Abstract
We retrospectively analyzed outcomes of 716 patients with multiple myeloma who were mobilized using CY and growth factor (n=370) or growth factor alone (n=346) before SCT. Patients receiving CY had higher stem cell yields than the growth factor only group (median number of apheresis sessions needed to achieve stem cell collection goals, two vs four sessions, respectively (P=0.001)). However, patients treated with CY required more time for engraftment of platelets and neutrophils (P<0.001 for both). For patients receiving CY, 75% achieved engraftment (defined as a platelet count of 50×109/l) by day 39, whereas 75% of patients not receiving CY achieved engraftment by day 18. Similar results were observed for neutrophil engraftment. These differences did not affect the duration of hospitalization, but patients treated with CY had a higher incidence of post transplant nonstaphylococcal bacteremia. For CY-mobilized patients, considerably faster platelet engraftment (5 fewer days) resulted if stem cell reinfusion occurred more than 30 days after the first apheresis session. Our data suggested that CY damaged the microenvironment and slowed engraftment. By lengthening the period between the completion of apheresis and stem cell reinfusion, the microenvironment may recover and result in faster engraftment.
Keywords: CY, engraftment, mobilization, multiple myeloma, SCT
Introduction
High-dose chemotherapy with stem cell reconstitution is an integral part of the management of patients with multiple myeloma.1,2 To ensure a safe outcome and low mortality rate, and to reduce the risk of serious infections and bleeding from protracted cytopenia, sufficient numbers of hematopoietic stem cells must be infused to achieve prompt engraftment.3,4 Since the early days of SCT, the standard mobilization technique has combined the administration of CY5 and a growth factor, and apheresis was initiated when the WBC count showed evidence of rebound from the chemotherapy-induced nadir.6,7 Subsequent studies showed that ex vivo purging of stem cell products to remove contamination by myeloma cells had no effect on patient outcome.8,9
After CD38+ plasma cells in the apheresis product (a marker of myeloma cells) were shown to have no effect on the outcome,10 the Myeloma Transplantation Group at Mayo Clinic made a policy change that eliminated CY from the stem cell mobilization regimen. Beginning in 2004, mobilization has been performed routinely with the growth factor alone. This new regimen has improved patient convenience; virtually all patients who undergo SCT at Mayo Clinic are nonresidents of Olmsted County, Minnesota (where the clinic is located), and eliminating CY from the mobilization regimen has decreased the duration of mobilization treatment by approximately 9 days.11 Subsequently, our group showed that the fraction of patients achieving a partial response after induction chemotherapy has no impact on PFS and overall survival after transplantation. 12 We also showed that PFS and overall survival was not affected by the use of CY during mobilization.13 This study, however, examines parameters such as time to engraftment, duration of hospitalization and incidence of infection in patients mobilized by the two techniques. Our findings suggest that CY potentially damages the BM microenvironment and causes delays in engraftment and increased bacteremia rates.
Materials and methods
This study was approved by the Mayo Clinic Institutional Review Board. All patients gave written consent in accordance with the Minnesota law and appropriate federal regulations. At our institution, patients with myeloma are monitored prospectively; a database continuously updates records with relevant demographic, clinical and laboratory information, including duration of hospitalization. We retrospectively reviewed records of 716 consecutive patients with multiple myeloma who underwent SCT from January 1, 2000, through November 1, 2007, at Mayo Clinic (Rochester, MN, USA). All patients who received high-dose chemotherapy were included. No patient was excluded from the analysis, and none were lost to follow-up.
The eligibility for SCT included biopsy-proven, symptomatic multiple myeloma (a response to induction therapy was not required). The baseline evaluation of all patients considered for transplantation included a BM examination and tests for β-2microglobulin and renal function.
Conditioning regimens (treatment in preparation for transplantation) were established by considering the risk of toxicity after high-dose chemotherapy. Melphalan (100 mg/m2 on each of the 2 consecutive days) was administered to most patients (89%); those with serum creatinine levels greater than 2mg per 100 ml and patients older than 70 years had the melphalan dosage reduced from 200 to 140 mg/m2 (Table 1).14 Conditioning chemotherapy and stem cell infusion were performed as outpatient services; patients were maintained as outpatients except when hospitalization became necessary to manage those with persistent refractory neutropenic fever, intractable mucositis with dehydration or a decline in performance status.
Table 1.
