Abstract
BACKGROUND
The precise role of androgen receptor (AR) in the normal development of prostate and the progression of prostate cancer (CaP) remains controversial. While AR expression and activity is associated with growth arrest and differentiation of normal prostate cells, it is maintained in CaP cells that are characterized by continued proliferation. Our objective was to determine the importance of AR signaling for survival and growth of CaP cells, particularly those with a hormone-refractory phenotype.
METHOD
AR expression was modulated in androgen-sensitive (AS) and androgen-insensitive (AI) CaP cells using RNAi and cDNA transduction. Resulting changes in AR transcriptional activity and cell growth were quantified.
RESULTS
Interference with AR expression in both AS and AI CaP cells by shRNA transduction demonstrated a direct correlation between residual AR expression and cell viability. CaP cells lacking AR expression undergo apoptosis several days after AR down-regulation. This delayed response suggests that AR regulates apoptosis likely through an indirect mechanism. Overexpression of AR or hyper-stimulation of AR with high levels of androgen was also poorly tolerated by CaP cells. Cells with elevated AR had a growth disadvantage due to G1 cell cycle arrest and induction of p21 and GADD45 expression.
CONCLUSIONS
CaP cells expressing endogenous AR are sensitive to both increases and decreases in AR expression levels and activity. AR in CaP cells is delicately regulated to provide a balance between cell death and continued proliferation. Thus, both approaches, inhibition and over-stimulation of AR activity, may have therapeutic value for treatment of prostate cancer.
Keywords: prostate cancer, androgen receptor
INTRODUCTION
Prostate cancer (CaP) is the most common neoplastic disease in men and the second leading cause of cancer-related deaths, claiming about 40,000 men each year in the United States. For more than 50 years, the preferred treatment for CaP has been androgen ablation therapy combined with surgical prostatectomy. Androgen ablation therapy is a specific and effective treatment for early stage CaP because of the unique hormone-dependency of the prostate gland [1–3]. Elimination of androgen production through castration results in dramatic and rapid changes in the prostate, leading to tissue atrophy through massive apoptosis of prostate epithelial cells [4,5]. Several mechanisms have been proposed to explain this phenomenon, including androgen receptor (AR)-dependent expression of anti-apoptotic factors [6–8]. Nevertheless, the exact mechanism by which androgen impacts the survival and proliferation of normal and cancerous prostate cells remains poorly understood. During normal prostate development, expression of AR and initiation of androgen-induced AR signaling are associated with growth arrest and differentiation of prostate epithelial cells [9]. In contrast to normal prostate epithelial cells, prostate tumor cells, which typically express AR and maintain active AR signaling, continue to proliferate. The reason for this difference in cellular response to AR signaling as well as the identity of key AR transcriptional target(s) that regulate the response are as yet undefined.
Advanced stage CaP is associated with progression of the tumor to a hormone-refractory or androgen-independent state in which tumor cells can grow in the absence of androgen, although in the majority of cases AR signaling is still active in such tumors [10,11]. This observation raises the questions of whether AR signaling is indispensable for development and progression of CaP [both androgen-sensitive (AS) and -insensitive (AI)] and what molecular mechanisms underlie the role of AR in CaP. Several lines of evidence indicate that AR function contributes to tumor cell survival after androgen ablation and to the growth of androgen-independent CaP [5,12].
As mentioned above, analysis of AR expression and function in CaP established the counterintuitive fact that hormone-independence of CaP is almost never associated with inactivation of AR signaling. Rather, tumor cells adapt through different mechanisms to maintain constant stimulation of the androgen pathway even in the absence of hormone stimulation [1,5,12]. Thus, the majority of prostate cancers retain their dependence on AR signaling even as they progress to androgen-independence, making AR an excellent prospective target for therapeutic intervention. There have been several recent reports demonstrating that inhibition of AR expression leads to attenuation of CaP cell growth in vitro and in vivo [23,24], but the mechanism underlying this phenomenon was unclear. In our study we have genetically validated the hypothesis that the ability of androgen-insensitive CaP cells to grow in the absence of androgen depends upon AR signaling independence of ligand stimulation. Inhibition of AR signaling through RNAi technology decreased survival of both androgen-dependent and -independent CaP cell lines through apoptotic cell death. We also demonstrate, however, that proliferation of these same CaP cells is suppressed following significant up-regulation of AR signaling through either supra-physiological hormone stimulation or overexpression of AR. Thus, the viability and proliferation of CaP cells require a strictly regulated level of AR activity: enough to prevent them from undergoing apoptosis, yet not so much as to arrest proliferation. The dual role played by AR in CaP cell growth that we have identified in this study suggests that modulation of AR signaling in either direction, inhibition or stimulation, might have therapeutic potential as a prostate cancer treatment. Importantly, this strategy would be able to target later stage AI cancers for which there is currently no effective treatment.
MATERIALS ANDMETHODS
Cells and Chemicals
LNCaP, 22Rv1, PC3, DU145, A293, Hela, and HT1080 cells were obtained from ATCC. C4-2 and CWR22R cells were provided by Warren Heston [Department of Cancer Biology, Cleveland Clinic Foundation (CCF), Cleveland, OH]. All prostate cancer cells were maintained in RPMI 1640 medium supplemented with 10% FBS, 1 mM sodium pyruvate, 10 mM Hepes buffer, 55 nM β-mercaptoethanol and antibiotics. Other cells were maintained in DMEM with 10% FBS and antibiotics. Charcoal-stripped serum (CSS) was purchased from Biosource. For experiments with CSS, phenol red-free medium was used with the additives listed above. Dehydrotestosterone (DHT) was obtained from the CCF Pharmacy Department.
Plasmids
The pARE-Luc luciferase reporter construct designed to measure AR transcriptional activity is shown schematically in Figure 1A. Retroviral shRNA vectors were generated by insertion of the H1 promoter and a cassette for cloning of shRNA into the right LTR of the pLPCHygro retroviral plasmid (Clontech) or pLSLPw lentiviral plasmid (kindly provided by P. Chumakov, Department of Molecular Genetics, CCF). shRNAs were designed as described in [13], using the following sequences: shAR1: gctcaaggatggaagtgca; shAR2: gctgctccgctgaccttaa; shAR3: tctctgtgcaagtgcccaa; shGFP [13]; shFasL:: tgcagcagcccttcaatta. For AR overexpression experiments, the full-length AR cDNA (kindly provided by AO Brinkmann, Department of Biochemistry, Erasmus University, Rotterdam, The Netherlands) was cloned into the pcDNA3.1hygro plasmid (Invitrogen) and the pLV-CMV lentiviral vector (provided by Inder Verma, Salk Institute, CA).
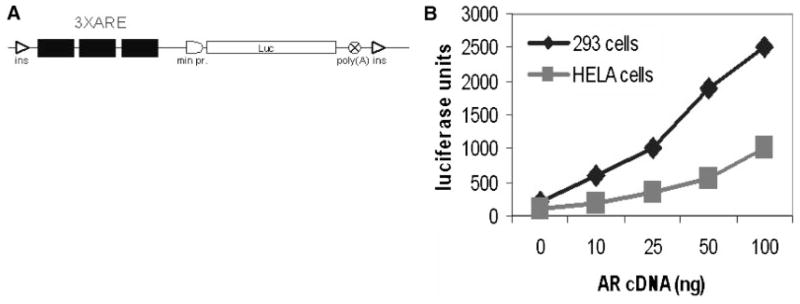
Fig. 1.
Structure and AR-responsiveness of the ARE-Luc reporter construct. A: A reporter construct sensitive to regulation by AR was generated by insertion of three repeated androgen responsive elements from the rat probasin gene promoter with flanking regions as described in Ref.[37] together with the minimal promoter from the human Hsp70 gene into plasmid pConA-Luc[15]. The engineered luciferase expression cassette was flanked with two insulator (ins) elements to diminish the effects of integration site. B: Responsiveness of the ARE-Luc reporter to different levels of AR. 293 and Hela cells were transfected with 0.5μg of pARE-Luc DNA and increasing amounts of AR cDNA. Luciferase activity was measured in cell lysates 48 hr after transfection. Neither Hela or 293 cells express endogenous AR. This experiment was performed in media containing regular FBS, but no added DHT.
