Abstract
Despite recent advances in NMR approaches for structural biology, determination of membrane protein dynamics in its native environment continues to be a monumental challenge as most NMR structural studies of membrane proteins are commonly carried out in either micelles or in vesicle systems under frozen conditions. To overcome this difficulty, we propose a solid-state NMR technique that allows for the determination of side chain dynamics from membrane proteins in lipid bilayers. This new technique, namely dipolar enhanced polarization transfer (DREPT), allows for a wide range of dipolar couplings to be encoded, providing high resolution and sensitivity for systems that undergo motional averaging such as that of amino acid side chains. NMR observables such as dipolar couplings and chemical shift anisotropy, which are highly sensitive to molecular motions, provide a direct way of probing protein dynamics over a wide range of timescales. Therefore, using an appropriate model it is possible to determine side chain dynamics that can provide additional information on the topology and function of a membrane protein in its native environment.
Dynamics is crucial to the function of membrane-associated molecules such as peptides and membrane proteins.1 In particular, the dynamics of amino acid side chains play a central role in the folding and oligomerization of membrane proteins as well as in their functions including ion transport and cell membrane disruption. Therefore, an amino acid sequence encodes not only the structure of a protein but also a range of accessible dynamics, which in most cases determines its function as well as its adaptability to various stimuli such as temperature, pH and ionic strength. While solving atomic-level resolution structures of membrane-associated proteins is a challenge, characterization of their dynamics at high-resolution is even more difficult. In particular, measurement of protein dynamics from physiologically-relevant fluid membranes is a major challenge as solid-state NMR structural studies of membrane proteins are commonly carried out using frozen model membranes such as multilamellar vesicles.2–4
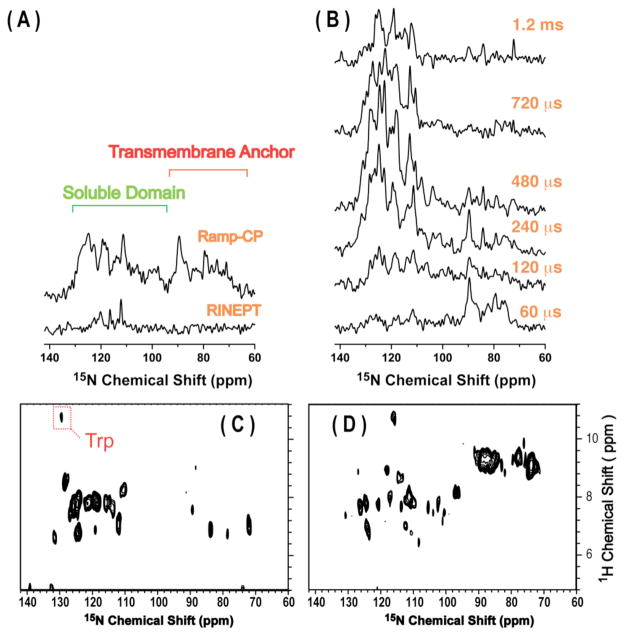
To overcome these limitations, we propose a 2D solid-state NMR technique that allows determination of side chain dynamics from a membrane protein in its near-native environment. This technique, namely Dipolar-Enhanced Polarization Transfer (DREPT), is a hybrid of PELF6 (Proton-Evolved Local Field and refocused-INEPT (Insensitive Nuclei Enhanced by Polarization Transfer).7 The schematic of the RF (radio frequency) pulse sequence is given in Figure S1. A homonuclear dipolar-decoupling pulse sequence is inserted in the delay periods of the refocused-INEPT (RINEPT) pulse sequence, allowing for the proton transverse magnetization to evolve under heteronuclear (scaled-dipolar and scalar) couplings during the incrementable t1 period. The efficiency of this technique is demonstrated using magnetically-aligned fluid lamellar-phase bicelles containing a 16.3 kDa mutant-cytochrome-b5 (mutant-Cyt-b5), a heme-containing membrane-anchored protein.8 The amino acid sequences of wild-type and mutant-Cyt-b5 are given in Figure S2. As shown in our previous study9, this protein exhibits dynamics covering a wide range of timescale (ns to ms) and therefore is an excellent system to examine the efficiency of the DREPT pulse sequence to selectively measure the dynamics of amino acid side chains in fluid lamellar phase lipid bilayers. Since the time period required to refocus the antiphase 15N magnetization is highly sensitive to the magnitude of the NH dipolar coupling, which depends on the mobility of the associated amino acid residue, spectral editing can be performed based on the dynamics of different segments of the protein as demonstrated in Figure 1. For example, a short delay of 60 μs is sufficient to refocus the antiphase 15N magnetization from residues in the transmembrane domain of the protein due to their large NH dipolar couplings (~3 to 5 kHz).9,10 On the other hand, due to a fast time scale of motion for residues in the soluble domain, their resonances only appear in the spectra collected