Patient characteristics (N = 716)
| Variable | CY (n = 370) | Growth factor only (n = 346) | P-value |
|---|---|---|---|
| Men, no. of patients (%) | 224 (61) | 202 (58) | 0.50 |
| Age, median (IQR), years | 58 (52–64) | 60 (53–65) | 0.11 |
| β-2Microglobulin, median (IQR), μg/ml | 2.7 (1.9–4.0) | 2.3 (1.9–3.2) | 0.01 |
| Creatinine, median (IQR), mg per 100 ml | 1.1 (0.9–1.3) | 1.0 (0.8–1.2) | 0.002 |
| Apheresis, median (IQR) collections, no. | 2 (1–3) | 4 (3–6) | 0.001 |
| Marrow plasma cells, % | 17 (5–34) | 5 (1–13) | 0.001 |
| CD34+ cells, median (IQR), cells/kg | |||
| Total collected | 10.3×106 (7.2×106–14.6×106) | 9.9×106 (7.6×106–11.9×106) | 0.01 |
| Infused | 5.6×106 (4.5×106–7.6×106) | 4.2×106 (3.8×106–5.0×106) | <0.001 |
| Duration of hospitalization, median (IQR), days | 4 (0–10) | 4 (0–9) | 0.92 |
| Nonstaphylococcal bacteremia, no. of patients (%) | 48 (13) | 25 (7) | 0.01 |
| Melphalan dosage, no. of patients (%) | 0.04 | ||
| 200 mg/m2 a | 337 (91) | 298 (86) | |
| 140 mg/m2 a | 33 (9) | 48 (14) | |
| Exposure before mobilization, no. of patients (%) | |||
| Melphalan | 45 (12) | 44 (13) | 0.50 |
| Lenalidomide | 14 (4) | 63 (18) | 0.01 |
Abbreviation: IQR = interquartile range.
Or equivalent dosage.
Although all patients in this study received treatment with growth factor, for simplicity of writing, we describe the patient cohorts throughout the text as receiving treatment ‘with CY’ or ‘with growth factor alone.’ Patients who received growth factor alone were treated with filgrastim (dosage, 10 μg/kg, daily), and CD34+ cell counts of the peripheral blood were performed on day 5. It has been suggested that the CD34+/CD33− fraction shows greater multilineage potential and is a better predictor of engraftment capacity,15 but it was not measured in this population. If the CD34+ cell count exceeded 0.01×109/l, apheresis was initiated. If this level was not achieved, most patients received successive increases in the filgrastim dosage (up to 16 μg/kg, twice daily). Patients undergoing stem cell mobilization with CY received 1.5 g/m2 daily for 2 consecutive days followed by sargramostim 5 μg/kg daily until apheresis was completed. After the total leukocyte count exceeded 0.5×109/l, the CD34+ cell count in the peripheral blood was measured daily until it exceeded 0.01×109/l. All CY-treated patients received growth factor on the third day of mobilization (the day after CY treatment was complete). Apheresis was performed by processing 11–14 l of blood in 4 h.16 All patients who underwent transplantation received a minimum of 2×106 CD34+ cells/kg.
The flow assessment of CD34 content did not change during the time course of this study. The goal for collection was 9×106 CD34+ cells/kg. This was to allow a tandem transplant with an infusion of three for each, which would permit the final three to be used for a salvage transplant at relapse. The protocol for the cryopreservation of cells, storage, thawing and reinfusion did not change appreciably during the study period.16
Standard supportive care after transplantation was administered; this included use of prophylactic fluoroquinolone antibiotics, acyclovir and fluconazole. All patients received growth factor beginning on day 5 (sargramostim, 5 μg/kg, daily) until engraftment of neutrophils was achieved. Engraftment was calculated from day 0 to the respective first date of neutrophil counts greater than 0.5×109/l and platelet counts greater than 50×109/l without transfusion in the preceding 48 h. Surveillance blood cultures were performed for all patients after catheter placement; subsequently they were not carried out on a routine basis and were performed only if a fever or skin erythema suggesting cellulitis developed. All patients were evaluated for bacteremia, which was defined as a positive blood culture at any time during the transplantation course, even if patients were asymptomatic. No attempt was made to distinguish contaminants from known pathogens.
Statistical analysis
The Kaplan–Meier method was used to analyze time to engraftment. Data from patients who died before engraftment were included in the analysis (shown in Figures 1-3 as ticks on each curve); however, they were censored on the date of death and classified as ‘not engrafted.’ Analysis of group differences was performed using the Kruskal–Wallis test for continuous variables and the Fisher exact test for categorical variables.
Figure 1.
