Abstract
Objective
To investigate the in-vivo cartilage contact biomechanics of the tibiofemoral joint following anterior cruciate ligament (ACL) injury.
Methods
Eight patients with an isolated ACL injury in one knee and the contralateral side intact participated in the study. Both knees were imaged using a specific MR sequence to create three-dimensional knee models of bone and cartilage. Next, each patient performed a lunge as images were recorded with a dual fluoroscopic system from 0° to 90° of flexion. The three-dimensional knee models and fluoroscopic images were used to reproduce the in-vivo knee position at each flexion angle. With these series of knee models, the location of tibiofemoral cartilage contact, size of contact area, cartilage thickness at the contact area, and magnitude of cartilage contact deformation were compared between the intact and ACL-deficient knees.
Results
Rupture of the ACL changed the cartilage contact biomechanics from 0° to 60° of flexion in the medial knee compartment. The location of peak cartilage contact deformation on the tibial plateaus was more posterior and lateral; the contact area was smaller; the average cartilage thickness at the tibial cartilage contact area was thinner; and the resultant magnitude of cartilage contact deformation was increased, compared with the contralateral knee. Similar changes were observed in the lateral compartment, with increased cartilage contact deformation from 0° to 30° of knee flexion in ACL deficiency.
Conclusion
ACL deficiency alters the in-vivo cartilage contact biomechanics, by shifting the contact location to smaller regions of thinner cartilage, and increasing the magnitude of cartilage contact deformation.
INTRODUCTION
Many experts have abandoned the long-held belief that knee osteoarthritis is a straightforward “wear and tear” disease of cartilage (1). Instead, the metabolic and structural changes of osteoarthritis are currently viewed as the adaptive response of synovial joints to a variety of genetic, constitutional, or biomechanical insults (2). Nevertheless, it remains widely accepted that knee joint instability is an important risk factor in the aetiopathogenesis of the disease (3–5). The assumption that abnormal kinematics and consequent abnormal loading within the joint initiate knee osteoarthritis underlies much of current osteoarthritis research and orthopaedic practice: transection of the anterior cruciate ligament (ACL) is a well-established animal model to induce osteoarthritis (6, 7); reconstruction of the ruptured ACL has become one of the most frequent orthopaedic procedures in an attempt to restore normal joint motion and prevent long-term complications (3); and a number of alignment-modifying therapeutic options, including bracing and osteotomy, might be used to alter the rate of osteoarthritis progression (8). However, even though osteoarthritis is widely believed to result, in part, from local mechanical factors acting within the context of systemic susceptibility, little is known regarding the extent of the mechanical alteration in the knee joint following rupture of the ACL. In general, the changes in kinematics that are observed in unstable knee joints are very minimal – in the order of millimeters (9–11). Yet, these minimal alterations in kinematics are believed to trigger the devastating destruction of the articular cartilage.
Measuring the articular cartilage function in the human knee joint with an acceptable accuracy is technically challenging. Cartilage morphology of the knee has been extensively investigated using both cadaveric and living knee specimens with various techniques, such as needle probes, ultrasound, stereophotogrammetry and magnetic resonance (MR) imaging (12–18). Recently, several studies have presented data on tibiofemoral contact kinematics in living subjects. Both open and closed MR imaging techniques have been used to determine tibiofemoral contact areas and locations for a variety of activities (19–21). Others have used a combination of either computer tomography (CT) or MR imaging with fluoroscopy to estimate cartilage contact locations during lunge in healthy (22, 23) and ACL-deficient knees (24, 25). In general, the cartilage contact location in these studies has been estimated based on the closest interarticular distance between the bony surfaces of the tibiofemoral joint (24), or the centroid of the tibiofemoral cartilage contact area (25). While these previous studies have provided valuable data on the cartilage contact location, essential in-vivo cartilage contact biomechanics such as cartilage contact area, cartilage thickness, and cartilage contact deformation in a knee joint at risk for osteoarthritis remain unclear.
In this study, we hypothesized that rupture of the ACL changes the cartilage contact biomechanics of the tibiofemoral joint, with a resultant increase in the magnitude of cartilage contact deformation. We used a combined dual fluoroscopic and MR imaging technique to analyze the effects of ACL deficiency on the location of tibiofemoral cartilage contact, the size of contact area, the cartilage thickness at the contact area, and the magnitude of cartilage contact deformation during in-vivo weight bearing knee flexion from 0° to 90°.
PATIENTS AND METHODS
PATIENT SELECTION
Eight patients (five males, three females; age range 19–38 years old) with complaints of knee laxity were included in the study. The included patients had diagnosed acute, isolated ACL ruptures documented by clinical examination (8 mm Lachman with no end point, a grade 2 pivot shift measured by the same orthopaedic surgeon, average International Knee Documentation Committee score 63.5 ± 8.7, average manual maximum injured-minus-intact knee displacement 4.2 ± 1.9 mm, measured with the KT-1000 arthrometer (MEDmetrics, San Diego, Ca) by the same physical therapist) and MR imaging. All patients had healthy contralateral knees. Patients had been injured within an average of 4.4 ± 3 months of testing. Injury to other ligaments, noticeable cartilage lesions, meniscal damage and injury to the underlying bone were reasons for exclusion from the study. Five of these eight patients have been included in our previous studies of the six degrees-of-freedom tibiofemoral kinematics (9) and the motion of the tibiofemoral cartilage contact points in ACL deficiency (25).
Each patient signed a consent form that had been approved by our institutional review board.
IMAGING PROCEDURE
MR and dual orthogonal fluoroscopic imaging techniques have been described in detail in previous publications (9, 25, 26). In short, both the left and right knee were imaged with an MR scanner to create three-dimensional (3D) meshed models of the knees using a protocol established in our laboratory (9). To reduce the effects of load history on cartilage thickness, patients were asked to refrain from all strenuous activity such as lifting, running, stair climbing for at least four hours prior to their visit, and to remain non-weight bearing for approximately one hour prior to the MR imaging of the knee. Each patient was asked to lay supine with their knee in a relaxed, extended position while sagittal plane images were acquired with a 3.0 Tesla MR scanner (Siemens, Malvern, PA). The MR scanner was equipped with a surface coil and employed a 3D double echo water excitation sequence (field-of-view=16×16×12 cm, voxel resolution=0.31×0.31×1.00 mm, time of repetition (TR)=24 ms, time of echo (TE)=6.5 ms, and flip angle=25°). Each scan lasted for approximately twelve minutes. The images were then imported into solid modeling software (Rhinoceros, Robert McNeel and Associates, Seattle, Wa) to construct 3D surface mesh models of the tibia, fibula, femur and articulating cartilage. The meshes were assembled using a point density of 80 vertices/cm2 and triangular facets with an average aspect ratio of two.
