Abstract
Changes in intrarenal oxygenation in rats during pharmacological stimuli were evaluated with a multiple gradient-recalled echo (mGRE) sequence. With administration of the loop diuretic furosemide, oxygenation in the medulla improved; acetazolamide, a proximal tubular diuretic, produced no significant change. These results are consistent with our previous studies in humans and resemble earlier studies of medullary oxygenation using oxygen microelectrodes in anesthetized rats. The technique may be useful in the evaluation of therapeutic strategies in animal models of pathophysiological states such as acute renal failure.
Index terms: magnetic resonance imaging, renal medullary hypoxia, pO2, blood oxygenation, BOLD, rats
Blood oxygenation level-dependent (bold) MRI has previously been shown to be useful in evaluating intrarenal oxygenation (1), which in turn is believed to be helpful in understanding the pathophysiology of ischemic renal diseases like acute renal failure (2). Because of the non-invasive nature of this technique, it is readily applicable to human subjects as well as animals and permits sequential studies to monitor pathophysiological changes and to evaluate therapeutic strategies.
We have suggested that changes in within human kidneys are related to changes in regional oxygenation (1). Renal medullary oxygenation improves following administration of the loop diuretic furosemide, which inhibits active transport in medullary tubules; minimal changes were observed with acetazolamide, which acts on proximal convoluted tubules located in the renal cortex. These results were consistent with earlier invasive microelectrode measurements performed in rats (3). The preliminary implementation of the BOLD MRI technique was done using echoplanar imaging (EPI) (1). Similar results were obtained with a multiple gradient-recalled echo (mGRE) sequence (4). The advantage of the mGRE sequence over EPI is that its implementation does not necessitate specialized hardware and software, making its availability more universal. In addition, the mGRE sequence allows improved spatial resolution. Conventional (single) gradient-recalled echo (GRE) sequences have been previously used for BOLD MRI in human brain (5) and in kidneys of anesthetized rats (6). These techniques monitor signal intensity changes in -weighted images. Our approach has been to use as a BOLD parameter, since it is more directly related to blood oxygenation compared with -weighted signal intensity. In principle, changes in would reflect both changes in regional R2 and/or changes in susceptibility (related to changes in blood oxygenation). In our previous study (1), we have demonstrated that only and not R2 showed a significant change when furosemide was administered, confirming that the observed changes in were related to changes in blood oxygenation. We have implemented a multiple GRE sequence, which allows calculation of maps at sufficiently high spatial resolution for application to rat kidneys.
The present study was undertaken to determine whether this technique is useful for evaluating intrarenal oxygenation in animal models, specifically rats. We considered this important because 1) previous studies using invasive microelectrodes to study effects of different diuretics were performed in rats (3); and 2) most of the pathophysiological models of ischemic renal diseases utilize rat kidneys (7–9). The availability of the proposed technique would allow serial measurements in a single animal to monitor pathophysiological changes. Specific aims for the present study were to determine whether 1) measurements at adequate spatial resolution for the application in rats can be obtained on a whole-body clinical scanner; and 2) as a qualitative validation of the technique, changes in following administration of furosemide and acetazolamide follow the same trends as previously seen in humans (1) and using microelectrode measurements, in rats (3).
MATERIALS AND METHODS
A total of 12 anesthetized (Inactin 100 mg/kg) Sprague-Dawley rats (423 ± 35 gs) were studied. Six rats were studied with administration of furosemide (10 mg/kg), a diuretic agent that inhibits reabsorption in thick ascending limbs, and six more with acetazolamide (100 mg/kg), an inhibitor of proximal tubular transport (3). The femoral vein was catheterized for administration of drugs. All experiments were approved by our institutional committee for animal studies.
