Abstract
Individualization of topotecan dosing reduces inter-patient variability in topotecan exposure, presumably reducing toxicity and increasing efficacy. However, logistical limitations (e.g., requirement for plasma, intensive bedside plasma processing) have prevented widespread application of this approach to dosing topotecan. Thus, the objectives of the present study were to develop and validate an HPLC with fluorescence detection method to measure topotecan lactone in whole blood samples and to evaluate its application to individualizing topotecan dosing. Plasma samples (200 μL) were prepared using methanolic precipitation, a filtration step, and then injection of 100 μL of the methanolic extract onto a Novapak® C18, 4μm, 3.9 × 150 mm column with an isocratic mobile phase. Analytes were detected with a Shimadzu Fluorescence RF-10AXL detector with an excitation and emission wavelength of 370 nm and 520 nm, respectively. This method has a lower limit of quantification of 1 ng/mL (S/N ≥5; RSD 4.9%), was validated over a linear range of 1 to 100 ng/mL, and results from a 5-day validation study demonstrated good within-day and between-day precision and accuracy. Data are presented to demonstrate that the present method can be used with whole blood samples to individualize topotecan dosing in children with cancer.
Keywords: topotecan, HPLC, whole blood, pharmacokinetics
INTRODUCTION
Topotecan, a topoisomerase I inhibitor, has been widely evaluated in many pediatric cancers including neuroblastoma, medulloblastoma, rhabdomyosarcoma, and acute leukemias (Furman et al., 2002, Houghton et al., 1995, Houghton et al., 1992, Pratt et al., 1994, Santana et al., 2003, Stewart et al., 2004). Due to the inherent variability in topotecan disposition, investigators have used pharmacokinetically guided dosing to individualize topotecan for patients based upon their systemic clearance and the goals of the clinical trial (Metzger et al., 2007; Montazeri et al., 2002; Santana et al., 2005; Stewart et al., 2004).
As depicted in Figure 1, topotecan undergoes a reversible, pH-dependent hydrolysis to an inactive carboxylate form. This complicates the approach to pharmacokinetically guided topotecan dosing since measurement of the lactone form is preferable as it is the pharmacological active moiety (Staker et al., 2002). Thus, in our institution topotecan dosing is individualized for each patient based upon that patient’s topotecan lactone systemic clearance with the goal to achieve a pre-defined drug exposure or area under the concentration-time profile (Metzger et al., 2007; Santana et al., 2005; Stewart et al., 2004).
Figure 1.
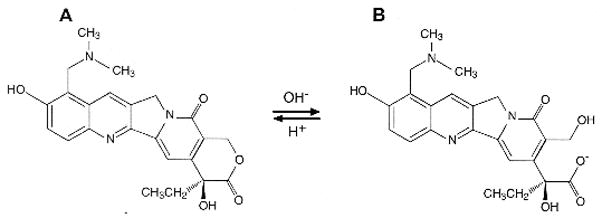
Structures of topotecan lactone (a) and carboxylate (b).
The response rate for this approach has been promising, but individualized dosing of topotecan has limitations. Measurement of topotecan plasma lactone concentrations requires immediate bedside processing into dry-ice methanol in order to minimize interconversion between lactone and carboxylate forms of the drug (Bai et al., 2003; Beijnen et al., 1990). This process is logistically demanding and requires well-trained personnel. Thus, the availability of trained personnel and the presence of equipment needed for immediate sample processing may hinder implementation of individualized topotecan dosing at an institution. To increase the likelihood that this approach can be incorporated into routine clinical practice, it will be important to make all aspects of the current dosing approach accessible to the clinician.
By removing the need for intensive bedside sample processing and using a biological matrix like whole blood, individualized topotecan dosing could be made much more accessible to clinicians and patients. An HPLC technique for the determination of topotecan in whole blood has been previously published (Loos et al., 2000a; Loos et. al., 2000b). However, this technique requires extensive sample preparation as well as a run time of 20 minutes. Hence, the objectives of the present study were to develop and validate an HPLC with fluorescence detection method for the measurement of topotecan lactone in whole blood samples and to evaluate its application to individualizing topotecan dosing in pediatric patients with cancer.