Transfection was done using Lipofectamine Plus reagent (Invitrogen) according to the manufacturer’s instructions.
Retroviral packaging and transduction were performed as described [14]. Briefly, Ampho packaging cells (Clontech) were transfected with the retroviral expression vector. Culture supernatants containing virus were collected at 48 hr post-transfection and immediately transferred onto target cells with the addition of 8 μg/ml polybrene (Sigma). Twenty-four hours later, the medium was changed to one containing the appropriate antibiotic for selection. After complete death of control-untransduced cells (typically 10–14 days in selection medium), the number of colonies was quantitated or cells were used for further experiments.
Lentiviral packaging and transduction were performed as previously described [15]. Briefly, 293 cells were transfected with equal amounts of lentiviral expression vector, packaging plasmid pLV-CMV-delta 8.2 (provided by Inder Verma) and pVSV-G plasmid (Clontech) for pseudotyping of viral capsid with VSV-G protein. Virus-containing supernatants were collected at 48 and 96 hr post-transfection and pooled. In some cases, virus was concentrated 20-fold by incubation of the supernatants overnight at 4°C in the presence of 40% PEG8000 followed centrifugation at 6,000 rpm. The resulting pellet of protein and virus was dissolved in cell culture medium and stored at −80°C. Target cells were transduced by incubation with virus-containing medium for 24 hr. Virus titer was determined either by using a green fluorescence protein (GFP)-encoding virus or by transduction of AR negative Hela cells with AR virus followed by immunofluorescent staining with anti-AR antibodies 48 hr after transduction.
siRNA transfection was done according to Dharmacon protocol using Dharmafect reagent. One hundred nano-molar of Dharmacon siRNA mixtures specific for either AR or GAPDH were used per well of six-well plates. siGLO (a scrambled non-specific control siRNA labeled with Cy5) from Dharmacon was added as 1/10th of the transfection mixture to monitor transfection efficiency.
Reporter Assay for AR Activity
Reporter assays were performed using two different protocols: (1) Cells were transiently transfected with the pARE-Luc plasmid and pcDNA-3.1 hygro plasmids (empty or AR-containing) or shRNA constructs in different proportions (see details in figure legends). Reporter activity was measured at 48 hr using the Luciferase Assay System (Promega). Transfection efficiency was normalized by cotransfection of pCMV-LacZ or pEGFP-mito (Clontech) by ONPG staining or FACS analysis respectively. (2) Cell lines possessing an integrated ARE-Luc reporter construct were generated by transfection of cells with pARE-Luc followed by selection using G418. Reporter activity was measured by Luciferase Assay System (Promega). Normalization in this case was based upon the total protein content of cell lysates (DC Protein Assay, BioRad).
Cell Survival Assay
To measure the androgen dependence of cell survival cells was plated in 12-well plates at 2 × 104/well in duplicate. The next day the media were removed, the cells were washed with PBS and then phenol red-free RPMI-1640 containing either FBS or CSS (and standard additives described above) was added. For wells with CSS-containing medium, DHT was added to the indicated final concentrations. Medium was changed every 48 hr. Two plates for each cell type were either (i) fixed and stained with 0.5 μg/ml methylene blue in 50% methanol solution for determination of relative cell number, or (ii) lysed with Cell Culture Reporter Lysis Reagent (Promega) for determination of AR activity by Luciferase Reporter Assay (Promega). Methylene blue staining was quantitated by extraction with 1%SDS in PBS solution and measurement of absorbance at λ = 600. Luciferase readings were normalized to the total protein content of each lysate (DC Protein Assay, BioRad). The data are representative of three independent experiments.
For lentiviral transduction experiments, cells were plated in six-well plates at 105 cells/well. The following day, cells were transduced with concentrated GFP- or AR-encoding lentiviruses, resulting in 50–100% transduction efficiency as measured by GFP fluorescence. Twenty-four hours later, the virus-containing media were removed, cells were washed with PBS and then phenol red-free RPMI-1640 with CSS (and the standard additives described above) with or without 0.3 nM DHT was added. The medium was changed every 48 hr. Cells were collected for Western blotting and luciferase assays at the indicated time points or fixed and stained with methylene blue on day 8 post-transduction.
Analysis of Cell Cycle Distribution
Approximately 105 cells were removed from culture dishes using trypsin, washed with PBS and resuspended in 300 μl 3%BSA in PBS. Five milliliters of 70% ethanol was then added dropwise. Cells were kept at −20°C for several hours and then stained with 10 μg/ml propidium iodide in the presence of 30 μg/ml RNase A at 37°C for 2 hr. Cell cycle distribution based upon propidium iodide staining of DNA content was analyzed using a FACS Calibur instrument (Becton Dickinson) and CellQuest software.
Western Blot Analysis
Cells were lysed in Cell Culture Reporter Lysis Reagent (Promega). Protein concentrations were determined with Dc Protein Assay (BioRad). Equal protein amounts were run on precast 4–20% gradient gels (Novex) and blotted onto PVDF membranes (Amersham). The following antibodies were used: anti-pAR—monoclonal mouse (Pharmigen, BD), anti-p21—monoclonal mouse F-5 (Santa-Cruz), anti-GADD45γ—mouse monoclonal (Santa-Cruz), anti-caspase 3—Rabbit polyclonal (Cell Signaling), HRP-conjugated secondary antibodies were purchased from Santa-Cruz. Proteins were visualized using ECL detection reagent (Amersham) and quantitation of the data was performed using Quantity One software from BioRad.
RESULTS ANDDISCUSSION
Growth of Prostate Cancer Cells In Vitro Depends Upon the Transcriptional Activity of AR
CaP cells can be divided into two categories based upon their androgen dependence: AS cells that display decreased proliferation in the absence of androgens and AI cells that continue to proliferate in the absence of androgens. The effects of androgens on cells are mediated through the activity of the AR. AR is a ligand-activated transcription factor that regulates expression of several dozen target genes. It is unclear, however, whether the effects of androgens on cellular proliferation result from the transcriptional activity of AR or from another as yet unidentified effects of AR. In AS cells, both the level of AR transcriptional activity and cellular proliferation are significantly reduced in the absence of androgens [16,17]. In contrast, the effect of androgen withdrawal on AR transcriptional activity in AI cells remains controversial [18]. Some studies report that androgen ablation leads to decreased AR transcriptional activity in AI cells, but that they continue to proliferate [19]. In other studies, however, AR activity as well as growth was shown to be androgen independent in AI cells [18,20]. We set out to resolve this issue by independently testing the effects of androgen on AR-mediated transcription and proliferation of AS and AI CaP cells. The effects of androgen were analyzed using media supplemented with regular FBS (typically containing 0.1–1 nM of DHT) as compared to CSS lacking DHT with or without known amounts of DHT added back. The role of AR itself was independently addressed by either inhibiting its expression using RNAi or inducing its overexpression using cDNA transduction protocols.
In order to both allow comparison with and expand upon previous studies, we chose well-characterized AS and AI CaP cell lines for our study. Although there are some AI CaP cell lines that have completely lost AR expression, for example, PC3 and DU145 [18], this does not reflect the AR status of most human tumors. Moreover, PC3 and DU145 cells do not express other prostate markers, including PSA and PSMA, known to be expressed in CaP. We, therefore, chose to use CaP cell lines that, like human tumors, retain AR expression and, presumably, AR signaling. This would allow us the opportunity to experimentally modulate expression of the endogenous AR. The cell lines used were initially isolated from patients with androgen dependent CaP but acquired androgen independency through experimental selection. These include AS LNCaP cells and their derivative, C4-2, which was isolated from the xenograft of LNCaP cells grown in castrated animals [21]. Two androgen-independent derivatives of CWR22 cells (grown only as xenografts in mice [22]), CWR22R, and 22Rv1 (grown routinely in culture [23]) were also chosen. All of these cells express endogenous AR as well as other prostate specific markers such as PSA or PSMA [24]. The AR gene in all of these cell lines possesses different mutations that frequently occur in CaP patients. These mutations, as well as most other AR mutations identified in CaP tumors, do not abrogate AR transactivation function, but rather make AR more promiscuous in terms of ligand type and concentration sufficient for activation [2]. The presence of AR mutations in primary tumor specimens indicates that the sequence changes may be functionally important. Moreover, the fact that the mutations typically enable AR activity suggests that AR signaling (whether androgen-activated or not) is crucial for CaP cell growth.