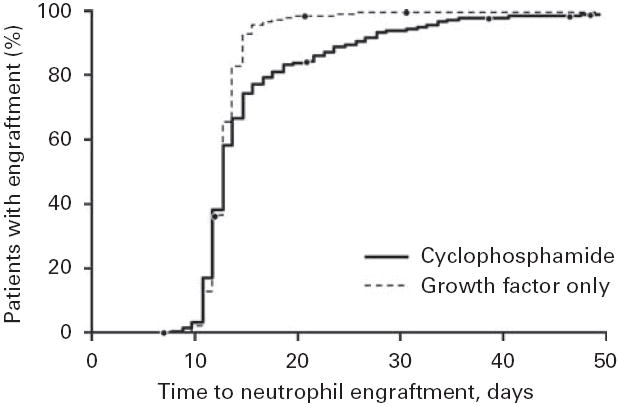
Time to neutrophil engraftment. The engraftment was defined as a neutrophil count of 0.5×109/l. Patients were treated with CY or with growth factor alone.
Figure 3.
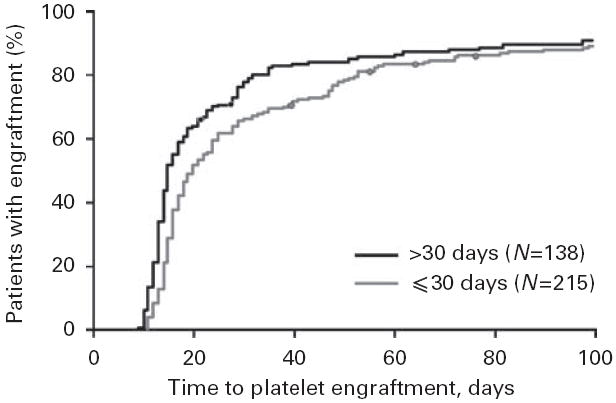
Time to platelet engraftment for CY-mobilized patients. The engraftment was defined as a platelet count of 50×109/l. Patients were categorized by the length of time between the initial stem cell collection and infusion.
Results
The Table 1 shows clinical characteristics of the 716 study patients. All had adequate cardiac and pulmonary function. In total, 370 patients were treated with CY, and 346 were treated with growth factor alone. Patients treated with CY required a median of two apheresis collections to achieve the target number of CD34+ cells. In contrast, patients treated with growth factor alone needed four collections to accrue the target number of CD34+ cells. The total number of CD34+ cells collected per patient was significantly higher in the CY group (P=0.01) because 111 patients (30%) exceeded the target number of CD34+ cells in one collection (median, 13.2×106 cells/kg). Of these patients, 83 (75%) collected greater than 9×106 cells/kg, and 28 (25%) collected greater than 21.9×106 cells/kg. For patients treated with growth factor alone, only 20 (6%) achieved the collection goal in one apheresis session (median, 11.5×106 cells/kg). Of these patients, 15 (75%) collected greater than 7.8×106 cells/kg, and five (25%) collected greater than 13.3×106 cells/kg. Of note, patients treated with CY had a higher percentage of plasma cells in the BM, a feature known to adversely affect mobilization.17 As the two groups were not balanced for the percentage involvement of plasma cells at the time of collection and plasmacytosis has been reported to delay engraftment,18 a bivariate analysis was performed comparing marrow plasma cells at the time of collection and the number of days to granulocyte engraftment. No differences were found (P=0.44). Similarly, no relationship was found between the total CD34+ cells collected and the percentage of marrow plasma cells at collection (P=0.41). The same analysis was performed for plasma cells and time to achieve a platelet count of 50×109/l, but no relationship was found (P>0.16).
This noncontemporaneous patient cohort reflected the shift in clinical practice that occurred at our institution in 2004, as described above. Of the 346 patients treated with growth factor alone, the ‘time midpoint’ (the date by which half the patients in a given treatment group had undergone transplantation) was 4 August 2005, with 51 patients undergoing stem cell collection and transplantation in 2007. In contrast, of the patients who received CY, the midpoint was 3 June 2002, and only 13 received CY in 2007 (usually to treat circulating cells or more aggressive relapse).
Given the limitations of a noncontemporaneous and nonrandomized patient cohort, engraftment data for neutrophils are shown in Figure 1. Both groups had an identical median time to engraftment (13 days), but 75% of patients in the CY group did not achieve a neutrophil count of 0.5×109/l until day 16, whereas 75% of patients treated with growth factor alone met the neutrophil engraftment goal by day 14. Even though the patients treated with CY had a significantly higher number of CD34+ cells infused at transplantation (5.6×106 vs 4.2×106 cells/kg; P<0.001), they required more time for engraftment. Ninety percent of patients in the growth factor group had neutrophil engraftment by day 15; however, 25 days elapsed before 90% of patients in the CY group had engraftment (P=0.001).