All studies were performed on Siemens 1.5 T Magnetom Vision (Siemens Medical Systems, Erlangen, Germany) whole-body scanner. We utilized a standard Siemens extremity (wrist) circularly polarized flexible coil (14″ × 6″) for signal reception. The body coil was used for transmission. The rat was placed in a right lateral position, and the flexible coil was wrapped around it. A 16-echo mGRE sequence was used with the following parameters: TR 76 msec, TE 7.5–61.7 msec, no. of acquisitions 4, flip angle 30°, field of view 78 × 125 mm2, matrix size 160 × 256, and slice thickness 3 mm. Single-slice images were acquired during free breathing in about 49 seconds. The slope of fitted ln (signal intensity) vs. echo time was performed at each pixel to measure . This calculation was done on-line as a part of the reconstruction algorithm. At the end of the acquisition, 17 images are output 16 -weighted GRE images and a calculated map. A selective water excitation pulse to eliminate fat signals was used (4,10).
Typically, four to six imaging slices to cover both the kidneys were obtained. This was performed before and after the injection of furosemide or acetazolamide. In a few animals, images at a single slice location were acquired every 2 minutes before, during, and after administration of the diuretic to observe the temporal signal changes.
The was measured using regions of interests (consisting of at least 6 pixels) in the renal medulla and cortex. Statistical significance of the differences in pre-vs. post-diuretic values was assessed based on a two-tailed Student’s t-test.
RESULTS
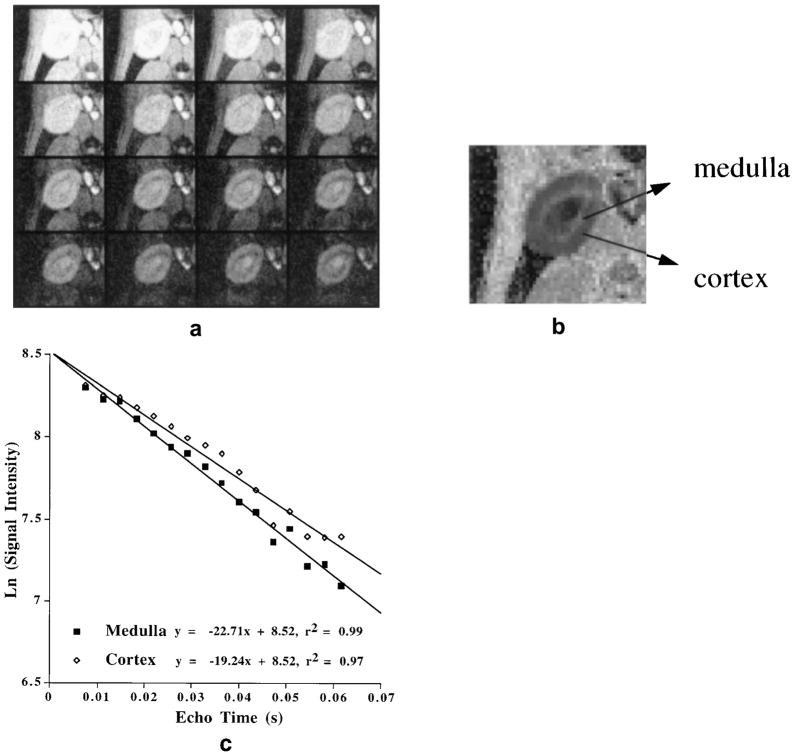
Figure 1a shows a typical data set consisting of 16 gradient-echo images with varying echo times obtained with the mGRE sequence. Figure 1b shows the calculated map obtained from the data set in Fig. 1a. One can clearly visualize the cortex and medulla owing to differences in regional oxygenation; a bright medulla indicates more deoxygenated blood. Figure 1c illustrates a typical example of numerical multiple echo data used for calculation of .
Figure 1.
a: A typical set of data acquired with mGRE sequence. Shown are the 16 images with TE of 7.5–61.7 msec. The signal-to-noise ratios were as follows: cortex = 32 and medulla = 31 (top left image); cortex = 12 and medulla (Image #16) = 9 (bottom right image). Signal was measured as the mean value of a region of interest, and noise was measured as the standard deviation in the background. b: The map calculated from a, performed as a part of the image reconstruction software on the scanner. The map was obtained by estimating the slope of the straight line fit to the ln (signal intensity) vs. echo time at each pixel. Note that the medulla appears brighter than the cortex, indicating less tissue oxygenation in the medulla. c: Plot of ln (signal intensity) vs. echo time, obtained by placing regions of interest in the medulla and cortex of each image. The slope of the straight line is used to estimate . The figure demonstrates the quality of the multiple echo data and the resultant fit.