EXPERIMENTAL
Chemicals and reagents
Topotecan used for preparation of standards and quality control samples was supplied by GlaxoSmithKline (Collegeville, PA, USA). HPLC grade methanol was purchased from Burdick & Jackson (Muskegon, MI, USA), whereas dioctylsulfosuccinate sodium salt, 96% (DOSS), was obtained from ACROS Organics (New Jersey, NJ, USA). Monobasic sodium phosphate, dibasic sodium phosphate, hydrochloric acid, and 85% o-phosphoric acid were acquired from Fisher Scientific (Fairlawn, NJ, USA). Triethylamine (TEA), 99%, was obtained from Sigma-Aldrich (St. Louis, MO, USA). Blank human whole blood was obtained from volunteers with informed consent. All water was distilled, deionized, and further purified via a Millipore Milli-QUV plus and Ultra-Pure Water System (resistance 18.2 MΩ) (Tokyo, Japan).
Equipment and chromatographic conditions
The HPLC system consisted of a Shimadzu LC-20AD pump, a Shimadzu SIL-20AC autoinjector which was cooled at 4°C and a Shimadzu Fluorescence RF-10AXL detector with an excitation and emission wavelength of 370 nm and 520 nm, respectively. All the modules were controlled by a Shimadzu CBM-20A communications bus module (Tokyo, Japan). A Shimadzu Class-VP chromatography data system was used for data acquisition and processing. Separations were obtained on a Novapak® C18, 4μm, 3.9 × 150 mm column (Waters, Milford, MA, USA) with a μBondapak C18, 3.9 × 20 mm, 10μm guard column (Waters, Milford, MA, USA) kept at ambient temperature. Analytes were isocratically eluted using a mobile phase delivered at 1 mL/min consisting of methanol-aqueous (58:42, v/v) mobile phase containing 36mM sodium phosphate buffer (pH 6.0), 8.75 mM DOSS, and 0.003% TEA, adjusted to pH 6.5 with phosphoric acid and filtered with a 0.2 μm PALL membrane (Port Washington, NY, USA). An Orion 250A pH meter (Beverly, MA, USA) was used for mobile phase preparation and a Labnet microcentrifuge (Edison, NJ, USA) was used for sample preparation.
Preparation of stock and working stock solutions
A 1 mg/mL stock solution was prepared by dissolving topotecan hydrochloride into 0.01 N HCl. Aliquots of the stock solution were stored in amber vials at −80°C. Appropriate dilutions of the stock solution were made in 0.01 N HCl to obtain working stock solutions at concentrations of 10, 1, and 0.1 μg/mL. When not in use, these working stock solutions were stored at −80°C.
Calibration curves
Whole blood calibration standards were prepared by adding topotecan working stock solutions into drug-free, lysed (frozen overnight at −20°C) whole blood to achieve the following concentrations: 1, 5, 20, 70, and 100 ng/mL. Quality control samples were prepared in the same manner to obtain final concentrations of 7, 30, and 80 ng/mL. Two separate calibration curves were obtained by using linear regression of peak height of the calibration standard samples weighted against the inverse square of the concentrations (1/X2). To consider an analytical run acceptable, the correlation coefficients (r2) of the calibration curves was at least 0.99, and quality control samples must be within 10% of their nominal values.
Sample preparation procedure
Calibrators and controls were deproteinized by adding 200 μL of spiked sample to 800 μL dry-ice cold methanol. After deproteinization, samples were vortexed for 10s, incubated on wet ice for 5 minutes, vortexed again for 10s, and centrifuged at 15000 g for 2 min (4°C). Methanolic supernatants were transferred to Spin-X HPLC microcentrifuge tubes (0.2 μm nylon filter, Costar, Corning, NY, USA) and re-centrifuged at 15000 g for 2 min (4°C) in order to separate out any particulates remaining in the sample. The filtrate was decanted to a screw-top vial and stored at −80°C. The supernatant was transferred into an HPLC sample vial and 100 μL aliquots were injected onto the HPLC system.
Specificity and selectivity
Drug-free whole blood obtained from three different healthy donors was processed and analyzed to determine whether endogenous whole blood constituents interfered with the topotecan lactone signal. In addition, drug-free whole blood was spiked with 50 ng/mL topotecan lactone in order to identify the retention time for topotecan.