In order to specifically and quantitatively monitor AR-mediated transactivation, we transfected cells with an AR-responsive reporter construct (illustrated schematically in Fig. 1A). In some experiments cells were transiently transfected with the reporter, while in others a drug-selected population of cells with an integrated reporter was used. Appropriate dose-responsive activity of the reporter was confirmed by transfecting of different doses of AR cDNA into AR-negative cells and by treating AR-expressing cells with different amounts of androgen (Figs. 1B and 2B).
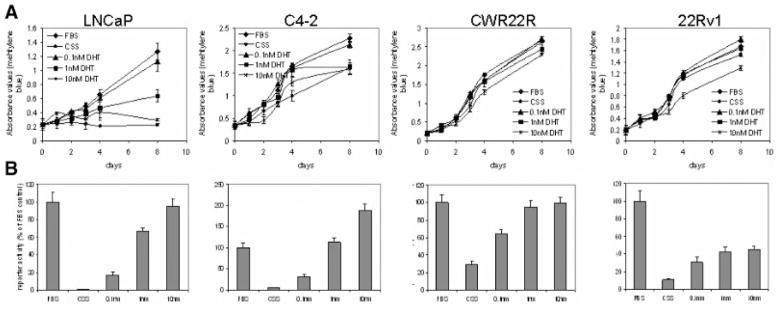
Fig. 2.
Regulation of growth and AR-mediated transcription by DHT in different CaP cell lines. Androgen sensitive LNCaP and androgen insensitive C4-2, CWR22R and 22Rv1 cells with integrated pARE-Luc were grown in media containing regular FBS or charcoal-stripped serum (CSS) with DHT added to the indicated concentration. A: Growth of CaP cells over time as measured by methylene blue staining. 104 cells/well were plated in 12-well plates. After the cells had attached (day 0), the media were changed to contain FBS, CSS, and DHT as indicated. Plates were fixed on the indicated days and stained with methylene blue. Quantitation was done by eluting of staining with 1% SDS and spectrophotometry at λ = 600 nm. B: Cells from the experiment described in A were lysed on day 8. Luciferase activity was measured and normalized to the total amount of protein in the lysates. Data are presented as the percentile of ARE-Luc reporter activity in FBS medium.
The androgen-dependence of cell growth and AR activity was analyzed for each of the cell lines (Fig. 2) by adding different concentrations of DHT to culture medium supplemented with CSS rather than FBS. Cell growth was monitored by determining cell number at different time points using methylene blue staining and AR-mediated transactivation of the pARE-Luc reporter was assessed using luciferase assays.
The data show that in LNCaP cells, the absence of DHT results in both reduced AR transactivation activity and elimination of cell growth (Figs. 2A and 2B). However, the dose-dependence of the two effects was very different. While DHT stimulated AR-dependent reporter activity in a dose-dependent manner (up to 250-fold with 10 nM DHT), proliferation of LNCaP cells was stimulated only by low physiological doses of DHT (0.1–1 nM). Higher levels of DHT (10 nM) did not stimulate proliferation of LNCaP cells.
The growth rate of C4-2 cells was somewhat retarded in the absence of androgen (Fig. 2A,B). DHT stimulated proliferation of these cells minimally, but induced significant AR-dependent reporter activity, although the effect was somewhat less than in LNCaP cells (45-fold induction of AR activity by 10 nM DHT).
The growth of CWR22R and 22Rv1 cells was only minimally affected by the absence or presence of DHT. Similarly, although AR activity was induced by DHT, the effect was much less striking than that observed in LNCaP and C4-2 cells. While 10 nM DHT resulted in 45- and 250-fold inductions of AR activity in C4-2 and LNCaP cells, respectively, this dose of DHT only activated AR ~3.5 to 9-fold in the truly AI CWR22R and 22Rv1 cell lines. Although physiological levels of DHT (0.1 and 1 nM) did not affect proliferation of CWR22R and 22Rv1 cells, high doses of DHT (10 nM) had a minor growth suppressing effect, particularly in 22Rv1 cells (Fig. 2A). Growth of AR-negative Hela cells was not affected by DHT and these cells showed no pARE-Luc reporter activity regardless of DHT dose (data not shown). This control indicates that AR is the primary cellular target of DHT and that modulation of AR activity underlies the DHT mediated regulation of CaP cells growth properties.
These experiments demonstrate that there is not a linear correlation between AR transcription and CaP cell proliferation, but rather that the relationship between these two cellular processes and their androgen-responsiveness is complicated. In fact, the data obtained in LNCaP and C4-2 cells clearly show that the effects of androgen on AR activity and on cell growth are inversely correlated. AR activity increases in a dose-dependent manner with 0.1, 1, and 10 nM DHT. In contrast, cell growth decreases over the same range of doses. These findings suggest that hyper-activation of AR is growth suppressive as will be discussed further below.
In addition, we find that the androgen-insensitivity of cell growth is not necessarily accompanied by complete androgen-independence of AR activity. That is, absence of androgen was found to decrease, but not completely silence AR signaling. The extent of this decrease correlated well with the proliferative responses of CaP cells in our experiments. The largest decrease in AR activity (more than 250-fold, compare FBS and CSS samples) occurred in the cell line showing the greatest degree of androgen-sensitivity for cell growth. C4-2 cells were intermediate in both transcriptional and growth responses to androgen (partially androgen sensitive, “PAS”) and the two AI cell lines, CWR22R and 22Rv1, demonstrated the smallest drop in AR activity following androgen removal and no effect of androgen on cell growth. Based upon these data, we conclude that the degree of androgen insensitivity for growth that is displayed by different CaP cells correlates with the level of residual AR activity in the absence of ligand stimulation. Based upon our idea that residual androgen-independent AR activity supports the viability and growth of PAS and AI CaP cells, we next sought to analyze the effect of blocking this residual AR activity.
Effect of AR Knockdown on the Growth of AS and AI CaP Cells
Based upon the fact that CaP cells very rarely lose AR signaling during cancer progression and our demonstration of the correlation between residual AR activity and CaP cell growth (see above), we proposed that complete inhibition of AR transcriptional activity would be lethal for both AS and AI CaP cells. While we were in the process of testing this hypothesis, several reports appeared confirming that inhibition of AR expression interferes with CaP cell growth in vitro and in vivo [25,26]. The mechanism underlying this growth suppression remains unclear, since one of these studies [24] showed that CaP cells with inhibited AR expression died from apoptosis and the other showed that cells were growth arrested [23]. To clarify this issue and, in particular, determine the effects of AR inhibition on the growth of AI CaP cells, we used RNAi technology to completely block AR activity in CaP cells insensitive to androgen withdrawal.
In order to specifically abrogate endogenous AR expression, we synthesized several shRNA constructs targeting different portions of the AR mRNA using the loop model described by others [13]. To choose the most active shRNA constructs from the synthesized pool, we tested the effects of AR shRNAs in cotransfection experiments using the AR-responsive reporter pARE-Luc in cells with endogenous AR (LNCaP), assuming that loss of AR expression would lead to a corresponding drop in luciferase activity. Control vectors directing expression of shRNAs targeting GFP or FasL were tested in parallel. As shown in Figure 3A, all three AR-specific shRNAs suppressed, to some degree, transactivation of the luciferase reporter by endogenous AR in LNCaP cells. In contrast, shRNAs targeting GFP and FasL did not reduce AR-dependent reporter activity, indicating that the effect of the AR shRNAs was specific. shAR1 was more effective than shAR2 and shAR3 in suppressing AR activity in all cell lines tested (Fig. 3A and data not shown). Cotransfection experiment set up did not allow us to demonstrate this by AR protein expression analysis due to low transfection efficiency in CaP cells (around 10%).