with longer refocusing delays. Spectra obtained from the DREPT pulse sequence are compared with those obtained from refocused-INEPT and ramp-CP in Figure 1. Our results suggest that the DREPT pulse sequence provides a better spectral sensitivity than the RINEPT pulse sequence. This is a direct consequence of the loss of proton transverse magnetization due to the 1H-1H dipolar interactions during the delay periods in the RINEPT pulse sequence, which are avoided in the DREPT pulse sequence by applying a windowless multiple-pulse sequence, namely BLEW.11 Therefore, the one-dimensional version of the DREPT pulse sequence can be used for spectral editing based on dynamical differences between different segments of a membrane protein. Interestingly, the signal intensity of resonances originating from the soluble-domain of the mutant-Cyt-b5 is significantly higher in the DREPT spectra compared to those obtained using ramp-CP (ramped-cross-polarization)12 with a contact time comparable to the refocusing delay of DREPT. This reflects the difference in the magnetization transfer efficiency between the two techniques. A broad timescale of dynamics associated with residues in the soluble-domain of the protein attenuates the ramp-CP efficiency. On the other hand, in the DREPT pulse sequence, the transfer of transverse magnetization from abundant proton nuclei to rare X nuclei (15N or 13C) is via a combination of dipolar and J couplings. A defined length of the delay period controls the efficiency of this magnetization transfer. While not all heteronuclear dipolar couplings can be satisfied by a given delay period in the DREPT sequence, a significant amount of magnetization transfer can still occur contributing to the overall intensity of the spectrum as seen in Figure 1. This versatile capability of the DREPT pulse sequence to act as a spectral editing method is of tremendous advantage in identifying the topology of different segments of a membrane protein in lipid bilayers and can also be used for resonance assignment when combined with other approaches. For example, 2D HETCOR (heteronuclear correlation of chemical shifts) spectra obtained using the DREPT pulse sequence are presented in Figure 1(C and D). By suitably varying the refocusing delay period, different regions of the protein can be selected in the HETCOR spectrum. For example, with a short refocusing delay (τ2), the HETCOR spectrum only shows the rigid part of the protein such as the resonances from the transmembrane domain. On the other hand, when a long refocusing delay was used, only the mobile region of the protein was observed in the HETCOR spectrum.
Figure 1.
A series of 15N chemical shift spectra acquired using ramp-CP and RINEPT (A), and DREPT (B) experiments on magnetically-aligned bicelles containing a uniformly-15N-labeled mutant-cytochrome-b5. The contact time used for ramp-CP was 800 μs. The delay periods used in RINEPT were τ1 = τ2 = 2ms. The refocusing delay (τ2) in the DREPT sequence used to obtain each spectra in (B) is indicated and in all these cases τ1 = τ2. All the spectra were acquired using 2000 scans and a 3s recycle delay. (C and D) 2D 1H-15N HETCOR spectra of the same sample obtained using the DREPT pulse sequence (Figure S1). These spectra were acquired using 20 t1 increments, 800 scans per increment, and a 3s recycle delay. The delay τ2 period used in these HETCOR spectra was 1ms for (C) and 80 μs for (D).
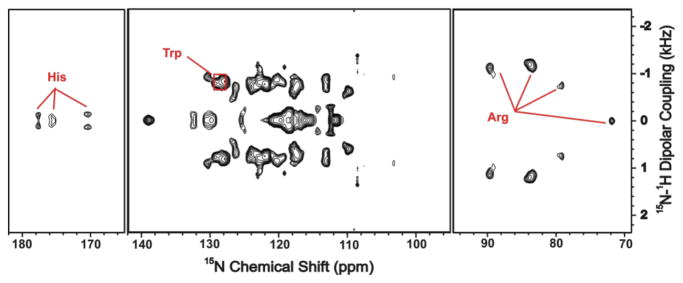
A 2D SLF version of the DREPT sequence shown in Figure S1 can be generated using the first delay (τ1), during which antiphase proton magnetizations are generated, as an incremental t1 period to measure NH dipolar couplings and to correlate with 15N chemical shift anisotropy (CSA) in the direct dimension. This manner of encoding the NH dipolar coupling is similar to that of the PELF sequence.10 Therefore, this 2D DREPT method provides high sensitivity and resolution for segments of a membrane protein that undergoes a rapid motional averaging and can be utilized in the measurement of NMR parameters pertaining to amino acid side chains by effectively suppressing resonances from immobile regions of the protein as demonstrated in Figure 2.