Lower limit of quantification (LLOQ)
To determine the lower limit of quantification (LLOQ), three separate samples within the lower range of the calibration curve (1, 2.5, and 5 ng/mL) were prepared in whole blood, extracted, and analyzed. The LLOQ was defined as the lowest concentration of the analyte that could be reproducibly quantified with suitable precision within (±) 10% relative standard deviation (RSD), an accuracy ± 10% deviation from the nominal value, and a signal to noise ratio ≥5.
Precision and accuracy
Intra-day precision and accuracy was evaluated by analyzing ten low (7 ng/mL), ten medium (30 ng/mL), and ten high (80 ng/mL) replicates of each concentration within a single analytical run in one day. Inter-day precision and accuracy was evaluated by analyzing five low (7 ng/mL), five medium (30 ng/mL), and five high (80 ng/ml) replicates of each concentration for three consecutive days. All samples were prepared in bulk and processed in the same manner as outlined in the sample preparation procedure section. The criteria for acceptability of the data included an accuracy ± 10% of deviation from the nominal value and a precision within ±10% of RSD.
Stability experiments
The short-term stability of topotecan lactone in lysed whole blood was determined by analyzing quality control (QC) whole blood samples at two levels (7 ng/mL and 80 ng/mL) kept at room temperature (~25°C) for 0, 1, and 3 hours, and at 4°C for 0, 1, 3 and 6 hours. Long-term stability of topotecan lactone in whole blood was assessed by analyzing the QC samples stored at −80°C for 0, 1, 7, 15, and 28 days. Topotecan stability in lysed whole blood was also assessed after two freeze-thaw cycles. In addition, the stability of topotecan in processed samples was determined by analyzing the extracted samples kept at 4°C in the autoinjector for up to 8 hours. All samples were prepared in bulk and processed in the same manner as outlined in the sample preparation procedure section. Sample stability limitations were defined as the last time point where values were ±10% of the value obtained from the freshly prepared solutions.
Application of method
The pharmacokinetic analysis of topotecan lactone in plasma versus topotecan lactone in whole blood was carried out in 14 pediatric patients. Serial blood samples (2mL) were obtained from a site contralateral to the site of infusion after an intravenous dose of topotecan between 1.7 and 3.5 mg/m2. Each blood sample was collected in a green-top (heparin sodium) vacutainer tube (Franklin Lakes, NJ, USA). Next, 500 μL of whole blood was removed and immediately placed in a polypropylene screw-top tube, then stored on dry ice until transferred to a −80°C freezer. The remaining whole blood was centrifuged at 15000 g for 2 minutes (4°C) to obtain plasma. Plasma was then deproteinized by adding 200 μL to 800 μL of dry-ice cold methanol, vortexed for 10 s, and centrifuged at 15000 g for 2 minutes (4°C). The supernatant was decanted into a polypropylene screw-top tube and stored at −80°C until analysis. On the day of analysis, stored whole blood samples were extracted using the method outlined previously in the sample preparation procedure section. The extracts of both topotecan lactone in whole blood and topotecan lactone in plasma were analyzed against freshly prepared calibration curves in their respective matrices.
To utilize the whole blood topotecan lactone values in our pharmacokinetic analysis, we had to use population priors in a maximum a priori Bayesian (MAP-Bayesian) analysis that were derived from plasma topotecan lactone values (see Discussion). Thus, we determined the relationship between topotecan whole blood and plasma lactone concentrations using linear mixed-effects modeling (Team, 2006). This relationship was used to convert the measured topotecan whole blood concentrations to equivalent plasma topotecan lactone concentrations.
As topotecan lactone clearance is the primary parameter used to estimate topotecan systemic exposure (i.e., topotecan area under the concentration-time curve from 0 to infinity; AUC0→∞), a two-compartment model was fit to concentration-time data using a maximum a posteriori (MAP)-Bayesian approach as implemented in ADAPT II (D’Argenio et al., 1997). The sample time points for the present group of patients were obtained from a previously developed limited sampling model described elsewhere (Turner et al., 2006). The topotecan lactone clearance was determined from the plasma concentration data using accepted pharmacokinetic equations. The topotecan whole blood values were adjusted to plasma topotecan lactone concentrations using an equation determined from linear mixed-effects modeling (see Application of Method). The topotecan lactone clearance values determined from the plasma concentration data were compared with the topotecan lactone clearance values determined from the whole blood adjusted to plasma topotecan lactone concentration data using linear mixed-effects modeling.
