Abstract
Diabetes is associated with increased incidence of heart failure even after controlling for coronary artery disease and hypertension. Thus, as diabetic cardiomyopathy has become an increasingly recognized entity among clinicians, a better understanding of its pathophysiology is necessary for early diagnosis and the development of treatment strategies for diabetes-associated cardiovascular dysfunction. We will review recent basic and clinical research into the manifestations and the pathophysiological mechanisms of diabetic cardiomyopathy. The discussion will be focused on the structural, functional and metabolic changes that occur in the myocardium in diabetes and how these changes may contribute to the development of diabetic cardiomyopathy in affected humans and relevant animal models.
Keywords: Diabetic cardiomyopathy, Diastolic dysfunction, Substrate utilization, Mitochondrial dysfunction, Uncoupling
1 Introduction
The concept of diabetic cardiomyopathy was first introduced by Rubler et al [1], and has subsequently been widely used by epidemiologists and clinicians. Diabetic cardiomyopathy describes diabetes-associated changes in the structure and function of the myocardium that is not directly attributable to other confounding factors such as coronary artery disease (CAD) or hypertension. It is important to note that in many patients, particularly those with type 2 diabetes, diabetes associated changes are amplified by the existence of these co-morbidities, which likely will augment the development of left ventricular hypertrophy, increase the susceptibility of the heart to ischemic injury and increase the overall likelihood of developing heart failure [2]. Several mechanisms have been implicated in the pathogenesis of diabetic cardiomyopathy. Changes in myocardial structure, calcium signaling and metabolism are early defects that have been described mainly in animal models and may precede clinically manifest cardiac dysfunction. However, subtle functional changes can be detected if specifically looked for.
2 Changes in structure and cell survival signaling pathways
2.1 Left ventricular hypertrophy (LVH)
Increased left ventricular (LV) mass is an independent risk factor for heart failure and may occur independently of arterial blood pressure in Type 2 diabetes, and may contribute to reduced myocardial compliance [3]. The Framingham study reported a significant increase in LV wall thickness only in women with diabetes [4]. In contrast, the Strong Heart Study conducted in Native Americans, found that both men and women with diabetes had higher LV mass and wall thickness [5]. Furthermore, in a multi-ethnic population, the likelihood of having LV mass that exceeds the 75th percentile is greater in patients with Type 2 diabetes, after adjusting for various covariates including hypertension [6]. Indeed, in this same population, increased LV mass was observed only in patients with diabetes but not in patients with impaired fasting glucose or impaired glucose tolerance [7], suggesting that changes in myocardial geometry in diabetes might not be an early defect but rather a consequence of long term diabetes-associated changes such as hyperglycemia and/or obesity. Eguchi et al. [6] described a significant interaction between diabetes and central obesity on the risk for LVH. Furthermore, obesity promotes concentric LVH independently of hypertension [8]. Emerging evidence has implicated cytokines, produced by the expanded adipose tissue of obesity, in the development of LVH. For example, leptin is linked to cardiac hypertrophy in obese humans and directly induces cardiomycyte hypertrophy in vitro [9]. The mechanisms by which leptin induces LVH is not fully characterized but might involve endothelin 1- mediated reactive oxygen species (ROS) generation [10]. Similarly, resistin, which is also an adipokine that is released from macrophages, was shown to induce cardiomyocyte hypertrophy in vitro via IRS-1 and MAPK signaling pathways [11]. Epidemiological studies have suggested a correlation between circulating levels of the inflammatory cytokine interleukin 6, and the risk of obesity-associated heart failure [12]. Insulin resistance and hyperinsulinemia have been correlated with increased LV mass and may partially account for the association of cardiac hypertrophy and obesity [13], and is also correlated with increased risk of heart failure [14]. An increase in IRS1-associated PI3K activity was recently reported in cardiac biopsies obtained from patients with Type 2 diabetes [15]. Insulin signaling might act as a growth factor in the heart, as genetic deletion of insulin receptors leads to reduced cardiac size [16]. Taken together, these observations raise the intriguing possibility that hyperinsulinemia might contribute to diabetes and obesity-related LV hypertrophy.