Figure 2.
A 2D DREPT-SLF spectrum of a uniformly-15N-labeled mutant-cytochrome-b5 in magnetically-aligned bicelles obtained using the pulse sequence given in Figure S1 with τ1 = τ2 = 400 μs. In this spectrum, dipolar couplings associated with different type of side chains can be identified. These results suggest that long refocusing delays suppress resonances from rigid parts of the protein (i.e. the transmembrane region) and allows for the identification resonances and measurement of NH dipolar couplings associated with side chains. Only those regions of the 2D Environment spectrum that contain peaks are shown.
Since NMR parameters such as dipolar couplings and CSA are highly sensitive to molecular motion, the DREPT method offers a direct means of monitoring protein dynamics covering a wide range of timescales. While a previous solid-state NMR study revealed the dynamical difference between the soluble and transmembrane domains of Cyt-b5 using backbone resonances, the measurement of side chain dynamics has not been possible due to poor spectral resolution.9 To demonstrate the utility of the 2D DREPT method in measuring NH dipolar couplings associated with amino acid side chains, a series of 2D SLF spectra were acquired from magnetically-aligned bicelles containing a uniformly-15N-labeled-Cyt-b5. Since the second-delay (τ2) period of the pulse sequence can be tuned to select resonances from specific regions of the spectrum, spectral regions corresponding to the side chains of a protein can be isolated and acquired for dynamical analysis. For instance, resonances at 80 and 90 ppm originate from the side chain of Arg, resonances at 129 and 130 ppm correspond to the side chain of Trp, and resonances from 169 to 177 ppm belong to the His side chain in the soluble domain. (Resonance assignment is shown in Figures 1(C and D) and S3.) However, due to the overlap of resonances, all three Trp residues in the membrane-spanning domain are not resolved. Due to the mobility of these side chains, the observed 15N chemical shift values are nearly equivalent to their isotropic chemical shift values, which facilitate the resonance assignment. Because of the large magnitude of NH dipolar couplings (~1 kHz) observed for Arg and Trp side chains, these residues are most likely located in the transmembrane region of the protein where the mobility is relatively restricted and therefore increases the order of an amino acid side chain.
Whole body motions of the helix coupled with side chain dynamics attenuate the observed dipolar couplings associated with side chains. Thus, the observed NH dipolar couplings from side chains are scaled according to the following equation [1].
| [1] |
Where DNH is the NH dipolar coupling in the rigid limit, SH is the order parameter of the helix, which for bicelles is estimated to be 0.8 from a previous study,10 and SSC is the order parameter of a side chain. SSC, therefore, describes the overall motion and fluctuations in the side chain orientation. Based on the SSC order parameters, it is possible to construct a model that describes the dynamics of amino acid side chains of a protein embedded in lipid bilayers. The SSC order parameter can be decomposed into two components as given below in equation [2].
| [2] |
Where ϑ is angle between the director axis (dsc) of a side chain and the helical axis (dH) and Slocal is the order parameter describing the fluctuation in the orientation of dsc; the term 〈….〉 denotes an average over the molecular motion, such as bond libration and rotation about the C-C bond in the side chain, that are fast relative to 1/DNH. Since large fluctuations are possible in a side chain, two models can be used to describe their motion: diffusion in a cone and orientational fluctuations. For the case of fluctuations in the orientation of the director axis of a side chain, the Slocal order parameter is expressed according to equation [3].
| [3] |
Where αf is the angle between the director axis of fluctuation (dm) and dsc. If dm is collinear with dsc, then Slocal will be equal to 1, which implies minor or negligible fluctuations in dsc. For the case of the “diffusion in a cone” model, the orientation of dsc is described as wobbling inside a cone defined by a semi cone angle αc.13
| [4] |
In this case, the Slocal order parameter is expressed according to equation [4]. Order parameters calculated from experimentally measured dipolar couplings are summarized in Table 1.
Table 1.