After the MR image-based computer models were constructed, both knees of each patient were simultaneously imaged using two orthogonally placed fluoroscopes (OEC 9800; GE, Salt Lake City, UT) as the patient performed a single leg quasi-static lunge at 0°, 15°, 30°, 60°, and 90° of flexion. At each flexion angle the patient was asked to pause for five seconds while simultaneous fluoroscopic images were taken. Throughout the experiment, the lower limb being tested supported the patient’s body weight, while the other limb provided stability. The time elapsed between the MR scan and the lunge activity was approximately fifteen minutes.
Next, the fluoroscopic images were imported into solid modeling software and placed in the orthogonal planes based on the position of the fluoroscopes during the imaging of the patient. Finally, the 3D MR image–based knee model of the patient was imported into the same software, viewed from the two orthogonal directions corresponding to the orthogonal fluoroscopic setup used to acquire the images, and independently manipulated in six degrees of freedom inside the software until the projections of the model match the outlines of the fluoroscopic images. When the projections matched the outlines of the images taken during in-vivo knee flexion, the model reproduced the in-vivo position of the knee. This system has an error of less than 0.1 mm and 0.3° in measuring tibiofemoral joint translations and rotations, respectively (9). When comparing the dual fluoroscopic model matching technique with tantalum bead matching – a technique similar to Roentgen Stereophotogrammetric Analysis, the difference between the two techniques in the proximodistal direction (i.e. analogous to the measured apparent penetration) was 0.075 ± 0.13 mm (27).
DATA ANALYSIS
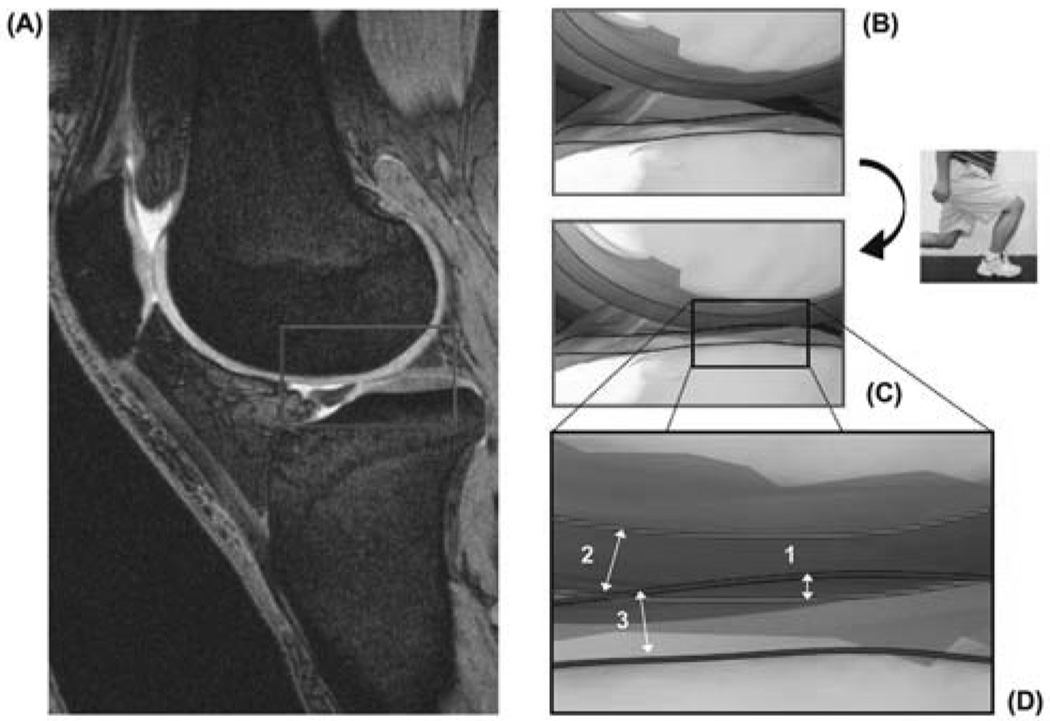
In this study, cartilage thickness was calculated by finding the smallest Euclidian distance connecting a vertex of the articular surface to the cartilage-bone interface of the 3D surface mesh models. The size of the contact area between the tibia and femur was determined by computing the area of tibial cartilage that intersected the femoral cartilage (26). The cartilage contact deformation was then defined for each vertex of the articular surface mesh as the amount of cartilage surface intersection (mm) (Figure 1) divided by the sum of the tibial and femoral cartilage surface thicknesses (mm), multiplied by 100 (26). In this study, cartilage contact location was defined as the location of peak cartilage deformation, referenced to Cartesian coordinate systems on the tibial plateaus (Figure 2) (23, 25, 28). The origin of each coordinate system was located by the center of a circle, which was fit to the posterior edge of each tibial compartment. The anteroposterior (AP) and mediolateral (ML) axes split each tibial plateau into quadrants. In the anteroposterior direction, a location anterior to the ML axis was considered positive. In the mediolateral direction, a location lateral to the AP axis was considered positive.
Figure 1.
Illustration of the calculation of compartmental contact deformation. The MR images of the knee joint after ~1 hour non-weight bearing (A) are used to determine the respective cartilage thicknesses of the femur (top) and tibia (bottom) at rest (B). After matching the MR models to the fluoroscopic images captured during weight bearing lunge (C), compartmental cartilage deformation is calculated by dividing the amount of penetration (1) by the sum of the femoral (2) and tibial (3) cartilage surface thicknesses, as illustrated in (D). (Reproduced from: Bingham JT et al. Rheumatology (Oxford). 2008 Nov;47(11):1622-7. Reprinted with permission)
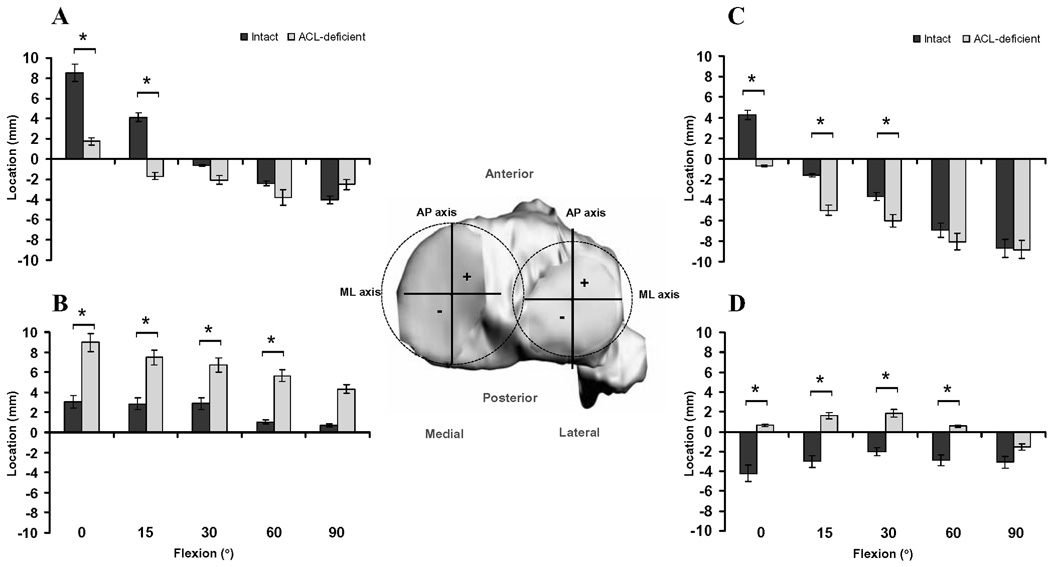
Figure 2.