RESULTS AND DISCUSSION
Optimization of the experimental conditions
To optimize the detection and selectivity for this method, the fluorescence detector excitation and emission settings were set to Ex=370 nm and Em=520 nm (Bai et al., 2003). To stabilize the topotecan lactone, the stock and working solutions were made in 0.01 N HCl instead of methanol. An additional sample preparation step was added to further clean the sample prior to injection onto the HPLC system.
Whole blood samples were not effectively cleaned with a simple methanolic precipitation, so an additional sample preparation step was necessary. A small amount of precipitant from the whole blood remained in the supernatant, which clogged the guard column and increased the pressure on the HPLC system. Thus, different approaches were evaluated including further pretreatment with chemicals such as zinc sulfate. The best results were obtained by centrifuging the methanolic supernatant obtained after spiking whole blood with known amounts of working stock solutions in 0.2 μm Spin-X HPLC microcentrifuge tubes at 15000 g for 2 min (4°C). Using this approach, a significant portion of unwanted precipitant was retained onto the nylon filter with minimal loss of sample volume to the membrane. This extra step sharpened up the topotecan lactone peak shape and minimized the pressure increase on the HPLC system.
Once the sample was processed and injected on the HPLC system, an endogenous peak that interfered with the topotecan peak was noted when using a mobile phase with a pH of 6.0. Thus, to allow for separation of the endogenous whole blood peak, the mobile phase pH was modified so that the final pH was 6.5 instead of 6.0. In addition, to achieve better topotecan lactone peak shape, a 4μm C18 analytical column was used.
Specificity and chromatography
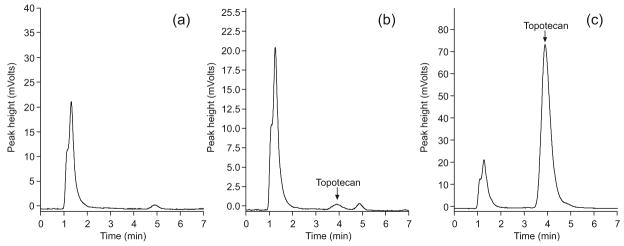
The retention time of topotecan lactone was 3.9 minutes, with a total run time of 7 minutes. Upon analysis of blank human whole blood, no interfering endogenous peaks were found. Chromatograms of representative processed blank whole blood and spiked whole blood with topotecan lactone are depicted in Figure 2. Additional studies were performed to determine whether or not topotecan carboxylate was detectable using this analytical method. Three 0.04 N borax stabilized topotecan carboxylate solutions at low and high concentrations (3 ng/mL and 90 ng/mL) were injected and no carboxylate peak was observed upon analysis of the chromatogram (data not shown). In addition, no conversion from the carboxylate form to the lactone form of topotecan was noted (data not shown).
Figure 2.
Chromatograms showing no interfering peaks in the blank whole blood sample (a), and topotecan elutes at a point separate from other peaks at low (b) and high (c) concentrations. (Note: the scales are different for each panel)
Calibration curves
Both calibration curves for topotecan lactone in human whole blood were linear over a concentration range of 1 ng/mL to 100 ng/mL with correlation coefficients (r2) of 0.995 (y=0.00134x +0.0635) and 0.997 (y=0.00133x +0.00161). Three quality control samples were run with each calibration curve, and these values were within 94–110% of their nominal values.
Lower limit of quantification (LLOQ)
The LLOQ of topotecan lactone in human whole blood was established at 1 ng/mL, with a precision within 4.9% RSD and an accuracy within 93% of the nominal value. In addition, the signal to noise ratio was ≥5. The LLOQ of 1 ng/ml is adequate to individualize topotecan dosages based upon published data from studies (Metzger et al., 2007; Santana et al., 2005; Santana et al., 2003; Stewart et al., 2004).