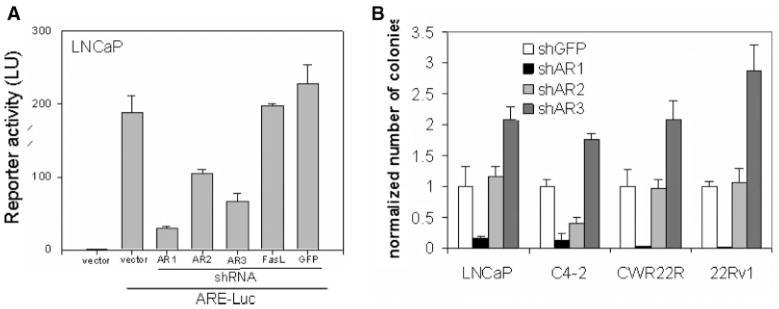
Fig. 3.
Effect of knockdown of AR expression in CaP cells. A: Effect of shRNA constructs on ARE-Luc reporter activity in LNCaP cells. LNCaP cells were cotransfected with pARE-Luc and the anti-AR shRNA constructs, shAR1, shAR2, and shAR3, or the control shRNA constructs, shGFP and shFasL. At 48 hr post-transfection, luciferase activity was measured in cell lysates. Transfection efficiency was normalized by cotransfection of pCMV-LacZ plasmid. Luciferase activity is shown relative to that of untransfected LNCaP cells (set at 1.0). This experiment was performed in media with regular FBS. B: Effect of anti-AR shRNA constructs on the growth of different CaP cells. The indicated cell lines were transduced with retroviral shRNA constructs and grown in selection media containing hygromycin for 2 weeks. This experiment was done in medium containing regular FBS. At the end of experiments plates with colonies of cells were stained with methylene blue. Quantitation of methylene blue staining was done by reading the absorbance of the eluted stain. The “normalized number of colonies” indicated on the y-axis was determined by setting the methylene blue absorbance of shGFP transduced cells at 1.0 for each cell line. Control of transduction efficiency was done by using AR negative DU145 (for quantitation number of colonies of each cell line was divided by number of colonies of DU145 cells transduced with corresponding virus).
To assess the dependence of CaP cells on AR expression, we transduced several CaP cell lines with retroviral vectors expressing the three different antiAR shRNAs and measured their ability to grow in selective medium containing hygromycin. As a negative control each cell line was also transduced with an anti-GFP shRNA vector. To demonstrate specificity, the AR-negative CaP cell line, DU145, was also transduced with the same panel of shRNA constructs. The results of this experiment, demonstrate that only AR1 shRNA significantly reduced growth of CaP cells (Fig. 3B) even in the presence of androgen (FBS-containing medium was used in this experiment). This was the AR shRNA construct that was most effective in reducing AR-dependent reporter activity (Fig. 3A). The growth of all tested CaP cells expressing endogenous AR was reduced by AR1 shRNA. Only C4-2 cells showed significant growth inhibition by shAR2 and no cell line was inhibited by the shAR3 construct. Although shAR3 construct did reduce pARE-Luc activity somewhat, it consistently led to increased cell growth, above and beyond control shGFP-transduced cells. This may indicate that weak suppression of AR expression and activity has a growth promoting effect, but this hypothesis requires additional testing since this effect may be an indirect artifact of the specific shRNA construct. Nevertheless, our data clearly demonstrate that only significant inhibition of AR expression is toxic to CaP cells, regardless of their androgen sensitivity status. Partial decreases in AR expression achieved with less potent shAR constructs are easily circumvented by CaP cells without an inhibitory effect on their growth.
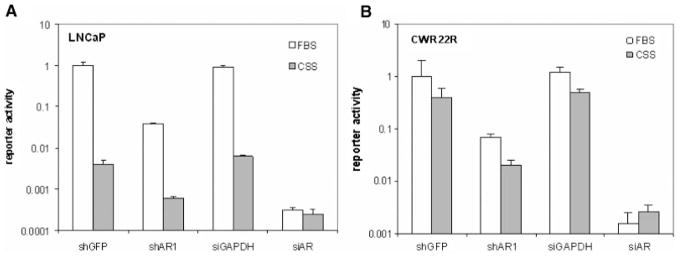
Thus, we have shown that suppression of AR expression by RNAi leads to growth inhibition of all CaP cells (AS and AI), while androgen withdrawal only inhibits AS LNCaP cell growth and not that of AI cell lines such as CWR22R and 22Rv1. We propose that this may be due to different degree of inhibition of AR function achieved with RNA silencing or androgen withdrawal in different cell lines. Efficient RNA silencing completely block AR expression and function, while androgen withdrawal effect depends on cell background and sensitivity of AR in certain cells to androgen stimulation. To compare the degree of AR transcription suppression achieved by RNAi and androgen withdrawal, AD (LNCaP, Fig. 4A) and AI (CWR22R, Fig. 4B) cells were cotransfected with the pARE-Luc reporter and shRNA vectors and grown in media supplemented with either regular FBS (DHT+) or CSS (DHT−). In addition to the shRNA vectors, we also tested siRNA mixtures from Dharmacon targeting AR and GAPDH as a control (On-Target Smart pool from Dharmacon) in this experiment. We expected that if either RNAi or androgen withdrawal produced partial inhibition of AR activity, simultaneous application of the other method would result in an increased level of inhibition. Complete inhibition of AR function, however, would be evident when neither method could increase the inhibitory effect of the other. Similar results were obtained in both cell lines, showing that only siAR RNA transfection completely inhibits AR function (Fig. 3B,C). This is illustrated by the similar extent of siAR-mediated inhibition of AR activity in FBS medium and in CSS medium. That is, androgen withdrawal cannot reduce AR activity any further than what is achieved by siAR transfection alone. These data allow us to conclude that only efficient inhibition of AR function (either by androgen withdrawal in LNCaP cells or by RNAi in CWR22R cells) results in significant growth inhibition.
Fig. 4.
Comparison of the effects of anti-AR shRNA and siRNA transfection with and without androgen withdrawal on AR-dependent reporter activity. LNCaP (A) and CWR22R (B) cells were cotransfected with either shRNA vectors (shAR1 or shGFP) or siRNA mixtures from Dharmacon (AR or GAPDH) together with the ARE-Luc reporter. Twenty-four hours after transfection cells were split and transferred to either FBS- or CSS-containing media. After an additional 24-hr incubation, luciferase activity was measured. Reporter activity is shown relative to that of cells transfected with shGFP and kept in FBS-containing medium (set at 1.0).