2.2 Myocardial lipotoxicity
Type 2 diabetes which is often associated with obesity, leads to myocardial lipotoxicity that may contribute to cell death and thus to cardiac dysfunction and this topic has recently been reviewed in detail by us [17]. Thirty years ago, Regan et al [18] identified lipofuscin deposits, which are brown lipid-containing pigment granules, in transmural LV biopsies obtained from patients with Type 2 diabetes. Furthermore, myocardial triglyceride (TG) and cholesterol content were significantly increased in these samples. Similarly, Oil Red O staining of heart sections of non-ischemic failing hearts, revealed increased lipid deposition that was exacerbated by diabetes [19]. Recent advances in magnetic resonance spectroscopy, has enabled non-invasive assessment of myocardial triglyceride content. Diabetes, obesity, insulin resistance and impaired glucose tolerance are associated with increased intra-myocardial lipid that is independent of circulating concentrations of triglycerides [20]. This increase in cardiac triglyceride accumulation is associated with diastolic but not systolic dysfunction [20, 21]. It is not clear if triglyceride accumulation is pathogenic per se or is a marker of the underlying metabolic milieu. Increased myocardial triglycerides were not observed in overweight but fairly well-controlled individuals with Type 2 diabetes [22]. This contrasts with the findings in obese diabetics with poorer control [20]. Improvement in cardiac function in these relatively well-controlled patients, in response to treatment with metformin or TZDs occurred independently of changes in myocardial triglyceride content [22].
An increase in myocardial fatty acid uptake and oxidation has been described in humans with both Type 1 and Type 2 diabetes, as well as in many animal models [17, 23, 24]. Transgenic mouse models have suggested that an isolated increase in myocardial lipid uptake is sufficient to precipitate cardiomyopathy in the absence of hyperglycemia. For example, over-expression of proteins involved in cardiac FA transport such as long-chain acyl-CoA synthetase, glycosylphosphatidylinositol (GPI) membrane-anchored form of lipoprotein lipase or FA transport protein 1 resulted in lipotoxic cardiomyopathy in mice [25, 26]. The exact mechanisms by which increased myocardial lipid uptake induces lipotoxicity and cardiac dysfunction are incompletely understood, but potential mechanisms have been recently reviewed [17]. Lipid-induced cell death might be an important contributor. For example, long-chain FA supplementation to chinese hamster ovary cells (CHO) at pathophysiologic concentrations induced cell death that was associated with increased de novo ceramide biosynthesis [27]. In parallel, inhibition of ceramide biosynthesis prevented lipotoxic cardiomyopathy in mice over-expressing a glycosylphosphatidylinositol (GPI) membrane-anchored form of lipoprotein lipase [28]. Since palmitate-induced cell death in CHO was not completely prevented by inhibition of ceramide biosynthesis [27], additional mechanisms by which FA induced-cell death were proposed. For example, long-chain fatty acids can change the dynamics of plasma and mitochondrial membranes by altering phospholipid composition. Detachment of cytochrome c from the mitochondrial inner membrane is a necessary step for cytochrome c release and initiation of apoptosis. The saturated long chain FA, palmitate, induces apoptosis in rat neonatal cardiomyocytes by diminishing the content of the mitochondrial anionic phospholipid, cardiolipin [29]. In addition, changes in the composition of endoplasmic reticulum (ER) membrane phospholipids have also been observed in lipotoxic conditions, which precipitate ER swelling and ER stress [30, 31].
2.3 Increased oxidative stress
Although many studies have suggested that oxidative stress may play a critical role in the development of diabetic cardiomyopathy, the mechanisms involved in reactive oxygen species (ROS) production in diabetic hearts are not well understood. Human and animal studies have suggested that increased oxidative stress correlates with lipid overload, suggesting a role for FA in the generation of ROS (Fig. 1). Indeed, oxidative stress is increased in hearts isolated from the db/db model of Type 2 diabetes, which are also characterized by cardiac lipid accumulation and increased mitochondrial FA oxidation [32]. In db/db hearts, oxidative stress is exacerbated in the presence of fatty acids, which we believe leads to mitochondrial uncoupling. The mechanisms for increased mitochondrial ROS in this model cannot be fully explained by increased FA flux, because independent models, such as the Akita mouse model of Type 1 diabetes do not exhibit increased mitochondrial ROS generation or evidence of mitochondrial uncoupling despite increased rates of FA oxidation [33]. An important distinction between the hearts of Akita mice versus db/db or ob/ob mice is the presence of myocardial insulin resistance in obese models with insulin resistance, whereas in Type 1 diabetes models, insulin sensitivity is preserved. Interestingly, in mice with cardiac-specific deletion of insulin receptors, hydrogen peroxide production was increased and mitochondria were uncoupled even at stage when myocardial FA oxidation was reduced [34]. These data raise the intriguing possibility that myocardial insulin resistance may specifically predispose cardiac mitochondria to ROS overproduction via mechanisms that remain to be elucidated.