Location of cartilage contact on the medial tibial plateau in the anteroposterior (A) and mediolateral (B) directions, and on the lateral tibial plateau in the anteroposterior (C) and mediolateral (D) directions, in the intact and ACL-deficient knees as a function of knee flexion angle. Insert illustrates the Cartesian coordinate system for the tibial plateau. AP = anteroposterior, ML = mediolateral. (* Denotes p<0.05)
In a recent study of the in-vivo cartilage contact deformation in the healthy human tibiofemoral joint, a lack of agreement of 14 ± 11% was found between the combined dual fluoroscopic – MR imaging technique and a silicone casting technique in calculating the cartilage contact area of 244 ± 131 mm2 (26). For the present study, we conducted an accuracy and precision analysis of the tibiofemoral cartilage reconstructions in which the measurement of cartilage thickness based on 3D MR image–based knee models was compared to direct cartilage thickness measurement on calibrated digital images of cross-sections of cadaveric specimens, and repeatedly measured for intra- and interobserver precision (see Appendix). The average absolute difference between the cartilage thickness values based on the 3D MR image–based knee models and those captured from the cadaveric specimens was 0.04 ± 0.01 mm, and excellent intra- and interobserver precision was obtained.
A two-way repeated measures analysis of variance and the Student-Newman-Keuls post hoc test were used to determine statistically significant differences in location, contact area, thickness, and cartilage contact deformation between the intact contralateral knees and the ACL-deficient knees at each flexion angle. Differences were considered significant at the level of p<0.05.
RESULTS
Location of cartilage contact
In general, the location of peak cartilage contact deformation on the tibial plateaus was more posterior and lateral in the ACL-deficient knees, as compared with the healthy contralateral knees. In the medial compartment of the ACL-deficient knees, cartilage contact shifted posteriorly by an average of 6.3 ± 0.7 mm at 0° and 15° of flexion (Figure 2A), and laterally by an average of 4.7 ± 0.9 mm between 0° and 60° of flexion (Figure 2B), as compared with the location in the intact knees. In the lateral compartment, cartilage contact shifted posteriorly by an average of 3.6 ± 1.3 mm between 0° and 30° of flexion (Figure 2C), and laterally by an average of 4.2 ± 0.6 mm between 0° and 60° of flexion (Figure 2D) following rupture of the ACL.
Size of contact area
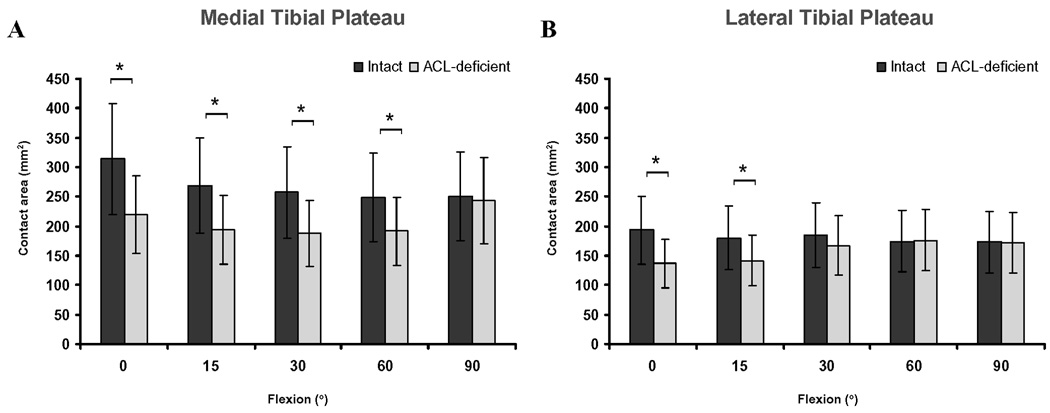
In the medial compartment, the cartilage contact area in the ACL-deficient knees was significantly smaller from 0° to 60° of flexion (p<0.05), compared with that in the intact knees (Figure 3A). The maximum decrease in cartilage contact area after ACL rupture occurred at 0° of flexion (314.4 ± 113.6 mm2 in the intact knee compared with 219.6 ± 69.4 mm2 in the ACL-deficient knee, p=0.0025). In the lateral compartment, a decrease in contact area in the ACL-deficient knees was observed at the lowest flexion angles (Figure 3B) (193.4 ± 75.2 mm2 and 180.0 ± 46.8 mm2 in the intact knee compared with 137.1 ± 64.1 mm2 and 141.7 ± 48.7 mm2 in the ACL-deficient knee, at 0° and 15° of flexion respectively).
Figure 3.
Cartilage contact area on the medial (A) and lateral (B) tibial plateaus in the intact and ACL-deficient knees as a function of knee flexion angle. (* Denotes p<0.05)
Cartilage thickness at the contact area
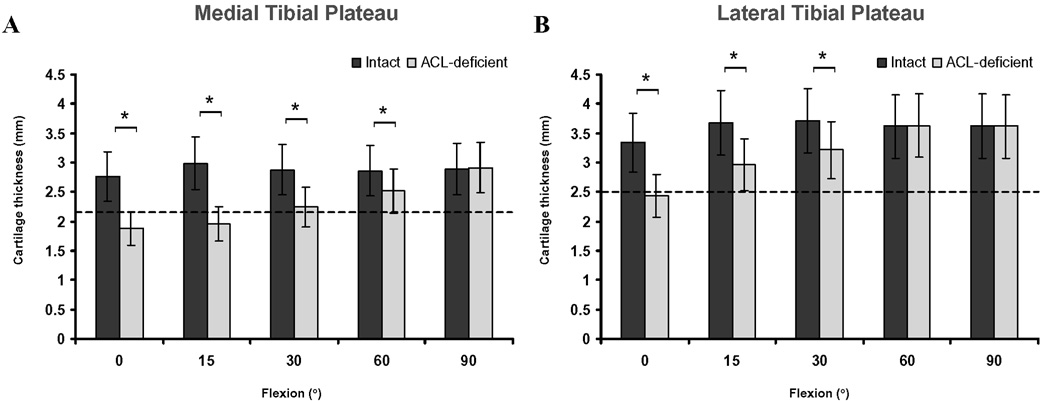
The total average thickness of cartilage of the studied knees was 2.2 ± 0.4 mm and 2.5 ± 0.5 mm, in the medial and lateral tibial plateaus respectively. In the intact contralateral knees, the cartilage thickness located in areas of contact was on average 1.4 times greater than the total average thickness. On the other hand, regions of contact for both the medial and lateral compartments in the ACL-deficient knees were on average 0.9 times thinner than the total average thickness. Cartilage thickness at contact was on average 0.7 ± 0.3 mm thinner between 0° and 60° of flexion in the medial compartment (Figure 4A), and on average 0.7 ± 0.2 mm thinner between 0° and 30° of flexion in the lateral compartment (Figure 4B), respectively, compared to the cartilage thickness at contact in the intact knees.
