Abstract
The pathogenesis of radiocontrast nephropathy is poorly understood. In an animal model, inhibition of the synthesis of nitric oxide and prostaglandins appears to predispose rats to severe renal injury following the administration of radiocontrast. Here we have investigated whether administration of radiocontrast, as well as changes in renal medullary oxygenation following pharmacologic inhibition of nitric oxide and prostaglandin synthesis, might be evaluated by blood oxygenation level-dependent (BOLD) MRI. Nineteen anesthetized (Inactin 100 mg/kg) rats were studied. BOLD MRI measurements were performed following administration of L-NAME (N-nitro-L-arginine methyl ester, 10 mg/kg), Indomethacin (10 mg/kg), and a radio-contrast agent (sodium iothalamate 60%, 6 mL/kg). Marked sequential changes in medullary , presumably reflecting decline in medullary pO2, were noted after each of the pharmacological interventions employed. These results, obtained by noninvasive MRI, are consistent with prior direct recordings of pO2 and doppler flow in the rat renal medulla after administration of L-NAME, Indomethacin and iothalamate. Medullary oxygenation in rats was reduced by inhibition of the synthesis of prostaglandins and nitric oxide, as well as by intravenous injection of radiocontrast agents. BOLD MRI can noninvasively evaluate changes in medullary oxygenation in rats that appear to predispose acute renal failure.
Index terms: renal medulla, oxygenation, radiocontrast, acute renal failure, MRI
Transient or irreversible renal failure induced by intravenous radiographic contrast agents (radiocontrast media induced nephropathy (RCIN)), is said to be the third most common cause of hospital acquired renal failure (1), accounting for more than 30% of all such patients with this iatrogenic disorder (2). Patients with pre-existing renal insufficiency, diabetes or congestive heart failure that are exposed to a large volume of high-osmolality radiocontrast (3) are at increased risk of developing RCIN. Renal hemodynamic changes are thought to play a key role in RCIN. The exact pathophysiological mechanisms are poorly understood, though disturbed endothelial dysfunction has been surmised (4). Nitric oxide and prostaglandins are important vasoactive mediators released by the vascular endothelium. Both play a role in the homeostasis of oxygen supply to the renal medulla, a region normally operating on the verge of hypoxia. Inhibition of these vasoconstrictors predisposed rats to severe renal injury following radiocontrast administration in studies employing invasive doppler flow probe measurements of regional blood flow (5).
The effects of radiocontrast agents on renal hemodynamics has been studied extensively in rat kidneys (6–12). The laboratory rat has also been used in a variety of experiments designed to study the mechanism of the other forms of acute renal failure (13–15) and their therapy (12,16–19). Such preventive strategies are usually designed to improve or maintain medullary oxygenation. Until now, a limitation in the effective testing of these hypotheses has been the absence of a suitable non-invasive, reproducible method to assess regional oxygenation of rat kidneys. BOLD MRI promises to be such a technique.
We have previously demonstrated that BOLD MRI can evaluate intra-renal oxygenation in a non-invasive way in humans (20,21). The technique is ideally suited to estimate sequential changes in medullary oxygenation owing to the relatively low tissue pO2 localized in this region (22). Our studies in humans have focused both on maneuvers that improve medullary oxygenation and circumstances that might block such improvement (23). In order to apply this technique to animal models, we have developed a BOLD MRI method to image rat kidneys (24). Here we investigated whether BOLD MRI would be sensitive both to reductions in renal medullary oxygenation that follow pharmacologic inhibition of the synthesis of nitric oxide and prostaglandins, and the administration of a radiocontrast agent (5).
METHODS
Animal Preparation
Nineteen anesthetized (inactin 100 mg/kg) Sprague-Dawley rats weighing 44 8 ± 9 g (mean ± s.e.) were studied. All studies were approved by the institutional animal care and use committee. A femoral vein was canulated for the administration of a nitric oxide synthase inhibitor (L-NAME), an inhibitor of prostaglandin synthesis (Indomethacin, 10 mg/kg), and a radiocontrast agent (Angio-Conray 60%, 6mL/kg). Four sets of experiments were performed in which these substances were administered in different order:
n = 3, Baseline, Indomethacin, L-NAME
n = 6, Baseline, L-NAME, Indomethacin
n = 7, Baseline, L-NAME, Indomethacin, radiocontrast
n = 3, radiocontrast alone
Imaging
All studies were performed on a 1.5 T whole-body MR scanner (Vision; Siemens Medical Systems, Erlangen, Germany) using a multiple gradient recalled echo (mGRE) sequence (TR/TE/FA= 60 msec/10–56 msec/30°) to acquire 16 -weighted images (24). Other relevant parameters were FOV = 128 mm, Matrix size = 256 × 256. The result was an effective pixel size of 0.5 × 0.5 mm, slice thickness = 3 mm. An map was reconstructed online, based on the signal of the sixteen -weighted images (24). A flexible wrist coil was used to receive the signal. Rats were placed on their right side to reduce the susceptibility artifacts on the kidneys caused by bowel gas. We obtained baseline BOLD MRI data and followed the changes every three minutes after administration of the pharmacological agents. We acquired images for 15 minutes following the injection of each of the pharmacological agents.