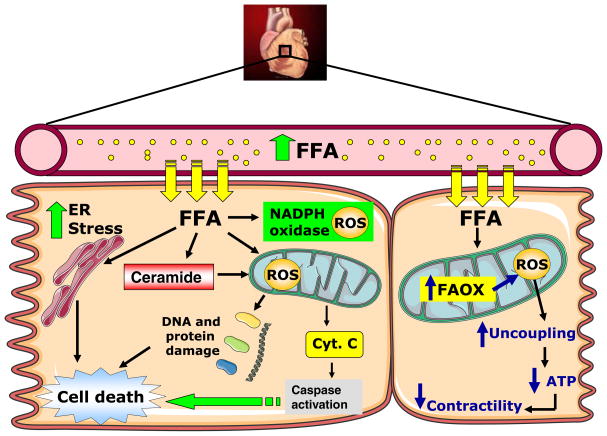
Fig. 1.
Mechanisms for FA-induced cardiac dysfunction in diabetes. Increased FA uptake in cardiomyocytes in vivo precipitates cardiomyocyte dysfunction by multiple mechanisms including increased mitochondrial and cytosolic ROS generation and ER stress. FA-mediated ROS generation leads to uncoupling of mitochondria, which reduces mitochondrial ATP production. FFA: free fatty acids; ROS: reactive oxygen species; ER: endoplasmic reticulum; Cyt. C: cytochrome c; FAOX: Fatty acid oxidation
Although a large fraction of total cellular ROS is generated in the mitochondria, enzymatic systems capable of generating ROS in the cytosol such as NADPH oxidase can be modulated by diabetes [35, 36]. ROS can also interact with other molecules such as nitric oxide (NO) to form nitrotyrosine species, which were found to be elevated in myocardial biopsies of humans with Type 2 diabetes [37]. Finally, in addition to ROS-induced cardiomyocyte cell death, these reactive molecules can also alter gene expression. In the diabetic fatty ZDF rats, increased ROS contributes to the switch in cardiac myosin heavy-chain gene expression from alpha to beta through the activation of NFkB, and antioxidant treatment was able to prevent this switch [38].
2.4 Cell death
When necrotic and apoptotic cell death have been evaluated in myocardial biopsies of diabetic subjects with heart failure that could not be attributed to myocardial ischemia, apoptosis and necrosis were increased in all cell populations within the heart. The co-existence of diabetes and hypertension, increased necrotic cell death further, whereas there was no additional increase in apoptosis [37]. Analysis of right atrial appendages obtained from Type 1 and Type 2 diabetic subjects, at the time of elective coronary artery bypass surgery, revealed an increase in apoptosis and necrosis at the time of tissue harvest, and an exaggerated increase when tissues from diabetics were subjected to ischemia and reperfusion [39]. Increased cardiomyocyte apoptosis has also been described in hearts of ob/ob and db/db mice [40]. The mechanisms for increased cell death are incompletely understood. A role for leptin deficiency has been postulated because treatment of ob/ob mice with leptin reduced apoptosis [40]. In addition to leptin deficiency, hyperglycemia has also been implicated in triggering cell death via a Rac1 mediated increase in NADPH and mitochondrial derived ROS in the hearts of db/db and STZ diabetic mice [41].
Activation of the renin-angiotensin system (RAS) correlated with increased oxidative stress, apoptosis and necrosis in cardiomyocytes and endothelial cells in the hearts of patients with diabetes and end stage heart failure, thereby representing another potential mechanism for cell death [37, 42]. In this regard, it is important to note that inhibition of the RAS, reduced the rate of first hospitalization from heart failure and improved echocardiographic indices of LV diastolic function in patients with Type 2 diabetes [43–45].
2.5 Interstitial fibrosis
Diabetic cardiomyopathy is characterized by interstitial and perivascular fibrosis. Regan et al. [18] found a significant increase in collagen deposition around intramural vessels and between myofibers in heart biopsies form diabetic patients. In addition, a significant increase in collagen type III but not type I or VI was found in endomyocardial biopsies obtained from patients with Type 2 diabetes, who did not have significant CAD and hypertension [46]. Furthermore, diastolic dysfunction detected in a population of uncomplicated Type 2 diabetes correlated with pro-collagen type I carboxy-terminal peptide [47, 48], suggesting a mechanistic role for myocardial fibrosis in myocardial dysfunction in diabetes. Similar to humans, some animal models with Type 2 diabetes also exhibited an increase in cardiac fibrosis even prior to the onset of hyperglycemia. Thus, increased extracellular fibrosis and collagen deposition was reported in the pre-diabetic stage in OLETF rats, a genetic model of diabetes that resembles human Type 2 diabetes [49]. The mechanisms for increased cardiac fibrosis in the diabetic heart are incompletely understood. A recent study reported an increase in TGFβ1 receptor II density in the diabetic myocardium. TGFβ is one of several cytokines the gene expression of which is enhanced by diabetes [50]. Increased CTGF expression and collagen deposition has also been observed in mouse models of STZ diabetes [51] that was associated with increased expression of PKCβ2. Similar changes were observed in the hearts of mice that lack insulin receptors in cardiomyocytes (CIRKO mice), and was further augmented by isoproterenol treatment suggesting a role for impaired insulin action in diabetes-associated cardiac fibrosis [52]. Cardiac fibrosis has not been a uniform characteristic of all mouse models however. For example myocardial fibrosis was absent in db/db mice [53].