Figure 4.
Thickness of cartilage in regions of contact for the medial (A) and lateral (B) tibial plateaus in the intact and ACL-deficient knees as a function of knee flexion angle. Dashed lines indicate total average cartilage thickness. (* Denotes p<0.05)
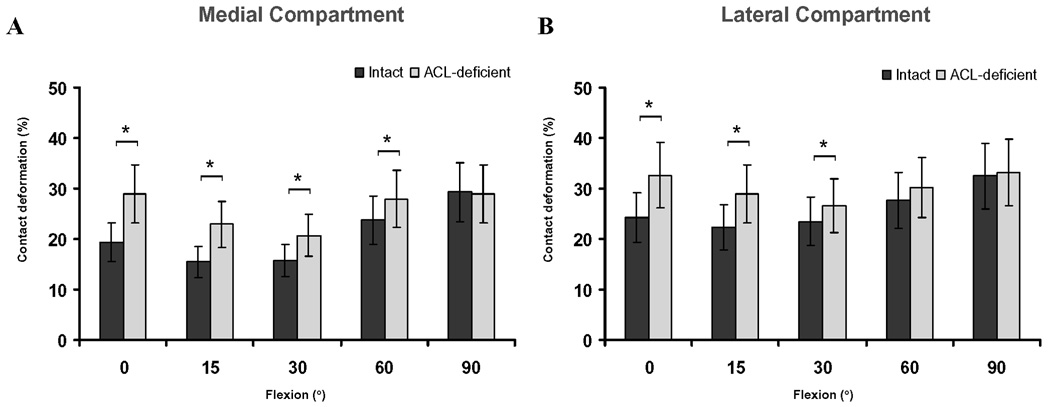
Magnitude of cartilage contact deformation
Rupture of the ACL significantly increased the deformation of cartilage from 0° to 60° of flexion in the medial compartment of the knee joint (Figure 5A) (p < 0.05). The maximum increase in compartmental cartilage deformation after ACL rupture occurred at 0° of flexion (19 ± 4% intact knee, 29 ± 9% ACL-deficient knee, p = 0.0138). The increase in cartilage deformation that was observed after ACL rupture gradually lessened with flexion. At 90° of flexion, there was no significant difference in cartilage deformation between the healthy and ACL-deficient knee.
Figure 5.
Peak cartilage contact deformation in the medial (A) and lateral (B) tibiofemoral compartments in the intact and ACL-deficient knees as a function of knee flexion angle. (* Denotes p<0.05)
In the lateral compartment, rupture of the ACL significantly increased the deformation of cartilage between 0° and 30° of knee flexion (Figure 5B) (p < 0.05). The maximum increase in cartilage deformation after ACL rupture occurred at 0° of flexion, where a deformation of 24 ± 9% was found in the healthy knee, and 33 ± 6% in the ACL-deficient knee (p = 0.0033).
DISCUSSION
Rupture of the ACL affects mostly patients under 30 years of age (29), and is clinically associated with an increased incidence (3, 4), an earlier onset (4), and a faster progression (30) of knee osteoarthritis. Using in-vivo T1ρ quantitative assessment of knee cartilage after ACL injury using 3.0 Tesla MR imaging, it has been demonstrated that cartilage abnormalities were already present following initial ACL injury in patients with underlying bone marrow edema-like lesions in the lateral tibia (15), suggesting a role in the pathogenesis of osteoarthritis in ACL deficiency (31, 32). Interestingly though, an increased prevalence of cartilage degeneration has been described in the medial compartment in the presence of an ACL injury (33–36), while the above-mentioned bone marrow edema-like lesions are usually found in the lateral compartment of the knee (37). It has been hypothesized, based on theoretical models, that the persistent abnormal kinematic behavior that is seen in isolated ACL-deficiency could alter the stress distributions in the cartilage overtime, hereby predisposing the knee to degenerative changes (38). The latter “wear and tear” theory could be supported by the efficacy of the classic animal models for knee osteoarthritis, in which transection of the ACL and subsequent joint instability, without injury to the other structures of the knee, triggers cartilage degeneration (39, 40). However, it remains poorly understood how, during in-vivo weight bearing flexion of the knee, the minimal changes in cartilage contact kinematics observed in unstable knee joints without manifest initial cartilage or underlying bone lesions (25) could contribute to the initiation of osteoarthritis.
In our previous in-vivo analysis of the tibiofemoral joint kinematics in ACL-deficiency, we found an increased anterior translation (approximately 3 mm) and internal rotation (approximately 2 degrees) of the tibia at low flexion angles, compared to the healthy control knee (9). Similar findings have been well documented in the literature (10, 11). ACL deficiency also caused an increased medial tibial translation of approximately 1 mm. These changes in the tibiofemoral kinematics after ACL injury were expected to lead to changes in the tibiofemoral cartilage contact characteristics. Specifically, the medial shift of the tibia after ACL deficiency would alter the contact stress distributions in the tibiofemoral cartilage near the medial tibial spine. Indeed, in the presence of ACL injury, the cartilage contact points not only shifted posteriorly –as was expected based on the increased anterior tibial translation- but also laterally on the surface of the tibial plateau (25). In the medial compartment, the contact points shift toward the medial tibial spine, a region where degeneration is observed in patients with chronic ACL injuries (33). The question remained as to whether the ~ 2mm shift in cartilage contact (determined based on the location of the centroid of the cartilage contact area) to incongruent articular regions of the knee joint affects the stress on the cartilage of the joint.