A summary of amino acid side chain chemical shifts and order parameters calculated from experimentally measured dipolar couplings from magnetically-aligned bicelles containing a 15N-labeled-mutant-Cyt-b5.
| Residue | 15N Chemical Shift (ppm) | NH Dipolar Coupling (kHz) | SSC |
|---|---|---|---|
| Trp | 129.7 | 0.80 | 0.15 |
| 128.2 | 0.82 | 0.17 | |
| Arg | 90.0 | 1.13 | 0.22 |
| 84.2 | 1.22 | 0.24 | |
| 79.2 | 0.77 | 0.14 | |
| His * | 177.7 | 0.12 | 0.05 |
| 175.7 | 0.09 | 0 | |
| 169.9 | 0.15 | 0.01 |
The His residue is in the soluble domain of the protein exhibits relatively a small degree of order.
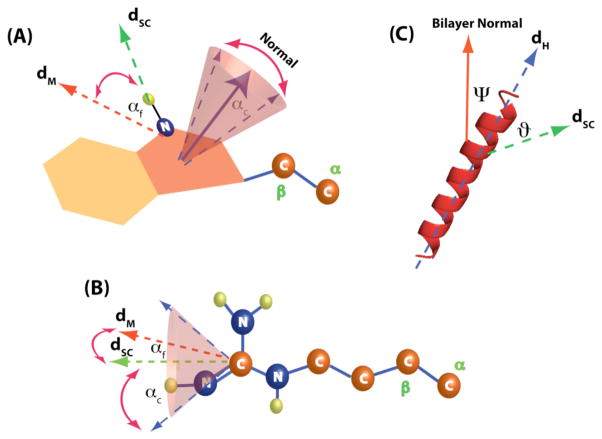
The orientation of various director axes of motions for different types of side chains is shown in Figure 3. Due to the rapid rotation about C-C bonds in Arg, dsc is collinear with the side chain. On the other hand, due to the rigidity of the indole ring in Trp, the observed NH dipolar coupling reflects only the orientation of the Nε-H bond vector with respect to the helical axis. Since the Nε-H bond vector is perpendicular to the indole-ring-normal, it is insensitive to the orientation of the indole ring. While the orientation of the indole ring cannot be deduced solely from Nε-H dipolar coupling, its dynamics is certainly encoded in the Slocal order parameter. Therefore, Slocal can be used to describe the dynamics of the indole ring. If αc or αf is equal to zero, implying a small degree of fluctuation in dsc, both models predict the orientation of Arg side chain to be 45–47° relative to the helical axis. For the case of Trp, the orientation of the Nε-H bond vector is estimated to be 50°. Interestingly, based on our simulations shown in Figure S4, only a large fluctuation (αc or αf > 30°) will change the orientation of side chains. Therefore, ϑ values determined from this study provide an upper limit for side chain orientations in lipid bilayers. This upper limit value is in agreement with the reported value for the methyl group orientation (~56°) of alanine, which experienced a limited side chain motion.14 Thus, our approach provides a viable means for determining side chain orientation of protein embedded in lipid bilayers.
Figure 3.
Schematics showing the orientation of the director axis dM, dsc and dH relative to the geometry of the protein and the side chains. The dipolar couplings of the side chains are projected along the dsc director axis due to the rapid internal motions inherent in the side chain, such as bond libation and isomerization, relative to the NMR timescale (1/DNH). dM is the director axis of motions describing the fluctuations of dsc. dH represents the director axis of the helix. (A) A diagram showing the orientation of dsc and dM director axis with respect to the indole ring. In this case, dsc is assumed to be collinear with the Nε-H bond vector. The angle αf defines the angle between dM, which describes the fluctuation in orientation of the Nε-H bond vector, and dsc. For the diffusion in a cone model, the size of the cone is defined by the cone semi angle αc, reflecting the wobbling motion of the indole ring. (B) A diagram showing the orientation of dsc with respect to the orientation of the Arg side chain. Due to the rapid motions around the side chain of Arg, the NH dipolar couplings are projected along dsc, which is collinear with the side chain. The angle αf defines the angle between dM, which describes the fluctuation in orientation of the side chain, and dsc. For the diffusion in a cone model, the size of the cone is defined by the cone semi angle angle αc and it reflects the wobbling motion of dsc director axis. (C) A diagram showing the orientation of dsc with respect to the helical director axis dH. In this case, the angle Ψ is the tilt angle of the helix and ϑ is the angle between dsc and dH.