More striking differences between the patient groups were observed with platelet engraftment (Figure 2). The overall median time to engraftment (defined as a platelet count of 50×109/l) was 16 days. For patients receiving growth factor alone, the median time was 15 days (data from seven patients were censored); for patients receiving CY, the median time was 18 days (data from 16 patients were censored). The percentage of patients who did not have platelet engraftment by day 18 was 25% for the growth factor group and 47% for the CY group. By day 27, 10% of patients in the growth factor group still had not achieved engraftment. However, 34% of patients in the CY group did not have engraftment by day 27, even though this group had a considerably higher median number of infused CD34+ cells.
Figure 2.
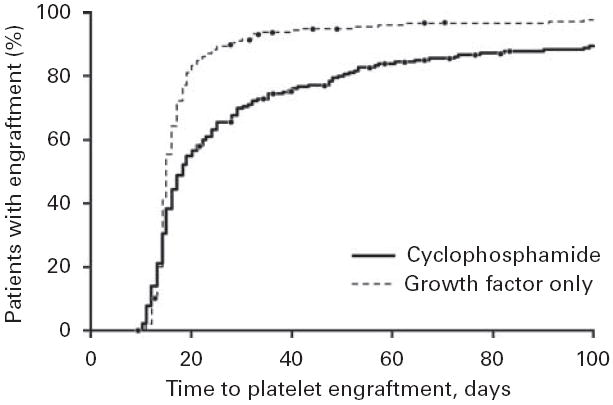
Time to platelet engraftment. The engraftment was defined as a platelet count of 50×109/l. Patients were treated with CY or with growth factor alone.
As noted in the Table 1, some patients had received melphalan or lenalidomide before apheresis. Both agents have been implicated in reducing postapheresis yields of stem cells.19,20 Both arms were balanced for the number of patients with precollection melphalan exposure; however, a disproportionate number of patients treated with growth factor alone had exposure to lenalidomide (18%). We therefore analyzed whether the lower CD34+ yield in the group that received growth factor alone was impacted by the minority of patients receiving lenalidomide. The median (interquartile range) CD34 yield in the group exposed to lenalidomide was 10.0×106 cells/kg (7.3×106–11.5×106 cells/kg); in the group not receiving lenalidomide, the yield was 9.76×106 cells/kg (7.0×106–12.0×106 cells/kg) (P=0.80). Presumably, the small fraction of patients exposed was insufficient to affect the values in the large cohort of patients studied.
To explore possible mechanisms that could delay engraftment in the CY-mobilized cohort, we separately examined the patients who were treated with CY (n=353). To determine whether CY itself could affect the BM microenvironment in a way that inhibited platelet engraftment, we categorized patients by the amount of time that elapsed between stem cell collection and infusion. Of the 353 patients, 138 had stem cell infusions greater than 30 days after the first collection, and 215 had infusion within 30 days of the first collection. Figure 3 shows a significant difference in time to platelet engraftment between the subgroups (P<0.001). For the early infusion group, the median time to engraftment was 20 days (data from eight patients were censored); the delayed infusion group had a median time of 15 days (data from seven patients were censored). Seventy-five percent of patients in the late infusion group achieved platelet engraftment by day 29, but 48 days elapsed before 75% of the early infusion group achieved engraftment. These data suggest that a delay in stem cell reinfusion promotes faster engraftment of platelets because it allows the effects of CY to abate.
To assess the clinical effect of delayed neutrophil engraftment, we reviewed the duration of hospitalization of both cohorts (CY and growth factor treatment groups). The median time for both groups was 4 days (P=0.92). We also questioned whether time to engraftment was affected by the total number of CD34+ cells infused. Of the 703 patient records showing CD34+ cell counts, only nine patients (1%) received less than 3×106 CD34+ cells/kg, and no patients underwent transplantation with fewer than 2×106 cells/kg. Because of the minimum threshold required for transplantation, we were unable to observe any association between the number of cells infused and time to engraftment. We also examined the incidence rate of nonstaphylococcal bacteremia, which occurred in 48 of the 370 CY-mobilized patients (13%) and 25 of the 346 growth factor-mobilized patients (7%) (P=0.01). Bacteremia could be used as a surrogate measure of morbidity, but a direct correlation between a longer time to engraftment and bacteremia could not be established with our data.