2.6 Changes in cardiac function
A number of studies have characterized functional changes that develop early in the course of diabetic cardiomyopathy. Early detection of these changes may play an important role in the design of clinical trials of novel or targeted therapeutic strategies and provide important parameters for monitoring the natural history of diabetic cardiomyopathy and its response to treatment.
2.7 Diastolic dysfunction (DD)
Diabetic cardiomyopathy in humans is characterized by DD, which may precede the development of systolic dysfunction. The use of flow and tissue Doppler techniques suggests a prevalence of DD as high as 40–75% in individuals with Type 1 and Type 2 diabetes without overt CAD [54, 55]. Indeed, indices for DD such as E/E′ and E/A ratios (where E and A are the mitral peak velocity of early and late ventricular filling respectively and E′ is the early diastolic mitral annular velocity) were impaired in Type 2 diabetic patients [54, 56]. Similarly, DD was found in animal models of Type 2 diabetes such as ob/ob and db/db mice and the ZDF rat. These animal models exhibit obesity, insulin resistance and mild or severe hyperglycemia and are suitable to investigate the effect of diabetes on cardiac function independently of micro and macro vascular complications given the absence of atherosclerosis in these models [57, 58]. Thus, DD has been shown in db/db hearts both in vivo (by echocardiography) [59] and ex vivo (in working heart preparations) [60, 61].
Several mechanisms have been proposed to explain Type 2 diabetes-associated DD such as increased cardiac lipid accumulation and altered calcium homeostasis. Diastolic but not systolic dysfunction was associated with increased cardiac triglyceride content in ob/ob mice [62]. Furthermore, these mice also exhibit impaired calcium reuptake that was associated with contractile dysfunction [53, 63]. Similarly, reduced contractility in cardiomyocytes isolated from sedentary db/db mice was associated with increased diastolic sarcoplasmic reticulum (SR)-Ca2+ leak, reduced synchrony of Ca2+ release, lower peak systolic and diastolic Ca2+ and caffeine-induced Ca2+ release, consistent with a role for calcium in the DD seen in Type 2 diabetes. Interestingly, exercise training in these animals was able to normalize these parameters and to reverse contractile dysfunction [64].
2.8 Systolic dysfunction (SD)
Systolic dysfunction is a later manifestation, usually occurring after DD develops. Subtle SD is often not detected using standard 2-dimensional echocardiography techniques. However, using tissue Doppler strain analysis and measurements of peak systolic velocity, subtle abnormalities in systolic function have been described in up to 24% of randomly selected patients with diabetes mellitus after excluding subjects with CAD or LVH [65, 66]. In vivo animal studies using MR imaging [67] or invasive catheterization with PV loop analysis, have revealed load dependent and independent indices of systolic dysfunction in various murine models [68]. Recent studies using PV loops have begun to compare contractile performance in Type 1 (STZ) versus Type 2 rodent models (Zucker Diabetic Fatty Rat). Both models revealed systolic dysfunction, but Type 1 diabetic models revealed delayed LV relaxation, whereas the Type 2 model revealed increased LV stiffness [69].