The was measured using regions of interest (ROI, consisting of at least six pixels) in the renal medulla and cortex. When evaluating the time series, the same ROI locations were utilized for each image in the series. Statistical significance of the differences in the pre- vs. post-pharamacologic maneuver was assessed based on a two-tailed paired Student’s t-test.
RESULTS
Figure 1a shows a set of maps of the right kidney before and after administration of L-NAME followed by Indomethacin and iothalamate. Figure 1b plots the corresponding values in the cortex and medulla. Table 1 summarizes and for cortex and medulla, measured at 15 minutes after administration of each of the pharmacological agents. Note the increase in values following administration of L-NAME and Indomethacin, signifying reductions in the oxygenation status of the medulla. After iothalamate was injected intravenously, there was an immediate but short lived fall in medullary (consistent with brief medullary hyperemia) and an increase in pO2. This was followed consistently by a rapid return to baseline hypoxic levels. This hyperemic response following iothalamate was seen whether or not the animals had been pretreated with Indomethacin and L-NAME, but the final levels of medullary , measured 15 minutes after injection, were much lower in the latter case (Table 1).
Figure 1.
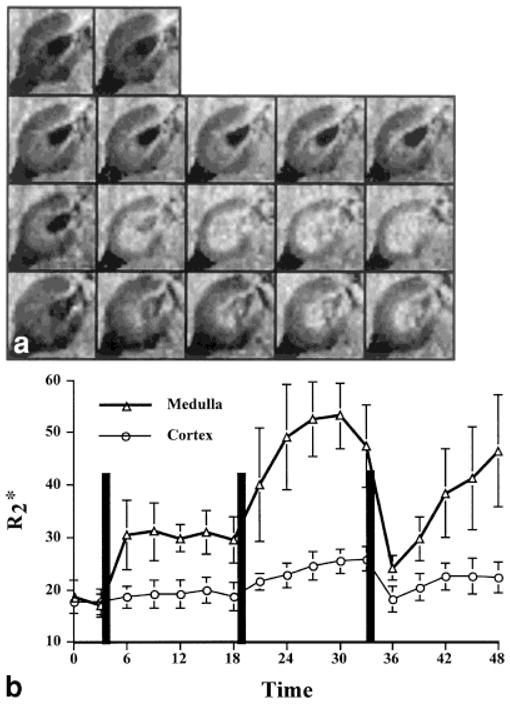
a: The map obtained at different time points in one representative animal. The top row shows the baseline images. The next three rows are images obtained after L-NAME, Indomethacin, and iothalamate, respectively. The five images in these rows were obtained at three-minute intervals for 15 minutes after administration of the agent. The brighter inner concentric region is the medulla, and the relatively darker outer region is the cortex. Higher values imply relatively lower blood pO2,, hence lower tissue pO2. b: Plot describing the temporal changes in for cortex and medulla following the administration of L-NAME, Indomethacin, and radiocontrast. The vertical lines indicate the time of administration of each of the agents. Error bars represent standard deviation within the ROI of this one representative animal.
Table 1.
of Medulla and Cortex Following Various Pharmacologic Agents
|
in Medulla (Hz) |
in Cortex (Hz) |
|
|||||
|---|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | Medulla | Cortex | ||
| Indomethacin, n= 3 | 21.6 ± 3.6 | 29.5 ± 6.1 | 18.4 ± 2.1 | 19.3 ± 2.3 | 7.9 ± 6.6 | 0.8 ± 0.5 | |
| Indomethacin + L-NAME, n = 3 | — | 40.2 ± 5.3 | — | 24.9 ± 0.7 | 18.6 ± 2.5 | 6.4 ± 2.6 | |
| L-NAME, n = 13 | 22.8 ± 1.0 | 32.2 ± 1.6a | 18.3 ± 0.6 | 21.6 ± 1.1a | 9.3 ± 1.3 | 3.8 ± 0.9 | |
| L-NAME + indomethacin, n = 13 | — | 39.9 ± 2.8b | — | 24.0 ± 1.5 | 15.5 ± 2.2 | 6.2 ± 1.0 | |
| L-NAME + indomethacin + Contrast, n = 7 | — | 37.4 ± 4.1 | — | 20.5 ± 1.4 | 15.3 ± 3.3 | 2.1 ± 1.2 | |
| Contrast, n = 3 | 26.4 ± 5.6 | 24.0 ± 2.9 | 21.0 ± 1.7 | 17.3 ± 2.8 | −2.4 ± 1.7 | −3.7 ± 1.2 | |
measured at 15 minutes after administration of the respective pharmacologic agent and (baseline) is the measurement made before any agent is administered. All values shown are mean ± s.e.