Precision and accuracy
As shown in Table 1, inter- and intra-day precision and accuracy for topotecan lactone in human whole blood were within acceptable limits. The accuracy of the quality control samples (7 ng/ml, 30 ng/mL, and 80 ng/ml) was close to 100 % (within 93.7 % of the nominal value, Table 1). The inter-and intra-day precision expressed as the relative standard deviation (RSD) of the measurements was less than 10%.
Table 1.
Inter- and Intra-day precision and accuracy of topotecan lactone in whole blood.
| Nominal concentration (ng/ml) | Mean (SD) Measured concentration (ng/ml) | Accuracy (%) | Within-day precision (%RSD) | Between-day precision (%RSD) |
|---|---|---|---|---|
| 7.0 | 7.2 (0.3) | 102.3 | 3.2 | 4.1 |
| 30.0 | 28.1 (2.1) | 93.7 | 2.9 | 7.6 |
| 80.0 | 75.8 (5.1) | 94.8 | 2.6 | 6.7 |
RSD. Relative standard deviation (SD*100/mean)
Stability in human whole blood
The results of the stability studies obtained for topotecan lactone in lysed human whole blood are presented in Table 2. The topotecan lactone concentration in lysed whole blood after 1 hour was approximately 75% of the baseline value (data not shown), and after 3 hours it was approximately 49% of the baseline value, suggesting topotecan lactone is not stable at room temperature for any length of time. Thus, whole blood samples used for this assay method should be kept for no more than 3 hours at 4°C or less until processed for analysis. This is because those samples kept at 4°C for 3 hours retained their concentrations within 92% of the nominal value, but did not retain their concentrations after 6 hours (approximately 82% of nominal value, data not shown). Furthermore, the long-term stability of topotecan lactone under storage conditions of −80°C for 28 days was acceptable (87.9% of the value obtained from a freshly prepared solution), showing a gradual degradation over time (data not shown). After two freeze-thaw cycles, the results were within the defined acceptance criteria as shown in Table 2. Processed topotecan lactone samples were stable at 4°C in the autoinjector for 8 hours and at −80°C for 28 days (Table 3).
Table 2.
Stability of topotecan lactone in lysed human whole blood.
| Nominal Concentration (ng/mL) | Storage condition | Measured Concentration. (ng/mL)a | Accuracy (%)b |
|---|---|---|---|
| 7.00 | 0 h (Baseline) | 6.5 | |
| RT (3 h) | 3.18 | 49.3 | |
| 4°C (3 h) | 6.04 | 93.6 | |
| day 0 (Baseline) | 6.80 | ||
| −80°C (28 days) | 5.98 | 87.9 | |
| 2 F-T | 6.77 | 99.6 | |
| 80.00 | 0 h (Baseline) | 76.2 | |
| RT (3 h) | 40.0 | 52.5 | |
| 4°C (3 h) | 70.4 | 92.4 | |
| day 0 (Baseline) | 78.6 | ||
| −80°C (28 days) | 73.9 | 94.0 | |
| 2 F-T | 76.9 | 97.8 |
F-T= freeze thaw-cycle
RT=Room (ambient) temperature
Average (n=2)
(Average measured concentration/average measured concentration at t=0) × 100
Table 3.
Stability of processed topotecan lactone in human whole blood samples.
| Nominal Concentration (ng/ml) | Storage condition | Measured Concentration (ng/ml)a | Accuracy (%)b |
|---|---|---|---|
| 7.00 | 0 h (Baseline) | 6.94 | |
| 4°C (8 h) | 7.06 | 101.7 | |
| day 0 (Baseline) | 6.82 | ||
| −80°C (28 days) | 6.12 | 89.7 | |
| 80.00 | 0 h (Baseline) | 75.6 | |
| 4°C (8h) | 83.7 | 110.7 | |
| day 0 (Baseline) | 78.6 | ||
| −80°C (28 days) | 74.7 | 95.0 |
Average (n=2)
(Average measured concentration/average measured concentration at t=0) × 100
Application of the method
The proposed HPLC method was used to measure topotecan lactone in whole blood samples obtained from pediatric patients with neuroblastoma and retinoblastoma to compare with topotecan lactone in plasma samples measured by our previously published method (Stewart et al., 1994; Zamboni et al., 1998; Zamboni et al., 1999). As depicted in Figure 3, we found a linear relationship between the topotecan lactone concentrations in both biological matrices. The regression equation that described the linear relation is shown in Figure 3, and on average the topotecan lactone whole blood concentrations were 17% greater compared to the plasma results. This result was expected as it has been previously reported that topotecan binds to red blood cells (Loos et al., 2000). We assessed the relationship between topotecan lactone concentrations in plasma and in whole blood using a linear mixed-effects model.