While the exact orientation of an amino acid side chain is difficult to determine due to its motional flexibility as shown in Figure S4, our approach provides a means of correlating its dynamics and orientation based on the observed dipolar coupling values. The orientations of Trp and Arg side chains determined from this study are consistent with values reported in the literature. For example, a solid-state NMR study reported ~52° for the orientation of the Nε-H bond vector of a Trp residue of a coat protein in magnetically-aligned filamentous bacteriophage particles.14 Another study on gramicidin estimated this angle to be between 40 to 60°.15 To the best of our knowledge, no specific orientational information on the Arg side chain has been reported so far in the literature. However, the order parameter obtained in our studies is consistent with reported values (0.2 ≤ SSC ≤ 0.3) for an antimicrobial peptide embedded in lipid bilayers.17 Based on its location in the amino acid sequence of the mutant-Cyt-b5 and the measured order parameter, the Arg residue (observed in Fig. 2) is likely to reside in the phospholipid glycerol backbone region of the lipid bilayer. Furthermore, due to the positive charge of the guanidine group in the Arg side chain, it preferentially binds to the negatively charged phosphate head group of the lipid.17 As a result, Arg side chain motion is considerably hindered as reflected in the observed relatively large NH dipolar couplings (0.77, 1.13 and 1.22 kHz) from three different NH groups (Table 1)). Therefore, such information on side chain dynamics can provide valuable insights into the topology of a membrane protein embedded in lipid bilayers. Interestingly, the role of Arg in a membrane protein is a subject of constant debate.18 Arg residues form an essential part of the voltage sensor domain of potassium channels, enzymes, and also in other cell membrane permeating peptides.18 It has also been demonstrated recently that the interaction between Arg and lipid bilayers can influence the orientation of a transmembrane helix.18 Therefore, the ability to provide information on Arg side chain dynamics will allow a better understanding of their role in the function of membrane proteins. Lastly, the imidazole side chain dynamics of His residues in the soluble domain are also observed using the DREPT pulse sequence. While their orientations are difficult to determine due to the motion in the soluble domain, the fact that they exhibit measurable dipolar couplings provides valuable information regarding their dynamics. Since imidazole side chains play important roles in the action of a number of enzymes, the ability to deduce their dynamics will provide insights into their role in various pH dependent enzyme catalysis.19
In conclusion, we have presented a solid-state NMR technique that enables the extraction of protein side chain dynamics in fluid lamellar phase lipid bilayers. The NH dipolar couplings of side chains can be carefully selected through fine-tuning of the delay period in the DREPT pulse sequence. We have shown that it is possible to characterize side chain motions by using an appropriate model. In conjunction with relaxation measurements, this can prove to be a powerful approach to determine side chain dynamics for a variety of amino acids. Since side chain interactions constitute the principle force dictating a protein’s function, it is expected that knowledge on their dynamics will have an immense impact on our understanding of their functional role. While previous studies have utilized selective isotopic-labeling approaches to measure dynamics of membrane proteins20–26, our approach has the unique advantage to study uniformly-15N and potentially 15N and 13C-labeled membrane proteins. It should be noted that the DREPT technique could also be used to characterize the dynamics of other aligned compounds such as liquid crystalline materials, single crystals, and fibers. The ability to measure a broad range of heteronuclear dipolar couplings from DREPT experiments further advocates its usefulness in extracting RDCs (residual dipolar couplings) from supramolecular complexes embedded in an aligned medium.
Supplementary Material
Acknowledgments
This study was supported by research funds from NIH (AI054515 and RR023597 to A.R. and GM035533 to L.W.), CRIF-NSF, and a VA Merit Review grant to L.W. The authors thank Dr. Shivani Ahuja for help with preparation of the manuscript.
Footnotes
Supporting Information Available: A list of abbreviations, materials and methods, pulse sequences, theory behind the DREPT pulse sequence, numerical simulation of side chain models, and amino acid sequence of wild-type and a mutant-Cyt-b5.