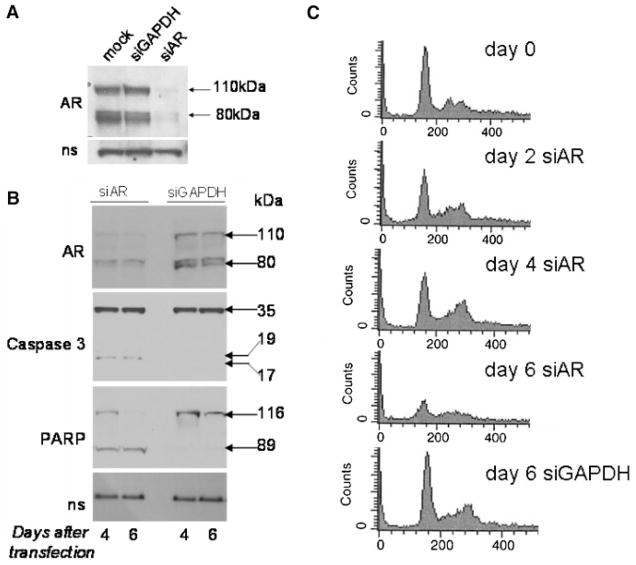
To determine whether the growth suppression observed upon down-regulation of AR activity was due to cell death (as opposed to growth arrest), we performed a number of apoptosis assay. We transfected the most androgen independent cell line, CWR22R, with the anti-AR siRNA mixture from Dharmacon that gave the most complete inhibition of AR function based upon the results shown in Figure 4B. Since siRNA-mediated inhibition of AR activity was not enhanced by androgen withdrawal (Fig. 4B), this experiment was run in standard FBS-containing medium. In contrast to viral transduction, siRNA transfection leads to a rapid and effective decrease in AR expression that is already evident 48 hr after transfection (Fig. 5A). To examine cell death, we first monitored the cell cycle distribution of transfected cells [as judged by fluorescent labeling of the siRNA (siGLO, Dharmacon)] by FACS analysis of propidium iodide stained cells (Fig. 5C). Changes in cell cycle distribution as well as visible cell death were observed starting from day 6 after transfection of siAR RNA mixture. We did not observe any changes in the cell cycle distribution of HT1080 cells transfected with siRNAs against either AR or GAPDH (data not shown). To determine whether the changes in cell cycle distribution after AR inhibition represented cells that had undergone apoptosis, we lysed cells on days 4 and 6 after siAR transfection for Western blot analysis of caspase 3 activation and PARP cleavage, two hallmarks of apoptotic cell death. Both apoptosis markers started to be seen already on day 4 after transfection but became more pronounced on day 6 (PARP cleavage) in cells transfected with anti-AR siRNA, yet absent in control cells transfected with anti-GAPDH siRNA. Importantly, apoptosis did not occur simultaneously with AR down-regulation, suggesting that AR prevents apoptosis through an indirect mechanism. Based upon these experiments, we conclude that down-regulation of AR expression in CaP cells results in their death through apoptosis, regardless of their androgen sensitivity. In this scenario, apoptosis may result from loss of AR-mediated expression of an anti-apoptotic factor, such bcl-xL shown by Liao et al. [25].
Fig. 5.
Inhibition of AR expression in CWR22R cells leads to cell death through apoptosis. CWR22R cells were transfected with siRNA mixtures targeting either AR or GAPDH or left untransfected (mock). siGLO (Dharmacon) was added to monitor transfection efficiency. Experiment was done in standard FBS containing medium. A: Western blot of cell lysates 48 hr after transfection probed with anti-AR antibody. Bands corresponding to the two polypeptides of the AR present in CWR22R cells [38] (110 and 80 kDa) are indicated by arrows. B: Western blots of cell lysates collected 4 or 6 days after transfection probed with anti-AR, anti-caspase 3, and anti-PARP antibodies. Cleavage of the 35 kDa Caspase 3 protein into 17 and 19 kDa fragments and of the 116 kDa PARP protein into an 89 kDa product is shown. C: FACS analysis of cells fixed and stained with propidium iodide 4 or 6 days after transfection.
CaP Cells Do Not Tolerate Overexpression of AR
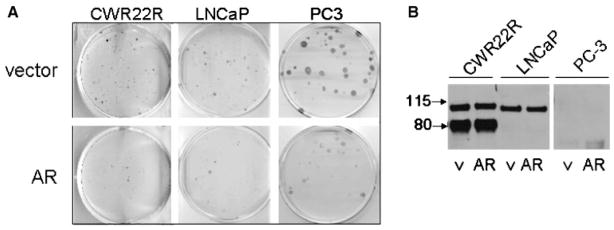
The data presented above and by others [25,26] show that AR expression is critical for CaP cell survival. Moreover, clinical data demonstrate that AR signaling is maintained in prostate tumor cells at all stages of cancer progression [2,5]. Taken together, these findings suggest that AR expression is an important determinant of prostate tumor cell survival. However, several other lines of evidence point to a growth suppressive role for AR. First, non-cancerous prostate epithelial cells (specifically the luminal secretory cells that probably give rise to prostate cancer) also express AR, yet they are quiescent both in vivo and under in vitro conditions. Second, studies have shown that it is surprisingly difficult to reconstitute AR expression in CaP cell lines that have lost expression of endogenous AR, such as PC3 and DU145 [27,28]. PC3 cells were only able to tolerate transfection of an AR expression construct that included its full-length 5′ UTR [29] resulting in a low level of AR expression and androgen stimulation of these cells resulted in growth arrest rather than proliferation [27]. Finally, in our own experiments on AR-expressing cells, high doses of DHT did not stimulate cell growth and, in some cases, actually reduced growth to the level observed in the complete absence of androgen (Fig. 2B). The mechanism underlying these apparent growth suppressive effects of AR is unclear. While at least some level of AR expression is required to protect tumor cells from apoptosis, the effects of AR overexpression on growth and survival have not been well studied. To address this question, we expressed AR in CaP cells that either have lost AR expression (PC3) or express endogenous AR with different levels of AR dependence (LNCaP, C4-2 and CWR22R). Following transfection with AR expression construct, cells were selected using hygromycin and the number of colonies was normalized for transfection efficiency based upon expression of a cotransfected pCMV-LacZ construct. In each case, expression of AR caused a significant reduction in the number of clones as compared to transfection of an empty control vector (Fig. 6A). Moreover, the rare clones that survived the selection process were found to not actually express or overexpress AR (Fig. 6B).
Fig. 6.
CaP cells do not tolerate overexpression of AR. A: Photographs of methylene blue-stained plates of cells. CWR22R (expressing endogenous mutant AR), LNCaP (expressing endogenous mutant AR), and PC3 (AR-null) cells were transfected with either AR cDNA or empty vector and selected in media containing hygromycin for 14 days. Transfection efficiency was normalized by cotransfection with pCMV-LacZ. B: Selected colonies that survived hygromycin selection following transfection with vector (v) or AR cDNA (AR) were expanded and analyzed by Western blot for AR protein levels.
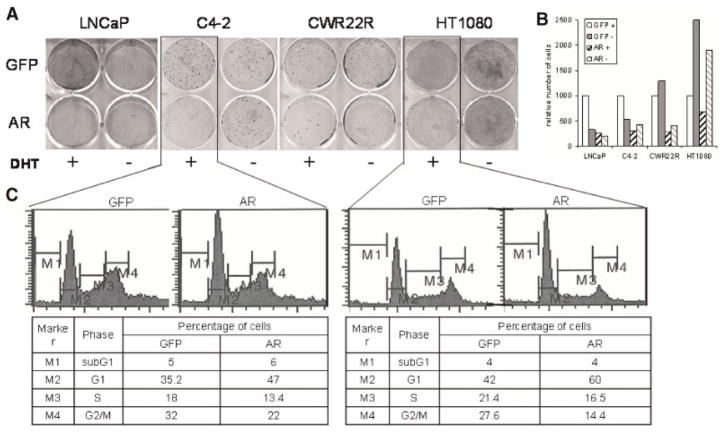
Similar results were obtained when we transduced cells with lentiviral vectors directing expression of AR or GFP as a control. This method resulted in 100% transduction efficiency as measured by GFP fluorescence. At 10 days post-transduction, the colonies of cells transduced with the AR expression construct were significantly smaller and fewer in number than those transduced with the control GFP expression construct (Fig. 7A,B, DHT(+) samples). These observations suggest that increased levels of AR in CaP cells lead to growth suppression, even in cells that express active endogenous AR and are sensitive to loss of AR expression. We also found that AR overexpression was growth suppressive in HT1080 fibrosarcoma cells that express a low level of endogenous AR (Fig. 7A,B). This may indicate that high levels of AR activity have a general growth suppressive effect on cells of different origins. Analysis of the cell cycle distribution of C4-2 CaP cells and HT1080 cells overexpressing AR revealed a higher proportion of cells in the G1 phase of the cell cycle and significantly lower proportions of cells in the S and G2/M phases (Fig. 7C). This cell cycle shift is typical of G1 growth arrest and supports the idea that overexpression of AR results in decreased cellular proliferation.
Fig. 7.