Figure 3.
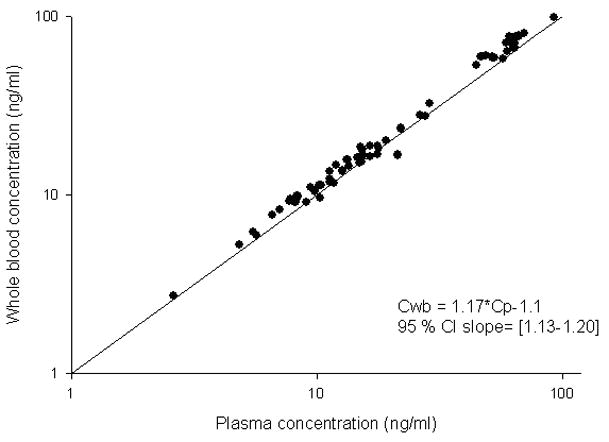
Relation between topotecan lactone measured in plasma and whole blood. Solid circles represent individual measurements and solid line represents the identity line.
Depicted in Figure 4 are the plasma and the converted whole blood concentration-vs-time profiles (using the relationship obtained for plasma and whole blood matched samples) for a representative patient. The actual observations are represented as solid circles while the line shows the simulated concentrations according to the pharmacokinetic parameter estimates obtained for that particular patient.
Figure 4.
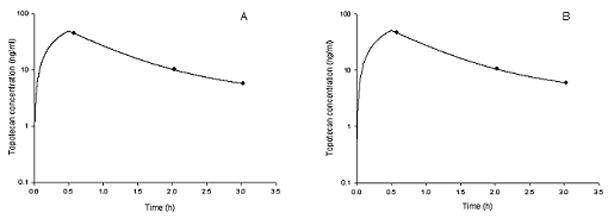
Topotecan lactone concentrations vs. time profile for a representative patient (A). Topotecan lactone in whole blood concentration (ng/ml) vs. time profile obtained from plasma samples (B). Concentration (ng/ml) vs. time profile was obtained from whole blood samples after transforming the concentrations by the relationship between plasma and whole blood concentrations in the same patient. The line represents the best-fit line from the model fit to the patient concentration data.
The median topotecan lactone clearance determined from the plasma concentration data was 133 ng*h/ml (range X to Y) compared with the topotecan lactone clearance value determined from the whole blood adjusted to plasma topotecan lactone concentration data which was 134 ng*h/ml. The mean %bias was 2.3%. Data from two different pharmacokinetic studies performed on the same patient were available for six patients. A good concordance was observed between the topotecan AUC values calculated from plasma and the corrected whole blood concentrations using the relationship found with linear mixed effects model. The %bias was 15% and 19% for two studies in one patient, but was less than 14% for the remainder of the studies. No statistically significant difference was found in the values between the two groups (p>0.05, linear mixed effects model).
Although a direct comparison of the topotecan lactone clearance values calculated from whole blood and plasma would be desirable, that was not possible for this particular example. The initial topotecan pharmacokinetic studies conducted over 10 years ago utilized extensive sampling (e.g., 6 to 8 samples per study per patient), but that was burdensome on the patient, families, and the laboratory. Thus, we developed a pharmacokinetic limited sampling model (LSM) to conduct future dosage individualization studies. This LSM utilizes only 2 or 3 plasma samples per patient per study and MAP-Bayesian pharmacokinetic modeling methods to determine the topotecan lactone clearance for the individual patient. The MAP-Bayesian method depends upon the use of population priors (mean and variance) determined from the extensive plasma sampling studies. Now that LSM are exclusively used in topotecan pharmacokinetic studies, it was not possible to obtain extensive sampling specifically for the purposes of evaluating the whole blood method. Thus, the approach utilized was to convert the whole blood topotecan lactone value to an equivalent topotecan plasma value to use the plasma population priors in the MAP-Bayesian analysis.