Figure 2 charts the changes in the signal in the medulla and cortex as a function of time after the injection of furosemide. There is a significant and sharp drop in the medullary value immediately following the injection of furosemide. The stays low over the 20 minute period in which data were collected. There is also a drop in cortical after the injection of furosemide, but its relative magnitude is less than that of the medulla.
Figure 2.
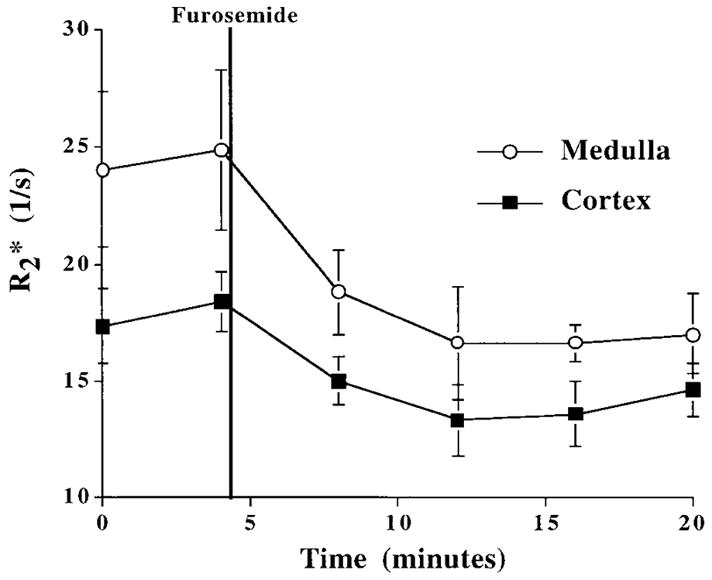
Changes in in the medulla and cortex after injection of furosemide. The first two points for both curves are baseline (pre-injection). Both medullary and cortical drop after the injection of furosemide and stay relatively constant over the 20 minute period of observation. Error bars represent standard deviation in the individual ROI measurements.
Figure 3 displays the average changes ( ) before and 10 minutes after the injection of furosemide or acetazolamide in all animals studied. After furosemide, there is a significant (P < 0.01) decrease in the of the medulla, suggesting increased medullary oxygenation during diuresis. This observation is consistent with the changes in human kidneys we have reported previously using EPI (1). There is also a smaller, although statistically significant, change in cortical . On the other hand, after the administration of acetazolamide, neither medullary nor cortical changed significantly.
Figure 3.
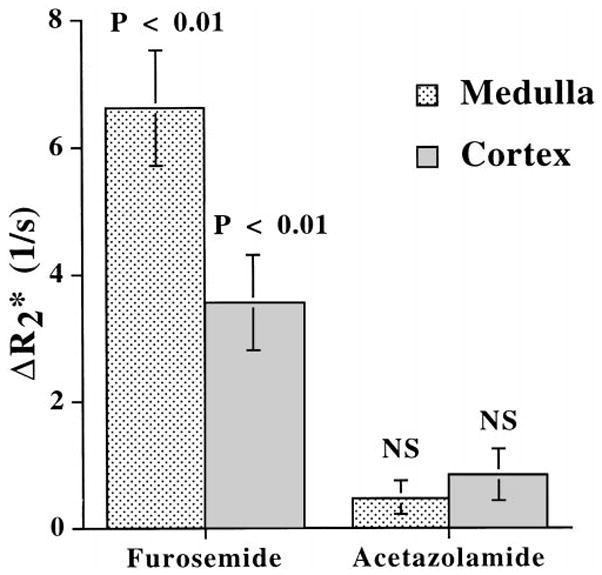
of the rat’s medulla and cortex after the administration of either furosemide or acetazolamide (n = 6 for each). Data are mean ± standard error. NS = P > 0.05.
Table 1 summarizes the measurements before and 10 minutes after administration of the diuretic agent.
Table 1.