Overexpression of AR suppresses cell growth. A: The indicated cells were transduced with high-titer lentiviruses encoding AR or GFP. Twenty-four hours later, cells were split with one half maintained in CSS medium (−DHT) and the other half in CSS medium with 0.3 nM DHT (+DHT). Cells were stained with methylene blue 8 days after transduction. B: The methylene blue staining shown in (A) was quantitated by spectrophotometry of eluted stain. For each cell line the effect of transduction with GFP or AR lentiviruses in the presence (+) or absence (−) of DHT is shown. Data are shown relative to the absorbance reading of GFP-transduced cells in the presence of DHT. C: C4-2 and HT1080 cells were transduced as in (A) and grown in CSS medium with 0.3 nM DHT for 3 days. Cells were fixed and stained with propidium iodide for FACS analysis of cell cycle distribution. The percentage of cells with DNA content indicative of each cell cycle stage (defined by gates M1–M4) is indicated in the Table.
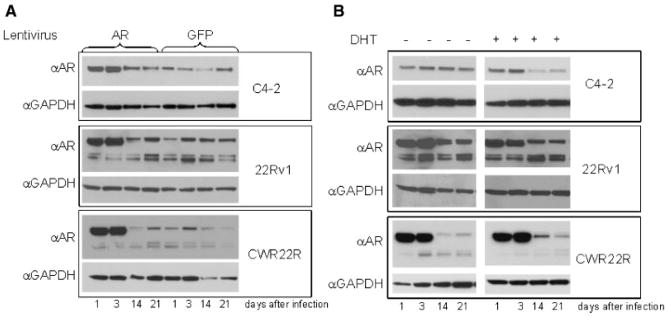
In order to explain the data shown in Figures 6 and 7, we proposed that cells overexpressing AR are lost in the process of growth due to a growth inhibitory effect of AR overexpression. To test this, we transduced cells with AR lentivirus at a dose that infected approximately 50% of the cell population. Cells were then propagated for 3 weeks in culture, with aliquots of cells taken for Western blotting throughout the entire time course. As shown in Figure 8A, the results of this experiment demonstrate that AR was overexpressed immediately following transduction but that, over time, expression gradually decreased down to the level of the GFP control cells. Importantly, there was no change over time in the level of GFP expression in pools of the same cells that were similarly transduced with a control GFP expression lentivirus (data not shown). Similar results were obtained using the same experimental protocol and examining pARE-Luc reporter activity. Transactivation by AR, after an initial increase in signal following transduction, also gradually decreased to the level of untransduced cells (Fig. 9).
Fig. 8.
AR protein levels in cells transduced with lentiviral AR cDNA decrease over time. A: The indicated cells were transduced with AR or GFP lentiviruses (at 50% transduction efficiency) in FBS medium. Cell lysates were collected at the indicated time points for Western blotting with anti-AR and anti-GAPDH antibodies. B: Part of cells transduced with lentiviral AR 24 hr after transduction were split into CSS-containing medium with (+) or without (−) DHT (0.5 nM). AR and GAPDH expression was analyzed by Western blotting of lysates collected on the indicated days.
Fig. 9.
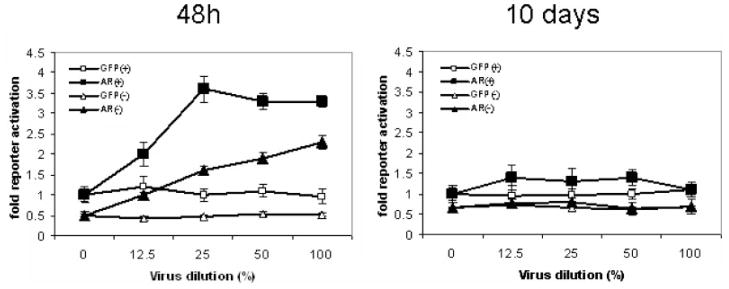
Induction of AR activity by transduction of lentiviral AR is not maintained over time. CWR22R cells stably transfected with pARE-Luc were transduced with AR- or GFP-encoding lentiviruses. Cells were maintained either in CSS (−) or in CSS +DHT(+) media. Viruses were diluted with appropriate medium as indicated on the X-axis. Luciferase activity was determined in lysates collected at 48 hr or 10 days after transduction and normalized against total protein content of each lysate. Data are shown relative to the reporter activity in mock-transduced (0%virus) cells in CSS + DHT medium.
To address the question of whether androgen-activated AR-dependent transactivation is important for the inhibitory effect of AR on cell growth, we split cells transduced with either AR or GFP lentivirus into CSS-containing media that lacks steroids (−DHT) and medium with 0.3 nM DHT (+DHT). In the absence of DHT, the growth of LNCaP and C4-2 cells over-expressing AR was not significantly different from the growth of GFP expressing cells [Fig. 7A,B, DHT(−) samples]. The growth of CWR22R cells overexpressing AR was inhibited (as compared to GFP expressing cells) even in CSS medium as it was in DHT(+) medium. These results are consistent with our proposal that the cumulative activity of mutant endogenous AR (highly active in CSS medium—Figs. 2 and 3) and wild-type exogenous AR is still high enough in these cells even in DHT(−) medium (especially taking into account the possibility of different AR variants forming hetero-dimers) to cause growth arrest in CWR22R cells. Growth of HT1080 cells was in principle better in DHT(−) medium in contrast to CaP cells, probably reflecting general growth inhibiting effect of AR function on cells of different origin.
The level of AR protein in AR-transduced C4-2 and 22Rv1 cells maintained in CSS was not as reduced in DHT(−) medium as it was in DHT(+) medium during 3 weeks of culture (Fig. 8B). CWR22R cells do not tolerate AR overexpression even if kept in DHT(−) medium in line with the results of experiment shown on Figure 7. The loss of exogenous AR expression observed in CWR22R cells even in the absence of DHT suggests that the cumulative transcriptional activity of exogenous and endogenous AR is sufficient for growth suppression. Exogenous wild-type AR is transcription-ally inactive in CSS medium in Hela, 293, LNCaP, and many other cells (data not shown), but it is active to some extent in CWR22R cells as shown on Figure 9A. Although we do not know how exactly it behaves in CWR22R cells in the presence of mutated endogenous one, we can propose that they form heteromers which are transcriptionally active in the absence of DHT.
Taken together, the data shown in Figures 6–9 indicate that CaP cells do not tolerate overexpression of AR. This results from the activation of AR-dependent transcription (as indicated by the effect of DHT shown in Fig. 8B), leading to the growth arrest of cells with high AR expression and activity. In situations in which AR transcription is not activated, that is, in the absence of androgens, the level of AR protein in cells can be elevated compared with the basal level specific for these cell types without an adverse effect on cell growth.
Conclusions drawn from our experiments are consistent with and provide insight into the phenomena observed in the study from the Liao laboratory [30]. This study showed that growth of “androgen-dependent” LNCaP-104S cells in the presence of the AR inhibitor casodex (CX) leads to selection of CX resistant cells with over-expressed AR. We conclude from our experiments that AR-expressing CaP cells cannot survive inhibition of AR signaling by any means. This is consistent with the reported selection of AR over-expressing cells following inhibition of AR by CX. In this case, the increased levels of AR would be expected to titrate out the AR inhibitor such that some residual AR activity could be maintained to allow cells to survive in the presence of the inhibitor. In a second step, the Liao group exposed the AR overexpressing CX resistant cells to androgen ligand, resulting in selection of cells with down-regulated AR expression. This result is consistent with our finding that CaP cells undergo growth arrest following overexpression or over-activation of AR. In our hands, this led to selection of cell subpopulations or clones that had practically lost AR expression. Thus, the different strategies used in the two studies to modulate AR expression and activity gave similar results demonstrating that CaP cells tolerate a rather narrow range of AR expression and activity.