Discussion
No optimal method for stem cell mobilization
The optimal method for mobilizing stem cells remains unclear. When transplantation first was used to treat multiple myeloma, CY was administered to enhance the recovery of PBSCs and possibly to promote in vivo purging of malignant cells from stem cell products.21 Clinicians hoped that CY would improve the outcome by inducing an incremental response in the patient’s myeloma and lower tumor mass before administration of high-dose chemotherapy. 22 However, subsequent studies have shown that ex vivo purging did not improve outcomes,8-10 and our own experience comparing patients who have an M protein reduction of 50% before transplant and patients who fail to achieve a 50% M protein reduction suggests that the extent of response to induction therapy before transplantation does not predict outcome.23 As a consequence, the Mayo Clinic Myeloma Transplantation Group elected to mobilize stem cells with growth factor alone,24 a strategy previously adopted for the treatment of patients with non-Hodgkin’s lymphoma. Both methods have advantages and disadvantages, and on the basis of the published data, neither appears to be the optimal choice.
Although the stem cell yield was greater with CY, approximately 10% of the patients required hospitalization for neutropenic fever. Also, red cell and platelet support was required in patients at the time of collection if their marrows were heavily infiltrated or if their induction therapy contained myelosuppressive agents. Hospitalization and transfusional support were rarely needed among patients who had mobilization with growth factors alone. As a matter of practice, owing to its safety and simplicity, the use of growth factors alone is now our standard method for CD34+ mobilization for myeloma patients unless there are circumstances that would a priori predict poor yield by standard methods, such as prior lenalidomide exposure.20,25
This study was an analysis of consecutive patients and not a randomized study; therefore, considerable imbalances existed between the two patient groups. The number of infused CD34+ cells was significantly different, with the CY group having higher collection levels and thus a greater number of infused cells (5.6×106 vs 4.2×106 cells/kg; P<0.001). The marked differences in serum β-2microglobulin levels suggested that the CY-mobilized cohort had more advanced disease. This also may reflect improved disease control before transplantation that was achieved with newer therapies. The serum creatinine levels were significantly different between the groups (P=0.002), although the clinically significant differences were small.
Patients who undergo stem cell mobilization with CY have an inherently increased length of stay at the transplant center; at least 9 days are needed for recovery from chemotherapy. Apheresis collections generally begin approximately 11–14 days after chemotherapy is initiated (in comparison, patients treated with growth factor alone can begin apheresis in 5 days). A subset of patients also will require transfusional support after a pulse treatment with CY, and a small proportion will require hospitalization for the management of neutropenic fever.26,27 However, CY mobilization may be more efficient. In this study, patients who received CY had an average of two fewer apheresis procedures than patients treated with growth factor alone. This CY group had a median of 10×106 cells/kg collected per patient, and 30% of patients met the target number of CD34+ cells in a single collection.
Nevertheless, these data also appear to indicate that CY treatment was associated with several clinically significant concerns after transplantation. In particular, time to engraftment of neutrophils and platelets was delayed when compared with that of patients mobilized with growth factor alone. Although this delay did not increase the duration of hospitalization, we did observe an association between treatment method and incidence of nonstaphylococcal bacteremia (13 and 7% for patients treated with CY or growth factor, respectively; P=0.01). In addition, delayed neutrophil engraftment uses more resources because additional growth factor is administered until engraftment is achieved. Increased antibiotic therapy may be necessary to treat higher bacteremia rates. The kinetics of neutrophil engraftment in the myeloma cohort was found to be nearly identical to that of our patients who undergo transplant for amyloidosis and do not receive post transplantation growth factor (medians, 13 and 14 days, respectively).28 As a consequence of these results, the transplantation group at Mayo Clinic, Rochester, elected to stop administering growth factors following transplantation in myeloma patients; the group’s reasoning was that the resource use did not compensate for 1 day of more rapid neutrophil engraftment.
Our data were not able to specifically indicate whether CY mobilization should be preferred over growth factor alone. Others have not reported delayed engraftment with CY mobilization,29 but the number of patients in that report was far smaller, and the study may not have been powered sufficiently to detect differences.
Effect of CY on the BM microenvironment
The longer time to engraftment of neutrophils and platelets for CY-mobilized patients is not explained readily. Because a greater number of stem cells were infused for CY-mobilized patients, faster engraftment in this cohort might be expected. However, the opposite was true for our patients and we therefore questioned how mobilization with CY could affect engraftment.