2.9 Impaired contractile reserve
Diabetic cardiomyopathy might be present even in asymptomatic subjects with normal resting LV dimensions and function. However in some of these individuals with early stage disease, LV dysfunction can be induced by exercise. In many of these subjects, impaired exercise-induced augmentation correlates with impaired myocardial sympathetic innervation [70]. Subsequent studies, revealed impaired exercise-induced augmentation in systolic performance in individuals with Type 1 and Type 2 Diabetes with no evidence of autonomic neuropathy or myocardial ischemia, and normal resting echocardiographic parameters (including tissue Doppler measurements) at baseline [71, 72]. Thus, impaired cardiac performance after exercise could be a potential tool to detect early contractile dysfunction in diabetes. However, before this approach can be widely adopted, there will need to be consensus regarding normal age and gender-specific exercise responses and cutoffs below which impaired contractile reserve can be confidently diagnosed. Moreover, it will be critical to exclude CAD, other structural abnormalities or neurohumoral abnormalities (such as thyroid dysfunction) that could independently impair contractile reserves. Similar studies in animal models are relatively sparse. Contractile reserve as assessed by inotropic stimulation was reported to be unchanged as in the OLETF rats [73] or reduced in ob/ob mice [62, 74]. It is important to note though that impaired inotropic reserves measured in vivo or ex vivo in animal models do not necessarily recapitulate the complex hemodynamic adaptations to exercise training.
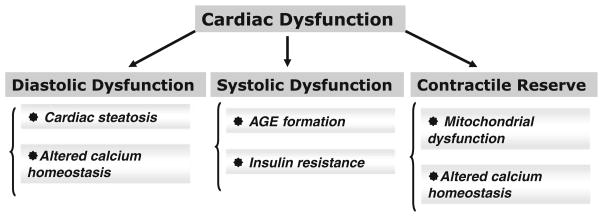
As summarized in Fig. 2 and recently reviewed by us [75], multiple mechanisms may lead to impaired diastolic and systolic function and reduced contractile reserves in diabetic cardiomyopathy. This includes accumulation of advanced glycation end products, [76], adipokines [77], impaired myocardial insulin signaling [34], altered calcium homeostasis [53, 63] and lipotoxicity [62].
Fig. 2.
Cellular mechanisms that contribute to cardiac contractile dysfunction in diabetes
2.10 Changes in myocardial metabolism
Many studies have implicated changes in myocardial substrate and energy metabolism in the pathogenesis of diabetic cardiomyopathy. In this section, we will discuss recent findings confirming the existence of metabolic changes in the heart of humans and animals with diabetes.
2.11 Altered substrate utilization
Diabetes is characterized by increased circulating concentrations of glucose and free fatty acid (FFA). Despite the presence of hyperglycemia, the diabetic heart relies almost completely on FFA utilization, with a coordinate decrease in glucose utilization. This pattern of substrate utilization has been described both in human and animal studies and have been reviewed in detail elsewhere [23, 24, 78]. There are a number of mechanisms that are responsible for this shift in substrate utilization. The earliest change that occurs in short term studies of high-fat fed mice is reduced myocardial GLUT4 content and a defect in GLUT4 translocation. This in turns leads to reduced rates of glycolysis and glucose oxidation. FA oxidation rates are subsequently increased most likely via the Randle cycle [79]. As high-fat feeding becomes more prolonged and diabetes ensues, increased delivery of FA substrates activate PPAR-alpha signaling pathways, which leads to transcriptional induction of enzymes involved in beta oxidation and increased expression of pyruvate dehydrogenase (PDH) kinase (PDK4), which further suppresses glucose oxidation by decreasing PDH activity [79, 80]. In humans with Type 2 diabetes and heart failure, myocardial lipotoxicity was associated with evidence of activation of the PPAR alpha target gene carnitine palmitoyl-transferase 1 (muscle isoform, mCPT1), which regulates mitochondrial FA uptake [19].
Similar changes in substrate utilization occur in Type 1 diabetes, where transcriptional repression of GLUT4 via down regulation of the expression of its regulator myocyte enhancer factor 2C (MEF2C) is well described in mice [81]. In a recent human study, GLUT4 and MEF2C mRNA were significantly down regulated in failing hearts from diabetic subjects as opposed to failing hearts from non-diabetics [82]. Because fatty acids are considered an inefficient substrate, increased FA oxidation in diabetic hearts is often accompanied by an increase in myocardial oxygen consumption (MVO2) and reduced cardiac efficiency in rodent models [83, 84], and in obese and insulin resistant humans, as well as humans with Type 1 diabetes [85, 86].
The challenge of future studies will be to determine if therapies that normalize myocardial substrate metabolism in diabetes mellitus will translate to lower prevalence of heart failure or improved long-term survival.