In the present study, we found a similar posterior and lateral shift in cartilage contact location on the surface of the tibial plateaus following ACL injury. However, when determining the location of cartilage contact based on the location where peak cartilage deformation occurred, we found that the magnitude of the posterior and lateral shift following ACL rupture (~ 5 mm) was greater than was previously reported (25). A possible explanation for the discrepancy in the magnitude of shift based on measurement methodology could be found in the regional variations in cartilage thickness. When determining the cartilage contact location based on the closest interarticular distance between the bony surfaces of the tibiofemoral joint (24), or the centroid of the tibiofemoral cartilage contact area (25), regional variations in the thickness of underlying cartilage are not taken into account. In the present study, the region of tibiofemoral cartilage contact was not only smaller following rupture of the ACL, but had also significantly thinner cartilage at contact in both medial and lateral compartments, compared to the thickness at contact in the intact knees. In other words, the minimal shift in the location of the cartilage contact area as was reported previously (25) to regions of thinner cartilage resulted in a considerable change in cartilage loading distribution within the knee joint. Finally, the posterior and lateral shift of cartilage contact to thinner articular regions increased the magnitude of cartilage deformation in those regions. The maximum relative increase in cartilage contact deformation after ACL rupture occurred at full extension in the medial compartment, where a deformation of 19 ± 4% was found in the healthy knees, and 29 ± 9% in the ACL-deficient knees, compared to 24 ± 9% and 33 ± 6% in the lateral compartment of the healthy and ACL-deficient knees, respectively. The relatively greater increase of cartilage deformation in the medial compartment compared to the lateral compartment relates to the increased development of osteoarthritis in the medial compartment of the knee joint, as was observed during arthroscopic examination of 130 ACL-deficient patients (34).
Limitations
Due to the constraints of the imaging technique, motion and deformation of the meniscus was not detectable in the fluoroscopic images. For the quantitative examination of the interaction of molecular changes in the meniscus and adjacent cartilage in ACL deficiency, recent developments in MR imaging techniques, for instance T1ρ mapping, represent potential means (41). However, based on our previous validation of the imaging technique (26), the present limitation in meniscal examination by the dual fluoroscopic technique did not affect the determination of cartilage-cartilage contact, as articular surface mesh penetration was only recorded at the location of in-vivo tibiofemoral cartilage contact.
An additional constraint of the present methodology is that any possible underlying physiochemical activities (e.g. cytokine elevation in the joint fluid (42)) which might occur in the knee joint following ACL rupture, could not be analyzed, thereby restricting the formulation of an inclusive insight in the pathogenesis of osteoarthritis in knee joint instability. In addition, regional variations in the mechanical properties of the articular cartilage were not taken into account when computing the cartilage contact deformation. This may be a limitation since in-vitro compression of tibial cartilage explants that were obtained from distinct regions of the joint has demonstrated that chondrocytes displayed region-specific baseline gene expression, and responded differently to in-vitro mechanical loading (43).
Patients with discernible cartilage lesions on 3.0 Tesla MRI at 4.5 ± 3 months of injury were excluded from the study. However, with our methodology we were unable to appreciate the extent of potential cartilage softening that existed at the time of the analysis, thereby unable to resolve the “chicken-egg” issue. Were our measured deformation differentials attributable to the ACL-deficient knee cartilage being more compliant, rather than increased deformation being responsible for the subsequent degeneration onset? Future studies should follow ACL-deficient patients who are treated conservatively for longer periods using a methodology similar to that used in this pilot study, with baseline and follow-up imaging biomarkers, such as T1ρ or delayed gadolinium-enhanced magnetic resonance imaging of cartilage. Tibiofemoral contact deformation and the health of the cartilage could therefore be monitored with time to quantify any possible biomechanical relationships.
We only acquired data from one functional activity, namely a single leg lunge, using a goniometer to measure the flexion angle. Measurement of the forces and strains in human tissues is currently impracticable, which impedes the extrapolation of the current findings to other weightbearing activities. It is conceivable that the knee joint anteroposterior shear forces during the single leg lunge might be higher than during normal gait (44), thus exaggerating the measured articular surface engagement differentials. However, it was found that the magnitude of the anteroposterior shear forces increased with knee flexion during the descent phase of a lunge performed with a 223-N (50-pound) barbell (45), and that the knee forces were minimum in the functional range between 0° and 50° of knee flexion (46). In the present study, the largest deformation differential was observed around 0° of knee flexion. In future studies, other in-vivo activities such as walking, running, and stair climbing should be considered to construct a comprehensive insight in the effect of ACL deficiency on the cartilage during daily activities.
While performing the lunge activity, the patients were asked to pause for five seconds at each flexion angle while simultaneous fluoroscopic images were taken. To the best of our knowledge, the real-time in-vivo creep of tibiofemoral cartilage has not been studied at this point. Based on the in-vivo creep compression curves for ankle cartilage using similar methodologies which showed that cartilage contact deformation continued to increase during the first 20 seconds of loading (47), it might be possible that the present data may be a conservative estimate of a potentially bigger differential if the patients were asked to pause longer at each flexion angle.
It should be noted that no ground reaction forces were measured in this study to document that global knee joint loading was reproduced reproducibly for both the intact and ACL-deficient knee. The patient performed the lunge activity with full body weight on the tested limb, while the untested limb was used for balance only, to ensure physiologic loading conditions.
Finally, the present analysis compared tibiofemoral cartilage contact deformation of the ACL-deficient and intact knees at each flexion angle, hereby ignoring potential interactions amongst the measured cartilage deformation and various knee flexion angles. Future research involving a larger study sample needs to be performed to confirm the present findings. Nonetheless, we believe the current findings provided a comprehensible insight in the changes in in-vivo tibiofemoral cartilage contact deformation following injury of the ACL, and identified important directions for future research.
We found that rupture of the ACL alters the in-vivo cartilage contact biomechanics of the tibiofemoral joint, by shifting the cartilage contact location to smaller regions of thinner cartilage, and increasing the magnitude of cartilage contact deformation.
Acknowledgments
Grant Support:
National Institutes of Health (NIH R01AR052408-02, NIH R21AR051078-01) (GL), the National Football League Charities Foundation (TJG), and the Belgian American Educational Foundation (SKV)
APPENDIX. ACCURACY AND PRECISION OF TIBIOFEMORAL CARTILAGE THICKNESS MEASUREMENT BY 3T MR IMAGING
An assessment of the accuracy and precision of cartilage thickness measurement based on 3D meshed knee models created with 3T MR images is critical for the appreciation of biomechanical parameters such as in-vivo cartilage contact deformation. In this validation study, we used calibrated digital images of a series of cadaveric cartilage cross-sections as the gold standard, because the actual cartilage boundaries can be clearly delineated from the specimens (Figure Appendix).