Application of this method has numerous advantages over the previously published methods, which will enhance the accessibility of individualized topotecan to the clinician. First, this method uses whole blood and does not require any processing of the blood in the clinic, which simplifies the sample collection step for the clinician. The one caveat determined from this study is that the whole blood sample must be kept at 4°C or less until processed for analysis to maintain the stability of the topotecan lactone. Second, the sample does not require any complex bedside processing to isolate the topotecan lactone form. The processing of the sample that is required for bioanalysis occurs in the laboratory, and only involves one additional step compared with the previous method used. Lastly, results of the pharmacokinetic analysis showed that the whole blood topotecan lactone data could be used to estimate the topotecan lactone AUC values, which is the ultimate test of the clinical utility of this method.
CONCLUSIONS
An HPLC with fluorescence detection method that can be used routinely to measure topotecan lactone whole blood concentration in patient samples has been successfully developed and validated. In addition, the applicability of this method was demonstrated by showing that the topotecan whole blood concentrations correlated linearly with plasma concentrations and could be used for pharmacokinetically guided drug dosing. Thus, application of this method will enhance the ability to individualize topotecan dosing in children with cancer. Furthermore, the simple collection of whole blood will simplify sample collection and provide for uniformity of sample processing, enhancing the likelihood that individualized topotecan dosing can be more routinely performed.
Acknowledgments
This work was supported in part by USPHS awards CA 23099, Cancer Center Support Grant CA 21765, a grant from GlaxoSmithKline, and by ALSAC.
References
- Bai F, Kirstein MN, Hanna SK, Iacono LC, Johnston B, Stewart CF. Determination of plasma topotecan and its metabolite N-desmethyl topotecan as both lactone and total form by reversed-phase liquid chromatography with fluorescence detection. Journal of Chromatography B Analytical Technologies in the Biomedical Life Sciences. 2003;784:225–232. doi: 10.1016/s1570-0232(02)00798-5. [DOI] [PubMed] [Google Scholar]
- Beijnen JH, Smith BR, Keijer WJ, Van Gijn R, Huinink WW, Vlasveld LT, Rodenhuis S, Underberg WJM. High-performance liquid chromatographic analysis of the new antitumour drug SK&F 104864-A (NSC 609699) in plasma. Journal of Pharmaceutical and Biomedical Analysis. 1990;8:789–794. doi: 10.1016/0731-7085(90)80122-6. [DOI] [PubMed] [Google Scholar]
- D’Argenio DZ, Schumitzky A. ADAPT II User’s Guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. Los Angeles: Biomedical Simulations Resource; 1997. [Google Scholar]
- Furman WL, Stewart CF, Kirstein M, Kepner JL, Bernstein ML, Kung F, Vietti TJ, Steuber CP, Becton DL, Baruchel S, Pratt C. Protracted intermittent schedule of topotecan in children with refractory acute leukemia: a pediatric oncology group study. Journal of Clinical Oncology. 2002;20:1617–1624. doi: 10.1200/JCO.2002.20.6.1617. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Cheshire PJ, Hallman JD, Lutz L, Friedman HS, Danks MK, Houghton JA. Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemotherapy and Pharmacology. 1995;36:393–403. doi: 10.1007/BF00686188. [DOI] [PubMed] [Google Scholar]
- Houghton PJ, Chesire PJ, Myers L, Stewart CF, Synold TW, Houghton JA. Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemotherapy and Pharmacology. 1992;31:229–239. doi: 10.1007/BF00685553. [DOI] [PubMed] [Google Scholar]
- Loos WJ, de Bruijn P, Verweij J, Sparreboom A. Determination of camptothecin analogs in biological matrices by high-performance liquid chromatography. Anticancer Drugs. 2000a;11:315–324. doi: 10.1097/00001813-200006000-00001. [DOI] [PubMed] [Google Scholar]