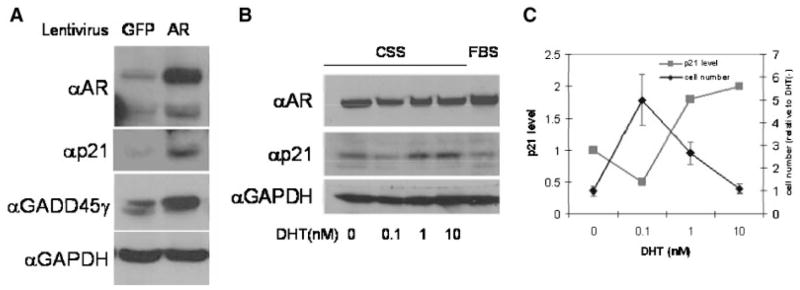
These observations can be accounted for by the hypothesis that AR controls expression of a putative growth inhibitory factor. At first glance, this hypothesis may appear at odds with the observation that AR is constitutively active in many CaP cells since expression of an anti-proliferative factor should result in loss of AR-expressing cells from the proliferative cell pool. However, there are several reports of tumor cells that express elevated levels of growth suppressive genes. For example, the CDK inhibitor p21 is expressed in many tumor cells and, moreover, protects these cells from apoptosis [31]. The growth inhibitory activity of p21 in tumor cells is typically overcome by overexpression of cyclin D [32]. Experimental induction of p21 in such cells leads to an imbalance in p21 and cyclin D levels resulting in growth arrest and subsequent elimination of p21-overexpressing cells from the proliferative pool [33,34] and our unpublished observations. While this manuscript was in preparation, Litvinov et al. [35] reported that introduction of AR into AR-negative CaP cells resulted in induction of p21 expression. Based upon this, we felt that it was important to examine the level of p21 in cells transduced with our AR lentivirus. We infected the most androgen insensitive CWR22R cells with lentiviral constructs directing expression of either AR or GFP as a control. Forty-eight hours post-transduction, cell lysates were prepared and p21 protein levels were determined by Western blot analysis (Fig. 10A). We observed a marked induction in p21 protein level that corresponded with AR transduction. We also observed that overexpression of AR resulted in increased expression of the growth suppressor GADD45γ, in agreement with work published by others [36].
Fig. 10.
Increased expression (A) or activity (B) of AR results in increased levels of p21 protein. A: CWR22R cells were infected with control (GFP-expressing) or AR-expressing lentiviruses in FBS containing medium. Cell lysates were analyzed by Western blotting for AR, p21, GADD45g, and GAPDH (control) protein levels. B: LNCaP cells expressing only endogenous AR were grown in medium containing FBS or CSS. DHT was added to CSS-containing cultures as indicated below each lane. Western blot analysis of AR, p21, and GAPDH protein levels is shown. C: Quantitation of changes of p21 level in experiment shown on a panel B in parallel with changes in cell number in the same experiment in dependence on DHT level in the medium.
To determine whether the observed induction of p21 protein is correlated with increased AR transcriptional activity or simply elevated AR protein levels, we measured p21 levels in LNCaP cells (expressing only endogenous AR) treated with increasing concentrations of DHT to induce AR-mediated transactivation. As shown in Figure 10B, the level of p21 was higher in cells treated with 1 or 10 nM DHT than in cells maintained in 0 or 0.1 nm of DHT or in medium with FBS (containing approximately 0.3 nM DHT). Notably, LNCaP cells maintained in 10 nM DHT proliferate slower than cells kept at lower hormone levels (Fig. 2). Thus, increased AR activity due to either its overexpression or hyper-stimulation with ligand inhibits cell growth, possibly through induction of the CDK inhibitor p21.
AR is a transcription factor constitutively expressed and active in differentiated cells of the male reproductive system. The target genes regulated by AR are involved in the differentiation and specialized function of cells within these organs. They also have important roles in maintaining survival of the highly differentiated epithelial cells within these organs, since androgen withdrawal leading to inactivation of AR causes massive death of epithelial cells in prostate. This has been experimentally demonstrated in animal models through castration or other means of androgen ablation and is also supported by clinical data. It is expected that specialized organ-specific transcription factors that function in non-proliferating differentiated cells, such as AR, would more likely serve to inhibit cell growth than promote it. However, the fact that AR expression and activity is maintained in proliferating CaP cells and that their proliferation is reduced following androgen withdrawal had suggested that AR is actually a growth promoting factor for these cells. Based upon our data and other recent work, we now believe that, in fact, AR might just maintain CaP cell survival rather than actually promote their growth. While the mechanism underlying this role remains undefined, it is possible that it involves transcriptional activation of anti-apoptotic factors, in absence of which CaP cells undergo apoptosis. A similar survival function has been defined for transcription factors in other highly differentiated cells (e.g., NF-kB in some lymphoid cells). As a counterpoint to the pro-survival role of AR in CaP, we have shown in our study that greatly increased activity of AR, leads to growth arrest in differentiated cells. This is accompanied by induction of p21 and potentially other cell cycle inhibitors. Thus, growth of CaP cells and the resulting progression of prostate cancer as a disease requires a precise balance between too little AR activity, which would induce apoptosis, and too much AR activity, which would result in growth arrest. This improved understanding of the dual role of AR in CaP cells suggests that not only inhibition, but also over-activation, of AR holds promise as a therapeutic approach for prostate cancer treatment.
Acknowledgments
This work was supported by NIH STTR grant 1R41CA110400-01 and a Prostate Cancer Foundation Award to K. Gurova and by grant from NIH CA098374 to A. Gudkov. We greatly appreciate help of Patty Baker in editing of this manuscript.
References
- 1.Gallagher E, Gapstur R. Hormone-refractory prostate cancer: A shifting paradigm in treatment. Clin J Oncol Nurs. 2006;10(2):233–240. doi: 10.1188/06.CJON.233-240. [DOI] [PubMed] [Google Scholar]
- 2.Kasper S, Cookson MS. Mechanisms leading to the development of hormone-resistant prostate cancer. Urol Clin North Am. 2006;33(2):201–210. vii. doi: 10.1016/j.ucl.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Saad F, Al Dejmah A, Perrotte P, McCormack M, Benard F, Valiquette L, Karakiewicz PI. Therapeutic approach to hormone-refractory prostate cancer. Can J Urol. 2006;13(Suppl 2):52–56. [PubMed] [Google Scholar]
- 4.Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996;28(4):251–265. doi: 10.1002/(SICI)1097-0045(199604)28:4<251::AID-PROS6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 5.Mitsiades CS, Koutsilieris M. Molecular biology and cellular physiology of refractoriness to androgen ablation therapy in advanced prostate cancer. Expert Opin Investig Drugs. 2001;10(6):1099–1115. doi: 10.1517/13543784.10.6.1099. [DOI] [PubMed] [Google Scholar]
- 6.Bruckheimer EM, Spurgers K, Weigel NL, Logothetis C, McDonnell TJ. Regulation of Bcl-2 expression by dihydrotestosterone in hormone sensitive LNCaP-FGC prostate cancer cells. J Urol. 2003;169(4):1553–1557. doi: 10.1097/01.ju.0000055140.91204.c7. [DOI] [PubMed] [Google Scholar]
- 7.Kirschenbaum A, Liu XH, Yao S, Narla G, Friedman SL, Martignetti JA, Levine AC. Sex steroids have differential effects on growth and gene expression in primary human prostatic epithelial cell cultures derived from the peripheral versus transition zones. Carcinogenesis. 2006;27(2):216–224. doi: 10.1093/carcin/bgi219. [DOI] [PubMed] [Google Scholar]