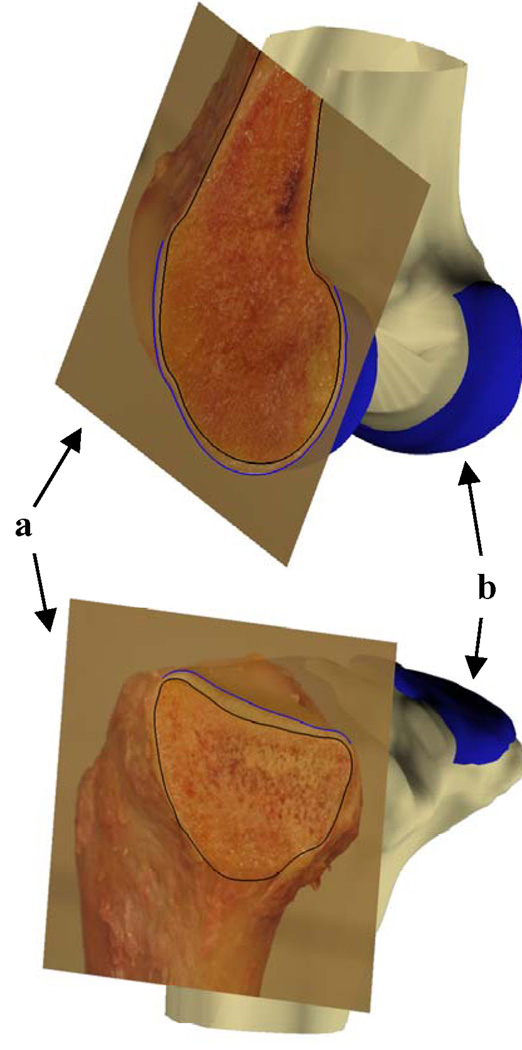
Figure Appendix.
Illustration of the comparison of direct measurement of cartilage thickness on cadaveric cross-sections with that on MR image based models. The digital images of the cadaver osteotomies (a) were matched to the respective cross-section planes of the MR image models (b). The black (bone mesh) and blue (cartilage mesh) lines on the digital images indicate the intersection of the cartilage mesh models with the digital image at the location of osteotomy. Only two cross-sections are shown for illustrative purposes.
Two fresh frozen cadaver knee specimens (male, 48 years, right knee; female, 48 years, left knee) were selected for this study. The specimens were stored at −20° C prior to the testing and thawed at room temperature for 24 hours before the experiment was conducted. Discernable cartilage damage was ruled out in each specimen upon fluoroscopic, MRI examination and visual inspection. Both knee specimens, with all the surrounding soft tissues intact, were then imaged with a 3.0 Tesla MR scanner to create 3D meshed models of the respective tibias, femurs, and articulating cartilage layers using the protocol described in the Patients and Methods section.
Following MR imaging, the knee specimens were stripped of all surrounding soft tissues and disarticulated to leave only the individual bones and articular cartilage surfaces. The cadaver bones were successively installed with the shaft centered in a rigid cylinder, and osteotomized through the midsagittal planes of the articulating surfaces of the medial and lateral condyles. Between each individual osteotomy and subsequent digital image capturing of the pertinent cross-section, the specimens were wrapped in a moist dressing to prevent desiccating of the articular cartilage. In this manner, a total of eight cadaveric cartilage cross-sections could be measured (i.e. two cross-sections for each tibia and femur of the two knee specimens). Digital images of the cross-sections were captured, and calibrated using the protocol designed in our laboratory to calibrate fluoroscopic images.
The next step was to determine the same cross-section of the respective tibial and femoral cartilage layers in the 3D surface mesh models. To do so, the osteotomized bones were CT scanned (LightSpeed Pro16, GE, Waukesha, WI) using high-resolution axial plane images. Images were obtained with a thickness of 0.625 mm and a gap of 0.625 mm, and with a resolution of 512 × 512 pixels. The CT images were then imported into Matlab (MathWorks, Natick, MA), and the contours of the osteotomized bones were digitized within each CT image based on a modified Canny edge detection method combining pixel magnitude to construct 3D anatomic mesh model of the osteotomized bones. The osteotomized bony models were then mapped to the MR image based models of the knee specimens using a customized code implemented in the Matlab based on the iterative closest point method. A plane was constructed along the cutting cross-section of the CT bony model. This plane was used to separate the MR image model at the location of the osteotomy.
Finally, the calibrated digital images that were captured of the cadaver cross-sections were matched to the respective cross-section planes of the MR image models. In this manner, cartilage thickness was measured at the same location on both the digital image and the MR image model. Ten equally distributed locations on each of the eight cartilage cross-sections were measured and compared using a paired student t-test with significance level set at p<0.05.
For the first specimen, the average cartilage thickness values measured from the digital images versus MR image models were 2.03 ± 0.26 mm and 2.04 ± 0.27 mm (medial femoral condyle, absolute difference 0.05 ± 0.03 mm, p=0.789); 2.00 ± 0.31 mm and 2.02 ± 0.30 mm (lateral femoral condyle, absolute difference 0.05 ± 0.03 mm, p=0.527); 2.24 ± 0.51 mm and 2.26 ± 0.48 mm (medial tibial condyle, absolute difference 0.05 ± 0.03 mm, p=0.378); and 2.16 ± 0.98 mm and 2.18 ± 1.00 mm (lateral tibial condyle, absolute difference 0.04 ± 0.03 mm, p=0.354), respectively.
For the second specimen, the average cartilage thickness values measured from the digital images versus MR image models were 2.15 ± 0.29 mm and 2.17 ± 0.30 mm (medial femoral condyle, absolute difference 0.04 ± 0.03 mm, p=0.357); 2.52 ± 0.49 mm and 2.52 ± 0.48 mm (lateral femoral condyle, absolute difference 0.03 ± 0.02 mm, p=0.826); 2.06 ± 0.22 mm and 2.04 ± 0.23 mm (medial tibial condyle, absolute difference 0.04 ± 0.01 mm, p=0.447); and 3.15 ± 0.81 mm and 3.17 ± 0.80 mm (lateral tibial condyle, absolute difference 0.03 ± 0.02 mm, p=0.521), respectively. The average absolute difference in cartilage thickness, measured at the ten locations along the eight section planes, between the direct measurement on the cadaveric specimens and the MR image based models was 0.04 ± 0.01 mm (corresponding to a 1.8 ± 1.6% difference). The above comparison demonstrated that the MR image based cartilage model was close to the actual cartilage, and sufficiently accurate for the determination of cartilage contact deformation differentials between intact and ACL-deficient knee joints.
To test the intraobserver precision of the tibiofemoral cartilage thickness measurement using the abovementioned MRI protocol and computer modeling, the cartilage layers that corresponded to the cutting planes were independently digitized ten times, with one day separating each re-segmentation, by three observers. Cartilage thickness was determined at ten equally distributed locations on each of the eight cartilage cross-sections. The analyses were based on the maximum differences between the digitizations provided by each observer (results from largest value minus smallest value for each location). The intraobserver precision was assessed with Pearson correlation coefficients and student t-tests of the paired differences of the observations. In this validation study, correlations between the ten digitizations were excellent. The Pearson correlation coefficients for intraobserver precision were >0.984 (P<0.0001) for each of the three readers at the measured locations.