P < 0.05 for two-tailed Student t-test when compared with baseline.
P < 0.05 for two-tailed Student t-test when compared with post-L-NAME.
DISCUSSION
The marked hypoxia of renal medulla, as compared with the renal cortex and most other body tissues, has important implications for human disease (22). In particular, it has been postulated that anoxic injury to renal medullary structures initiates the phenomenon of acute renal failure (25). If so, physiological or pharmacological maneuvers that adversely influence medullary oxygenation should confer susceptibility to acute renal failure. Consistent with this hypothesis, it has been shown in rats that inhibition of the synthesis of nitric oxide and prostaglandins predisposes renal failure from intravascular injection of iothalamate characterized by extensive necrosis of medullary, thick, ascending limbs (5). This model of radiocontrast nephropathy does not require preliminary uninephrectomy or chronic salt depletion (13,26).
As seen in Fig. 1 and Table 1, the BOLD MRI technique readily detects the reduction in medullary oxygenation that follows the administration of various pharmacologic agents. Owing to the relatively small number of animals used for Indomethacin and Indomethacin + LNAME, the changes in did not reach statistical significance. However, the changes after L-NAME and Indomethacin appear comparable, and their effects are additive, regardless of the order of administration. By comparison, the effect of these agents on cortical was much less pronounced. These changes in medullary values following administration of L-NAME and Indomethacin are consistent with the changes in regional blood flow that have been measured in rats (5). The observed medullary changes in are also consistent with previous invasive pO2 measurements following nitric-oxide inhibition by L-NMMA (26) and Indomethacin (27). BOLD MRI, however, may be less sensitive to changes in blood flow in the renal cortex than in the medulla. This is due to the relatively high baseline pO2 of the cortex and to the fact that oxygen utilization in the renal cortex tends to be proportional to blood flow.
The reason for the transient decrease in medullary values immediately following radiocontrast administration is not quite clear. Agmon et al. (5) report an increase in medullary blood flow (consistent with our observation of transient improvement in medullary pO2) following iothalamate without pretreatment. Conversely, Heyman et al. (27) noticed a drop in pO2 following iothalamate. Agmon et al. (5) also found a small drop in blood flow following iothalamate administered after pretreatment with L-NAME and Indomethacin. In the experiments of Heyman (27) and Agmon (5), however, the right kidney had been exposed through a long abdominal incision, then de-encapsulated and fixed in position, whereas the present data with BOLD MRI was obtained from intact rats not subjected to surgical stress. It seems possible that the transient increase in medullary pO2 observed directly after intravenous injection of iothalamate may have been a consequence of sudden expansion of the blood volume and, perhaps, osmotic diuresis.
The marked and sustained falls in medullary pO2 seen after Indomethacin or L-NAME have important clinical implications. Non-steroidal anti-inflammatory drugs that inhibit prostaglandin synthesis are known to predispose acute renal failure (28), and the chronic use of similar agents has been implicated in the papillary necrosis of analgesic nephropathy (29). A theoretical concern in the clinical use of inhibitors of nitric oxide synthase for septic shock and hepatorenal syndrome is the possible deleterious effect of such therapy on the oxygenation of the renal medulla. It seems possible that patients with disorders of renal synthesis of prostaglandin or nitric oxide may be unusually susceptible to ischemic renal injury in clinical situations that predispose acute renal failure. Finally, it would be prudent to ensure that drugs inhibiting prostaglandin synthesis are interdicted for patients scheduled to receive radiocontrast agents in diagnostic tests.
Further studies are warranted to correlate the observed BOLD MRI changes with both conventional renal function measurements and histologic changes in the medulla. Future studies are also necessary to elucidate the transient decrease in medullary following radio-contrast administration. It may also be possible to correlate the BOLD MRI responses to direct and simultaneous measurements of tissue pO2 and regional blood flow using novel optical probe technology (30).