2.12 Mitochondrial dysfunction
In contrast to skeletal muscle, studies examining mitochondrial function in cardiac muscle of humans with diabetes have been challenging. Our contribution to the understanding of mitochondrial bioenergetics comes mainly from animal models of obesity and diabetes, and have been extensively reviewed [75, 78, 87]. In brief we and others have observed striking changes in mitochondrial morphology, remodeling of the mitochondrial proteome and decreased respiratory capacity in models of Type 1 and type 2 diabetes [32, 33, 74, 88]. Thirty years ago, Reagan et al. [18] observed an increase in mitochondrial number with pleomorphism without swelling or distortion of cristae in the myocardium of patients with diabetes. Furthermore, using 31P nuclear magnetic resonance spectroscopy, a number of groups provided evidence for decreased cardiac energetics (decreased pCR/ATP ratios) in Type 1 and Type 2 diabetic patients who were free of overt CAD [89–91]. However reduced PCr/ATP ratios have not been seen in all studies [92]. A recent study, in which mitochondrial function was directly measured in right atrial appendages obtained from diabetics at the time of coronary artery bypass surgery revealed direct evidence of reduced mitochondrial oxygen consumption and increased H2O2 emission [93]. Studies related to the response of the diabetic heart to ischemic preconditioning (IPC) have also identified a defect in the mitochondrial ATP-sensitive potassium channel that may impair the ability of the diabetic heart to be preconditioned and may contribute to their increased risk for myocardial infarction [94].
Our studies of the ob/ob and db/db mouse models of Type 2 diabetes identified mitochondrial uncoupling as an additional defect that contributes to mitochondrial dysfunction in obesity and insulin resistance [32, 74]. We demonstrated increased state 4 respiration and reduced ATP synthesis in mitochondrial preparations obtained from ob/ob and db/db hearts that were pre-perfused with palmitate. This mitochondrial uncoupling further contributes to increasing oxygen consumption without a concomitant increase in ATP production, which contributes to decreased cardiac efficiency in these hearts. The mitochondrial uncoupling is largely mediated by uncoupling proteins and to a lesser extent by the adenine nucleotide translocase. Mitochondrial ROS generation or lipid peroxides such as hydroxynonenal have been shown to activate uncoupling proteins in heart muscle mitochondria [95] and were found to be increased in the hearts of db/db mice [32]. Interestingly, mitochondrial uncoupling was not observed in the hearts of mice with Type 1 diabetes [33, 96]. One difference between the hearts of models of Type 1 and Type 2 diabetes is the existence of myocardial insulin resistance in ob/ob mice versus normal insulin sensitivity in the hearts of the Akita model of Type 1 diabetes, raising the possibility that myocardial insulin resistance might be causally linked to mitochondrial uncoupling. Indeed, our recent study of mitochondrial function in the hearts of mice with genetic deletion of insulin receptors revealed the presence of mitochondrial uncoupling and oxidative stress in the absence of hyperglycemia [34].
3 Conclusion
Although the increase in cardiovascular mortality and heart failure is due in part to accelerated atherosclerosis, compelling epidemiological and clinical data indicate that diabetes mellitus increases the risk for cardiac dysfunction and heart failure independently of other risk factors such as CAD and hypertension. The existence of diabetic cardiomyopathy is becoming increasingly recognized and this review has summarized the associated structural, functional and metabolic changes. As the mechanisms responsible for diabetic cardiomyopathy continue to be elucidated, it is hoped that these insights will provide the impetus for novel therapies that are tailored to reduce the risk of heart failure in individuals with diabetes mellitus.
Acknowledgments
Dr. Boudina has been supported by the JDRF, and is currently supported by NIH P30 HL101310 and a Scientist Development Award from the American Heart Association. Dr. Abel is an Established Investigator of the American Heart Association and is supported by the American Diabetes Association and UO1 HL087947 (Animal Models of Diabetes Complications Consortium).