Interobserver agreement was then assessed by determining whether significant differences between the cartilage thickness measurements (the thickness values from the first digitization session were selected) made by the three observers existed, using the intra-class correlation (ICC) coefficients. Interobserver agreement was very high in this study, with ICC coefficients ranging from 0.989 to 0.999 (p<0.0001). These data are consistent with values reported in the literature (48) and indicate that the cartilage thickness measurement based on MR image based cartilage models could be performed with great precision.
REFERENCES
- 1.Aspden RM, Scheven BA, Hutchison JD. Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet. 2001;357(9262):1118–1120. doi: 10.1016/S0140-6736(00)04264-1. [DOI] [PubMed] [Google Scholar]
- 2.Brandt K, Lohmander L, Doherty M. The concept of osteoarthritis as failure of the diarthrodial joint. In: Brandt K, Doherty M, Lohmander L, editors. Osteoarthritis. Oxford: Oxford University Press; 1998. pp. 70–74. [Google Scholar]
- 3.Fithian DC, Paxton LW, Goltz DH. Fate of the anterior cruciate ligament-injured knee. Orthop Clin North Am. 2002;33(4):621–636. doi: 10.1016/s0030-5898(02)00015-9. v. [DOI] [PubMed] [Google Scholar]
- 4.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthritis Cartilage. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 5.Felson DT, Lawrence RC, Dieppe PA, Hirsch R, Helmick CG, Jordan JM, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133(8):635–646. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 6.Marshall KW, Chan AD. Arthroscopic anterior cruciate ligament transection induces canine osteoarthritis. J Rheumatol. 1996;23(2):338–343. [PubMed] [Google Scholar]
- 7.Brandt KD, Myers SL, Burr D, Albrecht M. Osteoarthritic changes in canine articular cartilage, subchondral bone, and synovium fifty-four months after transection of the anterior cruciate ligament. Arthritis Rheum. 1991;34(12):1560–1570. doi: 10.1002/art.1780341214. [DOI] [PubMed] [Google Scholar]
- 8.Hunter DJ, Sharma L, Skaife T. Alignment and osteoarthritis of the knee. J Bone Joint Surg Am. 2009;91 Suppl 1:85–89. doi: 10.2106/JBJS.H.01409. [DOI] [PubMed] [Google Scholar]
- 9.Defrate LE, Papannagari R, Gill TJ, Moses JM, Pathare NP, Li G. The 6 degrees of freedom kinematics of the knee after anterior cruciate ligament deficiency: an in vivo imaging analysis. Am J Sports Med. 2006;34(8):1240–1246. doi: 10.1177/0363546506287299. [DOI] [PubMed] [Google Scholar]
- 10.Georgoulis AD, Papadonikolakis A, Papageorgiou CD, Mitsou A, Stergiou N. Three-dimensional tibiofemoral kinematics of the anterior cruciate ligament-deficient and reconstructed knee during walking. Am J Sports Med. 2003;31(1):75–79. doi: 10.1177/03635465030310012401. [DOI] [PubMed] [Google Scholar]
- 11.Logan M, Dunstan E, Robinson J, Williams A, Gedroyc W, Freeman M. Tibiofemoral kinematics of the anterior cruciate ligament (ACL)-deficient weightbearing, living knee employing vertical access open "interventional" multiple resonance imaging. Am J Sports Med. 2004;32(3):720–726. doi: 10.1177/0095399703258771. [DOI] [PubMed] [Google Scholar]
- 12.Shepherd DE, Seedhom BB. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58(1):27–34. doi: 10.1136/ard.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones G, Glisson M, Hynes K, Cicuttini F. Sex and site differences in cartilage development: a possible explanation for variations in knee osteoarthritis in later life. Arthritis Rheum. 2000;43(11):2543–2549. doi: 10.1002/1529-0131(200011)43:11<2543::AID-ANR23>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 14.Ateshian GA, Soslowsky LJ, Mow VC. Quantitation of articular surface topography and cartilage thickness in knee joints using stereophotogrammetry. J Biomech. 1991;24(8):761–776. doi: 10.1016/0021-9290(91)90340-s. [DOI] [PubMed] [Google Scholar]
- 15.Bolbos RI, Ma CB, Link TM, Majumdar S, Li X. In vivo T1rho quantitative assessment of knee cartilage after anterior cruciate ligament injury using 3 Tesla magnetic resonance imaging. Invest Radiol. 2008;43(11):782–788. doi: 10.1097/RLI.0b013e318184a451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saadat E, Jobke B, Chu B, Lu Y, Cheng J, Li X, et al. Diagnostic performance of in vivo 3-T MRI for articular cartilage abnormalities in human osteoarthritic knees using histology as standard of reference. Eur Radiol. 2008;18(10):2292–2302. doi: 10.1007/s00330-008-0989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samosky JT, Burstein D, Eric Grimson W, Howe R, Martin S, Gray ML. Spatially-localized correlation of dGEMRIC-measured GAG distribution and mechanical stiffness in the human tibial plateau. J Orthop Res. 2005;23(1):93–101. doi: 10.1016/j.orthres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Taylor C, Carballido-Gamio J, Majumdar S, Li X. Comparison of quantitative imaging of cartilage for osteoarthritis: T2, T1rho, dGEMRIC and contrast-enhanced computed tomography. Magn Reson Imaging. 2009 doi: 10.1016/j.mri.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeman MA, Pinskerova V. The movement of the knee studied by magnetic resonance imaging. Clin Orthop Relat Res. 2003;(410):35–43. doi: 10.1097/01.blo.0000063598.67412.0d. [DOI] [PubMed] [Google Scholar]
- 20.Hinterwimmer S, Gotthardt M, von Eisenhart-Rothe R, Sauerland S, Siebert M, Vogl T, et al. In vivo contact areas of the knee in patients with patellar subluxation. J Biomech. 2005;38(10):2095–2101. doi: 10.1016/j.jbiomech.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 21.Wretenberg P, Ramsey DK, Nemeth G. Tibiofemoral contact points relative to flexion angle measured with MRI. Clin Biomech (Bristol, Avon) 2002;17(6):477–485. doi: 10.1016/s0268-0033(02)00036-0. [DOI] [PubMed] [Google Scholar]
- 22.Asano T, Akagi M, Tanaka K, Tamura J, Nakamura T. In vivo three-dimensional knee kinematics using a biplanar image-matching technique. Clin Orthop. 2001;(388):157–166. doi: 10.1097/00003086-200107000-00023. [DOI] [PubMed] [Google Scholar]
- 23.Li G, DeFrate LE, Park SE, Gill TJ, Rubash HE. In vivo articular cartilage contact kinematics of the knee: an investigation using dual-orthogonal fluoroscopy and magnetic resonance image-based computer models. Am J Sports Med. 2005;33(1):102–107. doi: 10.1177/0363546504265577. [DOI] [PubMed] [Google Scholar]