We hypothesize that CY transiently damages the BM microenvironment and that this damage delays recovery. Earlier reports support our hypothesis. Stromal reconstitution to normal levels was uncommon in adults undergoing allogeneic transplantation, showing that the marrow stromal microenvironment is seriously damaged after transplantation. 30 In addition to affecting long-term stem cell engraftment and maintenance of hematopoietic function, damage of the stromal microenvironment also injures the progenitor compartment for endosteal osteoblasts and causes osteoporosis. 31 Chemotherapy for ALL can induce functional deregulation within BM stromal cells with a reduction in the number of erythroid burst-forming units, which suggests a mechanism for the postchemotherapy anemia observed in most patients.32 Human mesenchymal stem cells are sensitive to various cytotoxic agents; the different (and sometimes delayed) recovery patterns could affect the ability of these cells to support hematopoiesis.33 Thrombopoiesis depends to some extent on signals from the marrow microenvironment, and both neutrophils and platelets in at least a subset of patients show impaired recovery related to altered signaling from the microenvironment.34
In a recent comparison of patients receiving reduced-intensity or standard conditioning,35 the latter had considerably impaired ability to generate stromal layers in vitro at days 30 and 100. In the univariate analysis, standard conditioning was the only factor that predicted stromal growth impairment after transplantation. We attempted to confirm these findings by assessing CY-mobilized patients and comparing those who received their stem cells within 30 days of the first apheresis session with those who received stem cells longer than 30 days after the first apheresis. When patients underwent transplantation more than 30 days after initiating CY mobilization, their median time to achieving a platelet count of 50×109/l was the same as those who were mobilized using growth factor alone (15 days). It is unlikely that time to transplantation was the sole explanation for the difference in time to platelet engraftment; however, 75% of the CY-mobilized patients who were infused less than 30 days after collection did not achieve the platelet count of 50×109/l until day 39, whereas those who underwent SCT more than 30 days after the first apheresis achieved this value at day 29. For patients with no exposure to CY, the platelet count was achieved by day 18. Thus, CY appears to produce some degree of microenvironmental damage that impedes the recovery of healthy infused CD34+ cells.
Conclusion
We suggest that the CY used for mobilization may affect the marrow microenvironment. CY improves yields but increases bacteremia rates after transplantation. CY increases time required to collect stem cells and slows engraftment.
References
- 1.Bjorkstrand B, Gahrton G. High-dose treatment with autologous stem cell transplantation in multiple myeloma: past, present, and future. Semin Hematol. 2007;44:227–233. doi: 10.1053/j.seminhematol.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 2.Jagannath S. Current standards for first-line therapy of multiple myeloma. Clin Lymphoma Myeloma. 2007;7(Suppl 5):S207–S214. doi: 10.3816/clm.2007.s.024. [DOI] [PubMed] [Google Scholar]
- 3.Bashey A, Donohue M, Liu L, Medina B, Corringham S, Ihasz A, et al. Peripheral blood progenitor cell mobilization with intermediate-dose cyclophosphamide, sequential granulocyte-macrophage-colony-stimulating factor and granulocyte-colony-stimulating factor, and scheduled commencement of leukapheresis in 225 patients undergoing autologous transplantation. Transfusion. 2007;47:2153–2160. doi: 10.1111/j.1537-2995.2007.01440.x. [DOI] [PubMed] [Google Scholar]
- 4.Jacoub JF, Suryadevara U, Pereyra V, Colon D, Fontelonga A, Mackintosh FR, et al. Mobilization strategies for the collection of peripheral blood progenitor cells: results from a pilot study of delayed addition G-CSF following chemotherapy and review of the literature. Exp Hematol. 2006;34:1443–1450. doi: 10.1016/j.exphem.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Goldschmidt H, Hegenbart U, Haas R, Hunstein W. Mobilization of peripheral blood progenitor cells with high-dose cyclophosphamide (4 or 7 g/m2) and granulocyte colony-stimulating factor in patients with multiple myeloma. Bone Marrow Transplant. 1996;17:691–697. [PubMed] [Google Scholar]