References
- 1.Baldwin AJ, Kay LE. Nat Chem Biol. 2009;5:808–814. doi: 10.1038/nchembio.238. [DOI] [PubMed] [Google Scholar]
- 2.McDermott AE. Curr Opin Struct Biol. 2004;14:554–561. doi: 10.1016/j.sbi.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Etzkorn M, Kneuper H, Dunnwald P, Vijayan V, Kramer J, Griesinger C, Becker S, Unden G, Baldus M. Nat Struct Mol Biol. 2008;15:1031–1039. doi: 10.1038/nsmb.1493. [DOI] [PubMed] [Google Scholar]
- 4.Ahuja S, Hornak V, Yan ECY, Syrett N, Goncalves JA, Hirshfeld A, Ziliox M, Sakmar TP, Sheves M, Reeves PJ, Smith SO, Eilers M. Nat Struct Mol Biol. 2009;16:168–175. doi: 10.1038/nsmb.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cady SD, Schmidt-Rohr K, Wang J, Soto CS, DeGrado WF, Hong M. Nature. 2010;463:689–U127. doi: 10.1038/nature08722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt-Rohr K, Nanz D, Emsley L, Pines A. J Phys Chem. 1994;98:6668–6670. [Google Scholar]
- 7.Morris GA, Freeman R. J Am Chem Soc. 1979;101:760–762. [Google Scholar]; Ramamoorthy A, Chandrakumar N. J Magn Reson. 1992;60:100. [Google Scholar]
- 8.Dürr UHN, Waskell L, Ramamoorthy A. Biochim Biophys Acta. 2007;1768:3235–3259. doi: 10.1016/j.bbamem.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Soong R, Smith PES, Xu J, Yamamoto K, Im SC, Waskell L, Ramamoorthy A. J Am Chem Soc. 2010;132:5779–5788. doi: 10.1021/ja910807e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dürr UHN, Yamamoto K, Im SC, Waskell L, Ramamoorthy A. J Am Chem Soc. 2007;129:6670–6671. doi: 10.1021/ja069028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burum D, Linder M, Ernst RR. J Magn Reson. 1981;44:173–188. [Google Scholar]
- 12.Metz G, Xiaoling X, Smith SO. J Magn Reson A. 1994;110:219–227. [Google Scholar]
- 13.Brainard JR, Szabo A. Biochemistry. 1981;20:4618–4628. doi: 10.1021/bi00519a016. [DOI] [PubMed] [Google Scholar]
- 14.Strandberg E, Ozdirekcan S, Rijkers DT, van der Wel PC, Koeppe RE, Liskamp RM, Killian JA. Biophys J. 2004;86:3709–3721. doi: 10.1529/biophysj.103.035402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross TA, Opella SJ. J Am Chem Soc. 1983;105:306–308. [Google Scholar]
- 16.Hu W, Lee KC, Cross TA. Biochemistry. 1993;32:7035–7047. doi: 10.1021/bi00078a032. [DOI] [PubMed] [Google Scholar]
- 17.Tang M, Waring AJ, Hong M. Chembiochem. 2008;9:1487–1492. doi: 10.1002/cbic.200800005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vostrikov VV, Hall BA, Greathouse DV, Koeppe RE, Sansom MSP. J Am Chem Soc. 2010;132:5803–5811. doi: 10.1021/ja100598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramamoorthy A, Wu CH, Opella SJ. J Am Chem Soc. 1997;119:10479–10486. [Google Scholar]
- 20.Ying WW, Irvine SE, Beekman RA, Siminovitch DJ, Smith SO. J Am Chem Soc. 2000;122:11125–11128. [Google Scholar]
- 21.Witter R, Nozirov F, Sternberg U, Cross TA, Ulrich AS, Fu RQ. J Am Chem Soc. 2008;130:918–924. doi: 10.1021/ja0754305. [DOI] [PubMed] [Google Scholar]
- 22.Vostrikov VV, Hall BA, Greathouse DV, Koeppe RE, Sansom MSP. J Am Chem Soc. 2010;132:5803–5811. doi: 10.1021/ja100598e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Killian JA, Taylor MJ, Koeppe RE. Biochemistry. 1992;31:11283–11290. doi: 10.1021/bi00161a004. [DOI] [PubMed] [Google Scholar]
- 24.Siarheyeva A, Lopez JJ, Lehner I, Hellmich UA, van Veen HW, Glaubitz C. Biochemistry. 2007;46:3075–3083. doi: 10.1021/bi062109a. [DOI] [PubMed] [Google Scholar]
- 25.Liu W, Crocker E, Siminovitch DJ, Smith SO. Biophys J. 2003;84:1263–1271. doi: 10.1016/S0006-3495(03)74941-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aisenbrey C, Prongidi-Fix L, Chenal A, Gillet D, Bechinger B. J Am Chem Soc. 2009;131:6340–6341. doi: 10.1021/ja900677b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.