- 24.Dennis DA, Mahfouz MR, Komistek RD, Hoff W. In vivo determination of normal and anterior cruciate ligament-deficient knee kinematics. J Biomech. 2005;38(2):241–253. doi: 10.1016/j.jbiomech.2004.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Moses JM, Papannagari R, Pathare NP, DeFrate LE, Gill TJ. Anterior cruciate ligament deficiency alters the in vivo motion of the tibiofemoral cartilage contact points in both the anteroposterior and mediolateral directions. J Bone Joint Surg Am. 2006;88(8):1826–1834. doi: 10.2106/JBJS.E.00539. [DOI] [PubMed] [Google Scholar]
- 26.Bingham JT, Papannagari R, Van de Velde SK, Gross C, Gill TJ, Felson DT, et al. In vivo cartilage contact deformation in the healthy human tibiofemoral joint. Rheumatology (Oxford) 2008;47(11):1622–1627. doi: 10.1093/rheumatology/ken345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Van de Velde SK, Bingham JT. Validation of a non-invasive fluoroscopic imaging technique for the measurement of dynamic knee joint motion. J Biomech. 2008;41(7):1616–1622. doi: 10.1016/j.jbiomech.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 28.Li G, Park SE, DeFrate LE, Schutzer ME, Ji L, Gill TJ, et al. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin Biomech (Bristol, Avon) 2005;20(7):736–744. doi: 10.1016/j.clinbiomech.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Griffin LY, Agel J, Albohm MJ, Arendt EA, Dick RW, Garrett WE, et al. Noncontact anterior cruciate ligament injuries: risk factors and prevention strategies. J Am Acad Orthop Surg. 2000;8(3):141–150. doi: 10.5435/00124635-200005000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kannus P, Jarvinen M. Posttraumatic anterior cruciate ligament insufficiency as a cause of osteoarthritis in a knee joint. Clin Rheumatol. 1989;8(2):251–260. doi: 10.1007/BF02030082. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Molina G, Guermazi A, Niu J, Gale D, Goggins J, Amin S, et al. Central bone marrow lesions in symptomatic knee osteoarthritis and their relationship to anterior cruciate ligament tears and cartilage loss. Arthritis Rheum. 2008;58(1):130–136. doi: 10.1002/art.23173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Ma BC, Bolbos RI, Stahl R, Lozano J, Zuo J, et al. Quantitative assessment of bone marrow edema-like lesion and overlying cartilage in knees with osteoarthritis and anterior cruciate ligament tear using MR imaging and spectroscopic imaging at 3 Tesla. J Magn Reson Imaging. 2008;28(2):453–461. doi: 10.1002/jmri.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fairclough JA, Graham GP, Dent CM. Radiological sign of chronic anterior cruciate ligament deficiency. Injury. 1990;21(6):401–402. doi: 10.1016/0020-1383(90)90130-m. [DOI] [PubMed] [Google Scholar]
- 34.Murrell GA, Maddali S, Horovitz L, Oakley SP, Warren RF. The effects of time course after anterior cruciate ligament injury in correlation with meniscal and cartilage loss. Am J Sports Med. 2001;29(1):9–14. doi: 10.1177/03635465010290012001. [DOI] [PubMed] [Google Scholar]
- 35.Amin S, Guermazi A, Lavalley MP, Niu J, Clancy M, Hunter DJ, et al. Complete anterior cruciate ligament tear and the risk for cartilage loss and progression of symptoms in men and women with knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(8):897–902. doi: 10.1016/j.joca.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52(3):794–799. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 37.Fowler PJ. Bone injuries associated with anterior cruciate ligament disruption. Arthroscopy. 1994;10(4):453–460. doi: 10.1016/s0749-8063(05)80198-7. [DOI] [PubMed] [Google Scholar]
- 38.Andriacchi TP, Mundermann A, Smith RL, Alexander EJ, Dyrby CO, Koo S. A framework for the in vivo pathomechanics of osteoarthritis at the knee. Ann Biomed Eng. 2004;32(3):447–457. doi: 10.1023/b:abme.0000017541.82498.37. [DOI] [PubMed] [Google Scholar]
- 39.Brandt KD. Transection of the anterior cruciate ligament in the dog: a model of osteoarthritis. Semin Arthritis Rheum. 1991;21(3 Suppl 2):22–32. doi: 10.1016/0049-0172(91)90037-z. [DOI] [PubMed] [Google Scholar]
- 40.Lozano J, Saadat E, Li X, Majumdar S, Ma CB. Magnetic resonance T(1rho) imaging of osteoarthritis: a rabbit ACL transection model. Magn Reson Imaging. 2008 doi: 10.1016/j.mri.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Bolbos RI, Link TM, Benjamin Ma C, Majumdar S, Li X. T1rho relaxation time of the meniscus and its relationship with T1rho of adjacent cartilage in knees with acute ACL injuries at 3T. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron M, Buchgraber A, Passler H, Vogt M, Thonar E, Fu F, et al. The natural history of the anterior cruciate ligament-deficient knee. Changes in synovial fluid cytokine and keratan sulfate concentrations. Am J Sports Med. 1997;25(6):751–754. doi: 10.1177/036354659702500605. [DOI] [PubMed] [Google Scholar]
- 43.Bevill SL, Briant PL, Levenston ME, Andriacchi TP. Central and peripheral region tibial plateau chondrocytes respond differently to in vitro dynamic compression. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 44.Shelburne KB, Torry MR, Pandy MG. Muscle, ligament, and joint-contact forces at the knee during walking. Med Sci Sports Exerc. 2005;37(11):1948–1956. doi: 10.1249/01.mss.0000180404.86078.ff. [DOI] [PubMed] [Google Scholar]
- 45.Stuart MJ, Meglan DA, Lutz GE, Growney ES, An KN. Comparison of intersegmental tibiofemoral joint forces and muscle activity during various closed kinetic chain exercises. Am J Sports Med. 1996;24(6):792–799. doi: 10.1177/036354659602400615. [DOI] [PubMed] [Google Scholar]
- 46.Escamilla RF. Knee biomechanics of the dynamic squat exercise. Med Sci Sports Exerc. 2001;33(1):127–141. doi: 10.1097/00005768-200101000-00020. [DOI] [PubMed] [Google Scholar]
- 47.Li G, Wan L, Kozanek M. Determination of real-time in-vivo cartilage contact deformation in the ankle joint. J Biomech. 2008;41(1):128–136. doi: 10.1016/j.jbiomech.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 48.Raynauld JP, Kauffmann C, Beaudoin G, Berthiaume MJ, de Guise JA, Bloch DA, et al. Reliability of a quantification imaging system using magnetic resonance images to measure cartilage thickness and volume in human normal and osteoarthritic knees. Osteoarthritis Cartilage. 2003;11(5):351–360. doi: 10.1016/s1063-4584(03)00029-3. [DOI] [PubMed] [Google Scholar]