- 8.Wang G, Reed E, Li QQ. Apoptosis in prostate cancer: Progressive and therapeutic implications (review) Int J Mol Med. 2004;14(1):23–34. [PubMed] [Google Scholar]
- 9.Bauman DR, Steckelbroeck S, Peehl DM, Penning TM. Transcript, profiling of the androgen signal in normal prostate, benign prostatic hyperplasia and prostate cancer. Endocrinology. 2006;147(12):5806–5816. doi: 10.1210/en.2006-0627. [DOI] [PubMed] [Google Scholar]
- 10.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J Clin Endocrinol Metab. 2003;88(7):2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 11.Isaacs JT. New strategies for the medical treatment of prostate cancer. BJU Int. 2005;96(Suppl 2):35–40. doi: 10.1111/j.1464-410X.2005.05945.x. [DOI] [PubMed] [Google Scholar]
- 12.Peehl DM. Basic science of hormonal therapy for prostate cancer. Rev Urol. 2001;3(Suppl 3):S15–S22. [PMC free article] [PubMed] [Google Scholar]
- 13.Brummelkamp TR, Bernards R, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296(5567):550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- 14.Gurova KV, Kwek SS, Koman IE, Komarov AP, Kandel E, Nikiforov MA, Gudkov AV. Apoptosis inhibitor as a suppressor of tumor progression: Expression of Bcl-2 eliminates selective advantages for p53-deficient cells in the tumor. Cancer Biol Ther. 2002;1(1):39–44. doi: 10.4161/cbt.1.1.39. [DOI] [PubMed] [Google Scholar]
- 15.Gurova KV, Hill JE, Razorenova OV, Chumakov PM, Gudkov AV. p53 pathway in renal cell carcinoma is repressed by a dominant mechanism. Cancer Res. 2004;64(6):1951–1958. doi: 10.1158/0008-5472.can-03-1541. [DOI] [PubMed] [Google Scholar]
- 16.Klus GT, Nakamura J, Li JS, Ling YZ, Son C, Kemppainen JA, Wilson EM, Brodie AM. Growth inhibition of human prostate cells in vitro by novel inhibitors of androgen synthesis. Cancer Res. 1996;56(21):4956–4964. [PubMed] [Google Scholar]
- 17.Long BJ, Grigoryev DN, Nnane IP, Liu Y, Ling YZ, Brodie AM. Antiandrogenic effects of novel androgen synthesis inhibitors on hormone-dependent prostate cancer. Cancer Res. 2000;60(23):6630–6640. [PubMed] [Google Scholar]
- 18.Kokontis J, Takakura K, Hay N, Liao S. Increased androgen receptor activity and altered c-myc expression in prostate cancer cells after long-term androgen deprivation. Cancer Res. 1994;54(6):1566–1573. [PubMed] [Google Scholar]
- 19.Tan J, Sharief Y, Hamil KG, Gregory CW, Zang DY, Sar M, Gumerlock PH, deVere White RW, Pretlow TG, Harris SE, Wilson EM, Mohler JL, French FS. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol Endocrinol. 1997;11(4):450–459. doi: 10.1210/mend.11.4.9906. [DOI] [PubMed] [Google Scholar]
- 20.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58(20):4640–4645. [PubMed] [Google Scholar]
- 21.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. Int J Cancer. 1994;57(3):406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 22.Wainstein MA, He F, Robinson D, Kung HJ, Schwartz S, Giaconia JM, Edgehouse NL, Pretlow TP, Bodner DR, Kursh ED, Resnick MI, Seftel A, Pretlow TG. CW R22:androgen-dependent xenograft model derived from a primary human prostatic carcinoma. Cancer Res. 1994;54(23):6049–6052. [PubMed] [Google Scholar]
- 23.Chen CT, Gan Y, Au JL, Wientjes MG. Androgen-dependent and -independent human prostate xenograft tumors as models for drug activity evaluation. Cancer Res. 1998;58(13):2777–2783. [PubMed] [Google Scholar]
- 24.Gregory CW, Hamil KG, Kim D, Hall SH, Pretlow TG, Mohler JL, French FS. Androgen receptor expression in androgen-independent prostate cancer is associated with increased expression of androgen-regulated genes. Cancer Res. 1998;58(24):5718–5724. [PubMed] [Google Scholar]
- 25.Liao X, Tang S, Thrasher JB, Griebling TL, Li B. Small-interfering RNA-induced androgen receptor silencing leads to apoptotic cell death in prostate cancer. Mol Cancer Ther. 2005;4(4):505–515. doi: 10.1158/1535-7163.MCT-04-0313. [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Fung KM, Day WV, Kropp BP, Lin HK. Androgen receptor signaling is required for androgen-sensitive human prostate cancer cell proliferation and survival. Cancer Cell Int. 2005;5(1):8. doi: 10.1186/1475-2867-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen R, Sumitomo M, Dai J, Harris A, Kaminetzky D, Gao M, Burnstein KL, Nanus DM. Androgen-induced growth inhibition of androgen receptor expressing androgen-independent prostate cancer cells is mediated by increased levels of neutral endopeptidase. Endocrinology. 2000;141(5):1699–1704. doi: 10.1210/endo.141.5.7463. [DOI] [PubMed] [Google Scholar]
- 28.Heisler LE, Evangelou A, Lew AM, Trachtenberg J, Elsholtz HP, Brown TJ. Androgen-dependent cell cycle arrest and apoptotic death in PC-3 prostatic cell cultures expressing a full-length human androgen receptor. Mol Cell Endocrinol. 1997;126(1):59–73. doi: 10.1016/s0303-7207(96)03970-6. [DOI] [PubMed] [Google Scholar]
- 29.Litvinov IV, Antony L, Isaacs JT. Molecular characterization of an improved vector for evaluation of the tumor suppressor versus oncogene abilities of the androgen receptor. Prostate. 2004;61(4):299–304. doi: 10.1002/pros.20187. [DOI] [PubMed] [Google Scholar]
- 30.Kokontis JM, Hsu S, Chuu CP, Dang M, Fukuchi J, Hiipakka RA, Liao S. Role of androgen receptor in the progression of human prostate tumor cells to androgen independence and insensitivity. Prostate. 2005;65(4):287–298. doi: 10.1002/pros.20285. [DOI] [PubMed] [Google Scholar]
- 31.Gartel AL, Radhakrishnan SK. Lost in transcription: p21 repression, mechanisms, and consequences. Cancer Res. 2005;65(10):3980–3985. doi: 10.1158/0008-5472.CAN-04-3995. [DOI] [PubMed] [Google Scholar]
- 32.Russell A, Hendley J, Germain D. Inhibitory effect of p21 in MCF-7 cells is overcome by its coordinated stabilization with D-type cyclins. Oncogene. 1999;18(47):6454–6459. doi: 10.1038/sj.onc.1203030. [DOI] [PubMed] [Google Scholar]
- 33.Chen WJ, Chang CY, Lin JK. Induction of G1 phase arrest in MCF human breast cancer cells by pentagalloylglucose through the down-regulation of CDK4 and CDK2 activities and up-regulation of the CDK inhibitors p27(Kip) and p21(Cip) Biochem Pharmacol. 2003;65(11):1777–1785. doi: 10.1016/s0006-2952(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 34.Tvrdik D, Djaborkhel R, Nagy A, Eckschlager T, Raska I, Muller J. Cyclin. D-cdk6 complex is targeted by p21(WAF) in growth-arrested lymphoma cells. J Struct Biol. 2002;140(1–3):49–56. doi: 10.1016/s1047-8477(02)00535-x. [DOI] [PubMed] [Google Scholar]
- 35.Litvinov IV, Antony L, Dalrymple SL, Becker R, Cheng L, Isaacs JT. PC3, but not DU145, human prostate cancer cells retain the coregulators required for tumor suppressor ability of androgen receptor. Prostate. 2006;66(12):1329–1338. doi: 10.1002/pros.20483. [DOI] [PubMed] [Google Scholar]
- 36.Jiang F, Wang Z. Gadd45gamma is androgen-responsive and growth-inhibitory in prostate cancer cells. Mol Cell Endocrinol. 2004;213(2):121–129. doi: 10.1016/j.mce.2003.10.050. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Thomas TZ, Kasper S, Matusik RJ. A small composite probasin promoter confers high levels of prostate-specific gene expression through regulation by androgens and glucocorticoids in vitro and in vivo. Endocrinology. 2000;141(12):4698–4710. doi: 10.1210/endo.141.12.7837. [DOI] [PubMed] [Google Scholar]
- 38.Tepper CG, Boucher DL, Ryan PE, Ma AH, Xia L, Lee LF, Pretlow TG, Kung HJ. Characterization of a novel androgen receptor mutation in a relapsed CWR22 prostate cancer xenograft and cell line. Cancer Res. 2002;62(22):6606–6614. [PubMed] [Google Scholar]