PRACTICAL APPLICATIONS
The BOLD MRI technique facilitates studies that elucidate pathophysiological mechanisms responsible for acute renal failure, both in humans and in animal models. The present data and our earlier studies in humans permit the conjecture that some day the application of the technique might help to “identify with greater sensitivity those patients at high risk for ischemic or nephrotoxic renal injury” (31).
Acknowledgments
This work was supported in part by a Grant-in-aid from the American Heart Association (PVP), and National Institutes of Health, DK 53221 (PVP) and DK 18078 (FHE). We thank Dr. Wei Li for his technical help.
Contract grant sponsor: American Heart Association; Contract grant sponsor: National Institutes of Health; Contract grant numbers: DK53221; DK18078.
Footnotes
Presented at the 7th Annual Meeting of ISMRM, Philadelphia, 1999.
References
- 1.Cohan RH, Dunnick NR. Intravascular contrast media: adverse reactions. AJR Am J Roentgenol. 1987;149:665–670. doi: 10.2214/ajr.149.4.665. [DOI] [PubMed] [Google Scholar]
- 2.Shusterman N, Strom BL, Murray TG, Morrison G, West SL, Maislin G. Risk factors and outcome of hospital-acquired acute renal failure. Clinical epidemiologic study. Am J Med. 1987;83:65–71. doi: 10.1016/0002-9343(87)90498-0. [DOI] [PubMed] [Google Scholar]
- 3.Lautin EM, Freeman NJ, Schoenfeld AH, Bakal CW, Haramati N, Friedman AC, Lautin JL, Braha S, Kadish EG, Haramiti N, et al. Radiocontrast-associated renal dysfunction: a comparison of lower-osmolality and conventional high-osmolality contrast media. [Published erratum appears in AJR Am J Roentgenol 1991;157:895; see comments] AJR Am J Roentgenol. 1991;157:59–65. doi: 10.2214/ajr.157.1.2048540. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg LS, Kurnik PB, Kurnik BR. Radiocontrast-induced nephropathy in humans: role of renal vasoconstriction. Kidney Int. 1992;41:1408–1415. doi: 10.1038/ki.1992.206. [DOI] [PubMed] [Google Scholar]
- 5.Agmon Y, Peleg H, Greenfeld Z, Rosen S, Brezis M. Nitric oxide and prostanoids protect the renal outer medulla from radiocontrast toxicity in the rat. J Clin Invest. 1994;94:1069–1075. doi: 10.1172/JCI117421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liss P, Nygren A, Olsson U, Ulfendahl HR, Erikson U. Effects of contrast media and mannitol on renal medullary blood flow and red cell aggregation in the rat kidney. Kidney Int. 1996;49:1268–1275. doi: 10.1038/ki.1996.181. [DOI] [PubMed] [Google Scholar]
- 7.Liss P, Nygren A, Revsbech NP, Ulfendahl HR. Measurements of oxygen tension in the rat kidney after contrast media using an oxygen microelectrode with a guard cathode. Adv Exp Med Biol. 1997;411:569–576. doi: 10.1007/978-1-4615-5865-1_70. [DOI] [PubMed] [Google Scholar]
- 8.Liss P, Nygren A, Hansell P. Influence of iotrolan on renal cortical and outer medullary blood flow in the rat. Acad Radiol. 1998;5(suppl 1):123–126. doi: 10.1016/s1076-6332(98)80080-9. [DOI] [PubMed] [Google Scholar]
- 9.Liss P, Nygren A, Hansell P. Hypoperfusion in the renal outer medulla after injection of contrast media in rats. Acta Radiol. 1999;40:521–527. doi: 10.3109/02841859909175578. [DOI] [PubMed] [Google Scholar]
- 10.Heyman SN, Brezis M, Epstein FH, Spokes K, Rosen S. Effect of glycine and hypertrophy on renal outer medullary hypoxic injury in ischemia reflow and contrast nephropathy. Am J Kidney Dis. 1992;19:578–586. doi: 10.1016/s0272-6386(12)80838-9. [DOI] [PubMed] [Google Scholar]
- 11.Brezis M, Greenfeld Z, Herman M, Meyer JJ, Heyman SN, Rosen S. Experimental nephrotoxicity of the radiocontrast agents iohexol, ioxaglate, and iothalamate. An in vitro and in vivo study. Invest Radiol. 1991;26:325–331. doi: 10.1097/00004424-199104000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Cantley L, Spokes K, Clark B, McMahon E, Carter J, Epstein F. Role of endothelin and prostaglandins in radiocontrast-induced renal artery constriction. Kidney Int. 1993;44:1217–1223. doi: 10.1038/ki.1993.371. [DOI] [PubMed] [Google Scholar]
