. doi: 10.1271/bbb.66.685. [DOI] [PubMed] [Google Scholar]
- 6.Jung HJ, Lee HB, Kim CJ, Rho JR, Shin J, Kwon HJ. J. Antibiot. 2003;56:492–496. doi: 10.7164/antibiotics.56.492. [DOI] [PubMed] [Google Scholar]
- 7.Tatsuta K, Masuda N, Nishida H. Tetrahedron Lett. 1998;39:83–86. [Google Scholar]
- 8.Tatsuta K, Masuda N. J. Antibiotics. 1998;51:602–606. doi: 10.7164/antibiotics.51.602. [DOI] [PubMed] [Google Scholar]
- 9.Myers AG, Siu M, Ren F. J. Am. Chem. Soc. 2002;124:4230–4232. doi: 10.1021/ja020072l. [DOI] [PubMed] [Google Scholar]
- 10.(a) Chan J, Jamison TF. J. Am. Chem. Soc. 2003;125:11514–11515. doi: 10.1021/ja0373925. [DOI] [PubMed] [Google Scholar]; (b) Chan J, Jamison TF. J. Am. Chem. Soc. 2004;126:10682–10691. doi: 10.1021/ja0470968. [DOI] [PubMed] [Google Scholar]
- 11.Berger GO, Tius MA. J. Org. Chem. 2007;72:6473–6480. doi: 10.1021/jo070923d. [DOI] [PubMed] [Google Scholar]
- 12.For a preliminary report of a portion of our work, see:Trost BM, Dong G, Vance JA. J. Am. Chem. Soc. 2007;129:4540–4541. doi: 10.1021/ja070571s.
- 13.Straneo C. Gazzetta Chimica Italiana. 1940;70:27–37. [Google Scholar]
- 14.For example, see: Ponaras AA. Tetrahedron Lett. 1980;21:4803–4806.
- 15.Trost BM, Schroeder GM. J. Am. Chem. Soc. 2000;122:3785–3786. [Google Scholar]
- 16.Besides this work, there is only another report of cyclopenten-1,2-diones with no substituents at C(3) and C(4), see: Martin HD, Kummer M, Martin G, Bartsch J, Brueck D, Heinrichs A, Mayer B, Roever S, Steigel A. Chem. Ber. 1987;120:1133–1149.
- 17.Yu J-Q, Wu H-C, Corey EJ. Org. Lett. 2005;7:1415–1417. doi: 10.1021/ol050284y. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaou KC, Montagnon T, Baran PS. Angew. Chem. Int. Eng. 2002;41:993–996. doi: 10.1002/1521-3773(20020315)41:6<993::aid-anie993>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 19.For a Seagusa oxidation of TMS ethers of diosphenols, see: Kim M, Applegate LA, Kawada K, Watt DS. Synth. Commun. 1990;20:989–997.
- 20.For an example of indium-catalyzed Sakurai allylation, see: Lee PH, Lee K, Sung S-Y, Chang S. J. Org. Chem. 2001;66:8646–8649. doi: 10.1021/jo0105641.
- 21.Kishi has reported a singular example of intramolecular 1,4-O addition into 4-substituted-cyclopenten-1,2-dione in the total synthesis of batrachotoxinin A: Kurosu M, Marcin LR, Grinsteiner TJ, Kishi Y. J. Am. Chem. Soc. 1998;120:6627–6628.
- 22.Fairlamb IJS, Dickinson JM, Pegg M. Tetrahedron Lett. 2001;42:2205–2208. [Google Scholar]
- 23.Liu Z, Lan J, Li Y. Tetrahedron: Asymmetry. 1998;9:3755–3762. [Google Scholar]
- 24.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953–956. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 25.For a recent example of constructing a polyunsaturated macrocycle via RCM, see: Fürstner A, Nevado C, Tremblay M, Chevrier C, Teplý F, Aïssa C, Waser M. Angew. Chem. Int. Ed. 2006;45:5837–5842. doi: 10.1002/anie.200601860.
- 26.The geometry of the newly formed E olefin was determined via NMR 1D nOe experiment between the C13 vinyl proton and the C11 proton. Isolation of the minor Z isomer proved difficult due to the contamination by other byproducts.
- 27.Nguyen ST, Johnson LK, Grubbs RH, Ziller JW. J. Am. Chem. Soc. 1992;114:3974–3965. [Google Scholar]
- 28.Onoda T, Shirai R, Iwasaki S. Tetrahedron Lett. 1997;38:1443–1446. [Google Scholar]
- 29.The yield for both steps was not optimized.
- 30.For an example of Pt catalyzed diboration of simple alkenes, see: Ishiyama T, Yamamoto M, Miyaura N. Chem. Commun. 1997:689–690.
- 31.Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- 32.Using CH3CN as solvent gave much decomposition.
- 33.When racemic Trost ligand was used, the dr was observed about 1:1.
- 34.AD-mix was freshly prepared by mixing all the ingredients.
- 35.For a related example, see: Andrus MB, Lepore SD, Sclafani JA. Tetrahedron Lett. 1997;38:4043–4046.
- 36.For preparation of 42, see: Haight AR, Stoner EJ, Peterson MJ, Grover VK. J. Org. Chem. 2003;68:8092–8096. doi: 10.1021/jo0301907.