- 6.To LB, Shepperd KM, Haylock DN, Dyson PG, Charles P, Thorp DL, et al. Single high doses of cyclophosphamide enable the collection of high numbers of hemopoietic stem cells from the peripheral blood. Exp Hematol. 1990;18:442–447. [PubMed] [Google Scholar]
- 7.Boiron JM, Marit G, Faberes C, Cony-Makhoul P, Foures C, Ferrer AM, et al. Collection of peripheral blood stem cells in multiple myeloma following single high-dose cyclophosphamide with and without recombinant human granulocyte-macrophage colony-stimulating factor (rhGM-CSF) Bone Marrow Transplant. 1993;12:49–55. [PubMed] [Google Scholar]
- 8.Vescio RA, Han EJ, Schiller GJ, Lee JC, Wu CH, Cao J, et al. Quantitative comparison of multiple myeloma tumor contamination in bone marrow harvest and leukapheresis autografts. Bone Marrow Transplant. 1996;18:103–110. [PubMed] [Google Scholar]
- 9.Vescio R, Schiller G, Stewart AK, Ballester O, Noga S, Rugo H, et al. Multicenter phase III trial to evaluate CD34+ selected versus unselected autologous peripheral blood progenitor cell transplantation in multiple myeloma. Blood. 1999;93:1858–1868. [PubMed] [Google Scholar]
- 10.Stewart AK, Vescio R, Schiller G, Ballester O, Noga S, Rugo H, et al. Purging of autologous peripheral-blood stem cells using CD34 selection does not improve overall or progression-free survival after high-dose chemotherapy for multiple myeloma: results of a multicenter randomized controlled trial. J Clin Oncol. 2001;19:3771–3779. doi: 10.1200/JCO.2001.19.17.3771. [DOI] [PubMed] [Google Scholar]
- 11.Hill QA, Buxton D, Pearce R, Gesinde MO, Smith GM, Cook G. An analysis of the optimal timing of peripheral blood stem cell harvesting following priming with cyclophosphamide and G-CSF. Bone Marrow Transplant. 2007;40:925–930. doi: 10.1038/sj.bmt.1705847. [DOI] [PubMed] [Google Scholar]
- 12.Kumar S, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Geyer S, et al. High-dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant. 2004;34:161–167. doi: 10.1038/sj.bmt.1704545. [DOI] [PubMed] [Google Scholar]
- 13.Dingli D, Nowakowski GS, Dispenzieri A, Lacy MQ, Hayman S, Litzow MR, et al. Cyclophosphamide mobilization does not improve outcome in patients receiving stem cell transplantation for multiple myeloma. Clin Lymphoma Myeloma. 2006;6:384–388. doi: 10.3816/CLM.2006.n.014. [DOI] [PubMed] [Google Scholar]
- 14.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar S, Leung N, et al. Impact of age and serum creatinine value on outcome after autologous blood stem cell transplantation for patients with multiple myeloma. Bone Marrow Transplant. 2007;39:605–611. doi: 10.1038/sj.bmt.1705627. [DOI] [PubMed] [Google Scholar]
- 15.Croockewit A, Raymakers RA, Trilsbeek C, Dolstra H, Pennings A, De Witte TJ, et al. Peripheral blood cell harvests yield primitive multilineage progenitor cells in the CD34+/33− fraction. Int J Artif Organs. 1993;16(Suppl 5):83–88. [PubMed] [Google Scholar]
- 16.Burgstaler EA, Pineda AA, Winters JL. Hematopoietic progenitor cell large volume leukapheresis (LVL) on the Fenwal Amicus blood separator. J Clin Apher. 2004;19:103–111. doi: 10.1002/jca.20011. [DOI] [PubMed] [Google Scholar]
- 17.Demirer T, Buckner CD, Gooley T, Appelbaum FR, Rowley S, Chauncey T, et al. Factors influencing collection of peripheral blood stem cells in patients with multiple myeloma. Bone Marrow Transplant. 1996;17:937–941. [PubMed] [Google Scholar]
- 18.Desikan KR, Tricot G, Munshi NC, Anaissie E, Spoon D, Fassas A, et al. Preceding chemotherapy, tumour load and age influence engraftment in multiple myeloma patients mobilized with granulocyte colony-stimulating factor alone. Br J Haematol. 2001;112:242–247. doi: 10.1046/j.1365-2141.2001.02498.x. [DOI] [PubMed] [Google Scholar]
- 19.Kumar S, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK, Gastineau DA, et al. Impact of lenalidomide therapy on stem cell mobilization and engraftment post-peripheral blood stem cell transplantation in patients with newly diagnosed myeloma. Leukemia. 2007;21:2035–2042. doi: 10.1038/sj.leu.2404801. [DOI] [PubMed] [Google Scholar]
- 20.Paripati H, Stewart AK, Cabou S, Dueck A, Zepeda VJ, Pirooz N, et al. Compromised stem cell mobilization following induction therapy with lenalidomide in myeloma. Leukemia. 2008;22:1282–1284. doi: 10.1038/sj.leu.2405100. [DOI] [PubMed] [Google Scholar]