- 13.Heyman SN, Brezis M, Reubinoff CA, Greenfeld Z, Lechene C, Epstein FH, Rosen S. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82:401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamoto M, Shapiro JI, Shanley PF, Chan L, Schrier RW. In vitro and in vivo protective effect of atriopeptin III on ischemic acute renal failure. J Clin Invest. 1987;80:698–705. doi: 10.1172/JCI113124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto K, Wilson DR, Baumal R. Outer medullary circulatory defect in ischemic acute renal failure. Am J Pathol. 1984;116:253–261. [PMC free article] [PubMed] [Google Scholar]
- 16.Shaw SG, Weidmann P, Hodler J, Zimmermann A, Paternostro A. Atrial natriuretic peptide protects against acute ischemic renal failure in the rat. J Clin Invest. 1987;80:1232–1237. doi: 10.1172/JCI113197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guan Z, Miller SB, Greenwald JE. Zaprinast accelerates recovery from established acute renal failure in the rat. Kidney Int. 1995;47:1569–1575. doi: 10.1038/ki.1995.220. [DOI] [PubMed] [Google Scholar]
- 18.Hammerman MR, Miller SB. Therapeutic use of growth factors in renal failure [editorial] J Am Soc Nephrol. 1994;5:1–11. doi: 10.1681/ASN.V511. [DOI] [PubMed] [Google Scholar]
- 19.Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci U S A. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI [see comments] Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 21.Prasad P, Chen Q, Goldfarb J, Epstein F, Edelman R. Breath-hold mapping with a multiple gradient recalled echo sequence: application to the evaluation of intra-renal oxygenation. J Magn Reson Imaging. 1997;7:1163–1165. doi: 10.1002/jmri.1880070633. [DOI] [PubMed] [Google Scholar]
- 22.Brezis M, Rosen S. Hypoxia of the renal medulla — its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- 23.Prasad PV, Epstein FH. Changes in renal medullary pO2 during water diuresis as evaluated by blood oxygenation level-dependent magnetic resonance imaging: effects of aging and cyclooxygenase inhibition. Kidney Int. 1999;55:294–298. doi: 10.1046/j.1523-1755.1999.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priatna A, Epstein FH, Spokes K, Prasad PV. Evaluation of changes in intrarenal oxygenation in rats using multiple gradient-recalled echo (mGRE) sequence. J Magn Reson Imaging. 1999;9:842–846. doi: 10.1002/(sici)1522-2586(199906)9:6<842::aid-jmri12>3.0.co;2-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brezis M, Rosen S, Epstein F. The pathophysiological implications of medullary hypoxia. Am J Kidney Dis. 1989;13:253. doi: 10.1016/s0272-6386(89)80062-9. [DOI] [PubMed] [Google Scholar]
- 26.Brezis M, Heyman SN, Dinour D, Epstein FH, Rosen S. Role of nitric oxide in renal medullary oxygenation. Studies in isolated and intact rat kidneys. J Clin Invest. 1991;88:390–395. doi: 10.1172/JCI115316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heyman SN, Brezis M, Epstein FH, Spokes K, Silva P, Rosen S. Early renal medullary hypoxic injury from radiocontrast and indomethacin. Kidney Int. 1991;40:632–642. doi: 10.1038/ki.1991.255. [DOI] [PubMed] [Google Scholar]
- 28.Baisac J, Henrich WL. Nephrotoxicity of nonsteroidal anti-inflammatory drugs. Miner Electrolyte Metab. 1994;20:187–192. [PubMed] [Google Scholar]
- 29.Gregg NJ, Elseviers MM, De Broe ME, Bach PH. Epidemiology and mechanistic basis of analgesic-associated nephropathy. Toxicol Lett. 1989;46:141–151. doi: 10.1016/0378-4274(89)90123-9. [DOI] [PubMed] [Google Scholar]
- 30.Maxwell R, Robinson S, McIntyre D, Griffiths J, Vojnovic B. Comparison of changes in gradient-echo 1H MR image intensity and pO2 in rodent tumours in response to carbogen breathing. Proceedings of the 7th Annual Meeting of ISMRM; Philadelphia. 1999. p. 495. [Google Scholar]
- 31.Kone B. A “BOLD” new approach to renal oxygen economy. Circulation. 1996;94:3067–3068. doi: 10.1161/01.cir.94.12.3067. [DOI] [PubMed] [Google Scholar]