R2* (s−1) Measurements (Mean ± SE) Pre- and Post-Injection of Furosemide and Acetazolamide in Rats With the mGRE Sequence
| Furosemide (n = 6) |
Acetazolamide (n = 6) |
|||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Medulla | 23.88 ± 0.84 | 17.26 ± 0.56 | 22.35 ± 0.98 | 21.89 ± 1.10 |
| (P < 0.01)* | NS** | |||
| Cortex | 18.41 ± 1.26 | 14.86 ± 0.39 | 18.37 ± 1.08 | 17.52 ± 0.9 |
| (P < 0.01)* | NS** | |||
Statistical significance based on two-tailed Student’s t-test.
NS = not significant, at P > 0.05.
DISCUSSION
We have demonstrated that the mGRE sequence can be used for evaluating changes in intrarenal oxygenation in small animals using a whole-body scanner. The ranges of echo times used were selected with specific interest for the kidneys ( values ~20 s−1). With the spatial resolution of 0.49 × 0.49 mm, the cortico-medullary differentiation in the maps is striking. The difference between the mGRE sequence designed for the animal models and that of the human studies lies in the echo times. The echo spacing was made longer to accommodate a smaller field of view. Owing to the relatively long echo times used, the mGRE sequence is sensitive to susceptibility artifacts, eg, due to the presence of bowel gas. To minimize these, the rats were placed on their right side so that the intestines no longer overlaid the kidneys. Because in this position, the two kidneys in rats usually do not lie in the same principal (x, y, or z) plane, this limits acquisition of data from both kidneys within the same transverse slice. We preferred the use of straight transverse slices over oblique orientation to make the image interpretation simpler. However, since the changes we are dealing with last much longer than the acquisition times for a single slice (~1 minute), we could still cover both kidneys with multiple slice positions. In principle, higher field strength would improve the sensitivity of the technique, and most small animal MRI imagers function at higher field strengths. However, application of the BOLD technique in the abdomen would have to take into consideration the inherent sensitivity to susceptibility artifacts. Thus the feasibility of using a commercial whole body scanner for this application in small animals is significant.
The large decrease observed in medullary following administration of furosemide implies an increase in oxygenation of the renal medulla, consistent with our previous results in humans (1). The BOLD MRI technique, as described here, is yet to be directly validated against simultaneous direct measurements of tissue oxygenation owing to current technical limitations. However, the results are in good agreement with previously reported intrarenal measurements using oxygen micro-electrodes performed in anesthetized rats (3). Recently, others have shown that BOLD MR data correlated well with oxygen microelectrode measurements in tumors (11). Unlike our results in humans, we found that cortical also falls significantly although less markedly in rat kidneys following administration of furosemide, perhaps because of the action of furosemide to decrease active transport in the ascending limbs located in medullary rays within the rat renal cortex. After administration of acetazolamide, on the other hand, we observed minimal change in , either in renal medulla or in cortex. Nevertheless, the mean change in following administration of acetazolamide was slightly higher in the cortex (P < 0.09) than in the medulla (P = 0.15), a trend consistent with the previous micro-electrode measurements (3) although not statistically significant.
Many experiments using rat models for ischemic renal disease and particularly acute renal failure have been reported (6,7,12,13); based on such models, therapeutic strategies have been evaluated (8,9,14–16). Such strategies are usually hypothesized to improve or to maintain medullary oxygenation status. Up to now, a limitation in the effective testing of these hypotheses has been the absence of a non-invasive, reproducible method to assess regional oxygenation of rat kidneys, a technique now available with BOLD MRI.
CONCLUSIONS
Changes in intrarenal oxygenation due to pharmacologic stimulation in rats can be evaluated with the multiple gradient-echo sequence. The changes in the of rat medulla and cortex resemble those previously demonstrated in human kidneys and earlier investigations of rat kidney using oxygen microelectrodes. The availability of this technique may allow studies on pathophysiological models of renal disease such as acute renal failure, to elucidate their pathophysiology and to evaluate means of prevention and treatment.
Acknowledgments
This work was supported by a grant-in-aid from the American Heart Association (to P.V.P.) and grants from the National Institutes of Health, DK 53221 (to P.V.P.) and DK 18078 (to F.H.E.).