- 21.Dyson PG, Horvath N, Joshua D, Barrow L, Van Holst NG, Brown R, et al. CD34+ selection of autologous peripheral blood stem cells for transplantation following sequential cycles of high-dose therapy and mobilization in multiple myeloma. Bone Marrow Transplant. 2000;25:1175–1184. doi: 10.1038/sj.bmt.1702408. [DOI] [PubMed] [Google Scholar]
- 22.Tricot G, Jagannath S, Vesole D, Nelson J, Tindle S, Miller L, et al. Peripheral blood stem cell transplants for multiple myeloma: identification of favorable variables for rapid engraftment in 225 patients. Blood. 1995;85:588–596. [PubMed] [Google Scholar]
- 23.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar SK. High-dose chemotherapy with autologous hematopoietic stem cell transplantation in patients with multiple myeloma. Expert Rev Anticancer Ther. 2006;6:343–360. doi: 10.1586/14737140.6.3.343. [DOI] [PubMed] [Google Scholar]
- 24.Alegre A, Tomas JF, Martinez-Chamorro C, Gil-Fernandez JJ, Fernandez-Villalta MJ, Arranz R, et al. Comparison of peripheral blood progenitor cell mobilization in patients with multiple myeloma: high-dose cyclophosphamide plus GM-CSF vs G-CSF alone. Bone Marrow Transplant. 1997;20:211–217. doi: 10.1038/sj.bmt.1700867. [DOI] [PubMed] [Google Scholar]
- 25.Mazumder A, Kaufman J, Niesvizky R, Lonial S, Vesole D, Jagannath S. Effect of lenalidomide therapy on mobilization of peripheral blood stem cells in previously untreated multiple myeloma patients. Leukemia. 2008;22:1280–1281. doi: 10.1038/sj.leu.2405035. author reply 1281–2. [DOI] [PubMed] [Google Scholar]
- 26.Tarella C, Boccadoro M, Omede P, Bondesan P, Caracciolo D, Frieri R, et al. Role of chemotherapy and GM-CSF on hemopoietic progenitor cell mobilization in multiple myeloma. Bone Marrow Transplant. 1993;11:271–277. [PubMed] [Google Scholar]
- 27.Scott MA, Ager S, Apperley JF, Jestice HK, Bloxham DM, Boraks P, et al. Peripheral blood progenitor cell harvesting in multiple myeloma and malignant lymphoma. Leuk Lymphoma. 1995;19:479–484. doi: 10.3109/10428199509112208. [DOI] [PubMed] [Google Scholar]
- 28.Gertz MA, Lacy MQ, Dispenzieri A, Hayman SR, Kumar SK, Leung N, et al. Transplantation without growth factor: engraftment kinetics after stem cell transplantation for primary systemic amyloidosis (AL) Bone Marrow Transplant. 2007;40:989–993. doi: 10.1038/sj.bmt.1705848. [DOI] [PubMed] [Google Scholar]
- 29.Narayanasami U, Kanteti R, Morelli J, Klekar A, Al-Olama A, Keating C, et al. Randomized trial of filgrastim versus chemotherapy and filgrastim mobilization of hematopoietic progenitor cells for rescue in autologous transplantation. Blood. 2001;98:2059–2064. doi: 10.1182/blood.v98.7.2059. [DOI] [PubMed] [Google Scholar]
- 30.Galotto M, Berisso G, Delfino L, Podesta M, Ottaggio L, Dallorso S, et al. Stromal damage as consequence of high-dose chemo/radiotherapy in bone marrow transplant recipients. Exp Hematol. 1999;27:1460–1466. doi: 10.1016/s0301-472x(99)00076-4. [DOI] [PubMed] [Google Scholar]
- 31.Banfi A, Bianchi G, Galotto M, Cancedda R, Quarto R. Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences and possibilities of treatment. Leuk Lymphoma. 2001;42:863–870. doi: 10.3109/10428190109097705. [DOI] [PubMed] [Google Scholar]
- 32.Corazza F, Hermans C, Ferster A, Fondu P, Demulder A, Sariban E. Bone marrow stroma damage induced by chemotherapy for acute lymphoblastic leukemia in children. Pediatr Res. 2004;55:152–158. doi: 10.1203/01.PDR.0000099773.71438.91. [DOI] [PubMed] [Google Scholar]
- 33.Li J, Law HK, Lau YL, Chan GC. Differential damage and recovery of human mesenchymal stem cells after exposure to chemotherapeutic agents. Br J Haematol. 2004;127:326–334. doi: 10.1111/j.1365-2141.2004.05200.x. [DOI] [PubMed] [Google Scholar]
- 34.Kushner BH, Gulati SC, O’Reilly RJ, Heller G, Cheung NK. Autografting with bone marrow exposed to multiple courses of very high dose cyclophosphamide in vivo and to 4-hydroperoxy-cyclophosphamide in vitro. Med Pediatr Oncol. 1990;18:454–458. doi: 10.1002/mpo.2950180604. [DOI] [PubMed] [Google Scholar]
- 35.Spyridonidis A, Kuttler T, Wasch R, Samek E, Waterhouse M, Behringer D, et al. Reduced intensity conditioning compared to standard conditioning preserves the in vitro growth capacity of bone marrow stroma, which remains of host origin. Stem Cells Dev. 2005;14:213–222. doi: 10.1089/scd.2005.14.213. [DOI] [PubMed] [Google Scholar]