References
- 1.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30(6):595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 2.Hayat SA, Patel B, Khattar RS, Malik RA. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004;107(6):539–57. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- 3.Aneja A, Tang WH, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121(9):748–57. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 4.Galderisi M, Anderson KM, Wilson PW, Levy D. Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy (the Framingham Heart Study) Am J Cardiol. 1991;68(1):85–9. doi: 10.1016/0002-9149(91)90716-x. [DOI] [PubMed] [Google Scholar]
- 5.Devereux RB, Roman MJ, Paranicas M, O’Grady MJ, Lee ET, Welty TK, et al. Impact of diabetes on cardiac structure and function: the strong heart study. Circulation. 2000;101(19):2271–6. doi: 10.1161/01.cir.101.19.2271. [DOI] [PubMed] [Google Scholar]
- 6.Eguchi K, Boden-Albala B, Jin Z, Rundek T, Sacco RL, Homma S, et al. Association between diabetes mellitus and left ventricular hypertrophy in a multiethnic population. Am J Cardiol. 2008;101 (12):1787–91. doi: 10.1016/j.amjcard.2008.02.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rerkpattanapipat P, D’Agostino RB, Jr, Link KM, Shahar E, Lima JA, Bluemke DA, et al. Location of arterial stiffening differs in those with impaired fasting glucose versus diabetes: implications for left ventricular hypertrophy from the Multi-Ethnic Study of Atherosclerosis. Diabetes. 2009;58(4):946–53. doi: 10.2337/db08-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodiwiss AJ, Libhaber CD, Majane OH, Libhaber E, Maseko M, Norton GR. Obesity promotes left ventricular concentric rather than eccentric geometric remodeling and hypertrophy independent of blood pressure. Am J Hypertens. 2008;21(10):1144–51. doi: 10.1038/ajh.2008.252. [DOI] [PubMed] [Google Scholar]
- 9.Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108(6):754–9. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 10.Xu FP, Chen MS, Wang YZ, Yi Q, Lin SB, Chen AF, et al. Leptin induces hypertrophy via endothelin-1-reactive oxygen species pathway in cultured neonatal rat cardiomyocytes. Circulation. 2004;110(10):1269–75. doi: 10.1161/01.CIR.0000140766.52771.6D. [DOI] [PubMed] [Google Scholar]
- 11.Kim M, Oh JK, Sakata S, Liang I, Park W, Hajjar RJ, et al. Role of resistin in cardiac contractility and hypertrophy. J Mol Cell Cardiol. 2008;45(2):270–80. doi: 10.1016/j.yjmcc.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bahrami H, Bluemke DA, Kronmal R, Bertoni AG, Lloyd-Jones DM, Shahar E, et al. Novel metabolic risk factors for incident heart failure and their relationship with obesity: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;51(18):1775–83. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 13.Karason K, Sjostrom L, Wallentin I, Peltonen M. Impact of blood pressure and insulin on the relationship between body fat and left ventricular structure. Eur Heart J. 2003;24(16):1500–5. doi: 10.1016/s0195-668x(03)00312-9. [DOI] [PubMed] [Google Scholar]
- 14.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. Jama. 2005;294 (3):334–41. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 15.Cook SA, Varela-Carver A, Mongillo M, Kleinert C, Khan MT, Leccisotti L, Strickland N, Matsui T, Das S, Rosenzweig A, et al. Abnormal myocardial insulin signalling in type 2 diabetes and left-ventricular dysfunction. Eur Heart J. 2009 doi: 10.1093/eurheartj/ehp396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belke DD, Betuing S, Tuttle MJ, Graveleau C, Young ME, Pham M, et al. Insulin signaling coordinately regulates cardiac size, metabolism, and contractile protein isoform expression. J Clin Invest. 2002;109(5):629–39. doi: 10.1172/JCI13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wende AR, Abel ED. Lipotoxicity in the heart. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Regan TJ, Lyons MM, Ahmed SS, Levinson GE, Oldewurtel HA, Ahmad MR, et al. Evidence for cardiomyopathy in familial diabetes mellitus. J Clin Invest. 1977;60(4):884–99. doi: 10.1172/JCI108843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S, Adrogue JV, Golfman L, Uray I, Lemm J, Youker K, et al. Intramyocardial lipid accumulation in the failing human heart resembles the lipotoxic rat heart. Faseb J. 2004;18(14):1692–700. doi: 10.1096/fj.04-2263com. [DOI] [PubMed] [Google Scholar]
- 20.McGavock JM, Lingvay I, Zib I, Tillery T, Salas N, Unger R, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116(10):1170–5. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 21.Rijzewijk LJ, van der Meer RW, Smit JW, Diamant M, Bax JJ, Hammer S, et al. Myocardial steatosis is an independent predictor of diastolic dysfunction in type 2 diabetes mellitus. J Am Coll Cardiol. 2008;52(22):1793–9. doi: 10.1016/j.jacc.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 22.van der Meer RW, Rijzewijk LJ, de Jong HW, Lamb HJ, Lubberink M, Romijn JA, et al. Pioglitazone improves cardiac function and alters myocardial substrate metabolism without affecting cardiac triglyceride accumulation and high-energy phosphate metabolism in patients with well-controlled type 2 diabetes mellitus. Circulation. 2009;119(15):2069–77. doi: 10.1161/CIRCULATIONAHA.108.803916. [DOI] [PubMed] [Google Scholar]