References
- 1.Prasad PV, Edelman RR, Epstein FH. Noninvasive evaluation of intrarenal oxygenation with BOLD MRI. Circulation. 1996;94:3271–3275. doi: 10.1161/01.cir.94.12.3271. [DOI] [PubMed] [Google Scholar]
- 2.Kone B. A ‘BOLD’ new approach to renal oxygen economy. Circulation. 1996;94:3067–3068. doi: 10.1161/01.cir.94.12.3067. [DOI] [PubMed] [Google Scholar]
- 3.Brezis ML, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation, 1: effects of diuretics. Am J Physiol. 1994;267:F1059–F1062. doi: 10.1152/ajprenal.1994.267.6.F1059. [DOI] [PubMed] [Google Scholar]
- 4.Prasad PV, Chen Q, Goldfarb JW, Epstein FH, Edelman RR. Breathold R2* mapping with a multiple gradient-recalled echo sequence: application to the evaluation of intrarenal oxygenation. J Magn Reson Imaging. 1997;7:1163–1165. doi: 10.1002/jmri.1880070633. [DOI] [PubMed] [Google Scholar]
- 5.Haacke EM, Hopkins AL, Lai S, et al. 2D and 3D high resolution gradient echo functional imaging of the brain: venous contributions to signal in motor cortex studies. NMR Biomed. 1994;7:54–62. doi: 10.1002/nbm.1940070109. [DOI] [PubMed] [Google Scholar]
- 6.Trillaud H, Delalande C, Quesson B, Combe C, Moonen C, Grenier N. BOLD MR signal changes due to hypoxia and renal vasoconstriction in rat kidney. Proceedings of the 6th Annual Meeting of the International Society of Magnetic Resonance in Medicine; Sydney: International Society of Magnetic Resonance in Medicine; 1998. p. 396. [Google Scholar]
- 7.Heyman SN, Brezis M, Reubinoff CA, et al. Acute renal failure with selective medullary injury in the rat. J Clin Invest. 1988;82:401–412. doi: 10.1172/JCI113612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaw SG, Weidman P, Hodler J, Zimmerman A, Paternostro A. Atrial natriuretic peptide protects against acute ischemic renal failure in the rat. J Clin Invest. 1987;80:1232–1237. doi: 10.1172/JCI113197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan Z, Miller SB, Greenwald JE. Zaprinast accelerates recovery from established acute renal failure in the rat. Kidney Int. 1995;47:1569–1575. doi: 10.1038/ki.1995.220. [DOI] [PubMed] [Google Scholar]
- 10.Thomasson DM, Moore JR, Purdy DE, Finn JP. Optimized water excitation using phase modulated 1-2-1 binomial pulse train (abstract). Proceedings of the 2nd Annual Meeting of the Society of Magnetic Resonance; San Francisco: Society of Magnetic Resonance; 1994. p. 797. [Google Scholar]
- 11.Al-Hallaq HA, River JN, Zamora M, Oikawa H, Karczmar GS. Correlation of magnetic resonance and oxygen microelectrode measurements of carbogen-induced changes in tumor oxygenation. Int J Radiat Oncol Biol Phys. 41:151–159. doi: 10.1016/s0360-3016(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 12.Yamamoto K, Wilson DR, Baumal R. Outer medullary circulatory defect in ischemic acute renal failure. Am J Pathol. 1984;116920:253–261. [PMC free article] [PubMed] [Google Scholar]
- 13.Nakamoto M, Shapiro JI, Shanley PF, Chan L, Schrier RW. In vitro and in vivo protective effect of atriopeptin III on ischemic acute renal failure. J Clin Invest. 1987;80:698–705. doi: 10.1172/JCI113124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cantley LG, Spokes K, Clark B, McMahon EG, Carter J, Epstein FH. Role of endothelin and prostaglandins in radiocontrast-induced renal artery constriction. Kidney Int. 1993;44:1217. doi: 10.1038/ki.1993.371. [DOI] [PubMed] [Google Scholar]
- 15.Kelly KJ, Williams WW, Colvin RB, et al. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci USA. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammerman MR, Miller SB. Therapeutic use of growth factors in renal failure [Editorial] J Am Soc Nephrol. 1994;5:1–11. doi: 10.1681/ASN.V511. [DOI] [PubMed] [Google Scholar]