- Loos WJ, Gelderblom HJ, Verweij J, Brouwer E, de Jonge MJ, Sparreboom A. Gender-dependent pharmacokinetics of topotecan in adult patients. Anticancer Drugs. 2000b;11:673–680. doi: 10.1097/00001813-200010000-00001. [DOI] [PubMed] [Google Scholar]
- Metzger ML, Stewart CF, Freeman BB, III, Billups CA, Hoffer FA, Wu J, Coppes MJ, Grant R, Chintagumpala M, Mullen EA, Alvarado C, Daw NC, Dome JS. Topotecan is active against Wilms’ tumor: results of a multi-institutional phase II study. Journal of Clinical Oncology. 2007;25:3130–3136. doi: 10.1200/JCO.2007.10.9298. [DOI] [PubMed] [Google Scholar]
- Montazeri A, Culine S, Laguerre B, Pinguet F, Lokiec F, Albin N, Goupil A, Deporte-Fety R, Bugat R, Canal P, Chatelut E. Individual adaptive dosing of topotecan in ovarian cancer. Clinical Cancer Research. 2002;8:394–399. [PubMed] [Google Scholar]
- Pratt CB, Stewart CF, Santana VM, Bowman L, Furman W, Ochs J, Marina N, Kuttesch JF, Heideman R, Sandlund JT, Avery L, Meyer WH. Phase I study of topotecan for pediatric patients with malignant solid tumors. Journal of Clinical Oncology. 1994;12:539–543. doi: 10.1200/JCO.1994.12.3.539. [DOI] [PubMed] [Google Scholar]
- Santana VM, Furman WL, Billups CA, Hoffer FA, Davidoff AM, Houghton PJ, Stewart CF. Improved response in high-risk neuroblastoma with protracted topotecan administration using a pharmacokinetically guided dosing approach. Journal of Clinical Oncology. 2005;23:4039–4047. doi: 10.1200/JCO.2005.02.097. [DOI] [PubMed] [Google Scholar]
- Santana VM, Zamboni WC, Kirstein MN, Tan M, Liu T, Gajjar A, Houghton PJ, Stewart CF. A pilot study of protracted topotecan dosing using a pharmacokinetically guided dosing approach in children with solid tumors. Clinical Cancer Research. 2003;9:633–640. [PubMed] [Google Scholar]
- Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Jr, Stewart L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Procedings of the National Academy of Sciences USA. 2002;99:15387–15392. doi: 10.1073/pnas.242259599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart CF, Baker SD, Heideman RL, Jones D, Crom WR, Pratt CB. Clinical pharmacodynamics of continuous infusion topotecan in children: systemic exposure predicts hematologic toxicity. Journal of Clinical Oncology. 1994;12:1946–1954. doi: 10.1200/JCO.1994.12.9.1946. [DOI] [PubMed] [Google Scholar]
- Stewart CF, Iacono LC, Chintagumpala M, Kellie SJ, Ashley D, Zamboni WC, Kirstein MN, Fouladi M, Seele LG, Wallace D, Houghton PJ, Gajjar A. Results of a phase II upfront window of pharmacokinetically guided topotecan in high-risk medulloblastoma and supratentorial primitive neuroectodermal tumor. Journal of Clinical Oncology. 2004;22:3357–3365. doi: 10.1200/JCO.2004.10.103. [DOI] [PubMed] [Google Scholar]
- Team RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Turner PK, Iacono LC, Panetta JC, Santana VM, Daw NC, Gajjar A, Stewart CF. Development and validation of limited sampling models for topotecan lactone pharmacokinetic studies in children. Cancer Chemotherapy and Pharmacology. 2006;57:475–482. doi: 10.1007/s00280-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Zamboni WC, Gajjar AJ, Heideman RL, Beijnen JH, Rosing H, Houghton PJ, Stewart CF. Phenytoin alters the disposition of topotecan and N-desmethyl topotecan in a patient with medulloblastoma. Clinical Cancer Research. 1998;4:783–789. [PubMed] [Google Scholar]
- Zamboni WC, Houghton PJ, Hulstein JL, Kirstein M, Walsh J, Cheshire PJ, Hanna SK, Danks MK, Stewart CF. Relationship between tumor extracellular fluid exposure to topotecan and tumor response in human neuroblastoma xenograft and cell lines. Cancer Chemotherapy and Pharmacology. 1999;43:269–276. doi: 10.1007/s002800050894. [DOI] [PubMed] [Google Scholar]