- 23.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88(2):389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115(25):3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 25.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, et al. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107(7):813–22. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yagyu H, Chen G, Yokoyama M, Hirata K, Augustus A, Kako Y, et al. Lipoprotein lipase (LpL) on the surface of cardiomyocytes increases lipid uptake and produces a cardiomyopathy. J Clin Invest. 2003;111(3):419–26. doi: 10.1172/JCI16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276(18):14890–5. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 28.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, et al. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49(10):2101–12. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrander DB, Sparagna GC, Amoscato AA, McMillin JB, Dowhan W. Decreased cardiolipin synthesis corresponds with cytochrome c release in palmitate-induced cardiomyocyte apoptosis. J Biol Chem. 2001;276(41):38061–7. doi: 10.1074/jbc.M107067200. [DOI] [PubMed] [Google Scholar]
- 30.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47(12):2726–37. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Brookheart RT, Michel CI, Listenberger LL, Ory DS, Schaffer JE. The non-coding RNA gadd7 is a regulator of lipid-induced oxidative and endoplasmic reticulum stress. J Biol Chem. 2009;284(12):7446–54. doi: 10.1074/jbc.M806209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56(10):2457–66. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 33.Bugger H, Boudina S, Hu XX, Tuinei J, Zaha VG, Theobald HA, et al. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57(11):2924–32. doi: 10.2337/db08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boudina S, Bugger H, Sena S, O’Neill BT, Zaha VG, Ilkun O, et al. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation. 2009;119(9):1272–83. doi: 10.1161/CIRCULATIONAHA.108.792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L, Renier G. Activation of nicotinamide adenine dinucleotide phosphate (reduced form) oxidase by advanced glycation end products links oxidative stress to altered retinal vascular endothelial growth factor expression. Metabolism. 2006;55(11):1516–23. doi: 10.1016/j.metabol.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Serpillon S, Floyd BC, Gupte RS, George S, Kozicky M, Neito V, et al. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297(1):H153–62. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, et al. Myocardial cell death in human diabetes. Circ Res. 2000;87 (12):1123–32. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- 38.Aragno M, Mastrocola R, Medana C, Catalano MG, Vercellinatto I, Danni O, et al. Oxidative stress-dependent impairment of cardiac-specific transcription factors in experimental diabetes. Endocrinology. 2006;147(12):5967–74. doi: 10.1210/en.2006-0728. [DOI] [PubMed] [Google Scholar]
- 39.Chowdhry MF, Vohra HA, Galinanes M. Diabetes increases apoptosis and necrosis in both ischemic and nonischemic human myocardium: role of caspases and poly-adenosine diphosphate-ribose polymerase. J Thorac Cardiovasc Surg. 2007;134(1):124–31. 131, e121–123. doi: 10.1016/j.jtcvs.2006.12.059. [DOI] [PubMed] [Google Scholar]
- 40.Barouch LA, Gao D, Chen L, Miller KL, Xu W, Phan AC, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98(1):119–24. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 41.Shen E, Li Y, Shan L, Zhu H, Feng Q, Arnold JM, et al. Rac1 is required for cardiomyocyte apoptosis during hyperglycemia. Diabetes. 2009;58(10):2386–95. doi: 10.2337/db08-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhalla NS, Liu X, Panagia V, Takeda N. Subcellular remodeling and heart dysfunction in chronic diabetes. Cardiovasc Res. 1998;40(2):239–47. doi: 10.1016/s0008-6363(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 43.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 44.Symeonides P, Koulouris S, Vratsista E, Triantafyllou K, Ioannidis G, Thalassinos N, et al. Both ramipril and telmisartan reverse indices of early diabetic cardiomyopathy: a comparative study. Eur J Echocardiog. 2007;8(6):480–6. doi: 10.1016/j.euje.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Tsutsui H, Matsushima S, Kinugawa S, Ide T, Inoue N, Ohta Y, et al. Angiotensin II type 1 receptor blocker attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Hypertens Res. 2007;30(5):439–49. doi: 10.1291/hypres.30.439. [DOI] [PubMed] [Google Scholar]
- 46.Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46(1):32–6. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez-Vilchez F, Ayuela J, Ares M, Pi J, Castillo L, Martin-Duran R. Oxidative stress and fibrosis in incipient myocardial dysfunction in type 2 diabetic patients. Int J Cardiol. 2005;101 (1):53–8. doi: 10.1016/j.ijcard.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Ihm SH, Youn HJ, Shin DI, Jang SW, Park CS, Kim PJ, et al. Serum carboxy-terminal propeptide of type I procollagen (PIP) is a marker of diastolic dysfunction in patients with early type 2 diabetes mellitus. Int J Cardiol. 2007;122(3):e36–8. doi: 10.1016/j.ijcard.2007.07.057. [DOI] [PubMed] [Google Scholar]
- 49.Mizushige K, Yao L, Noma T, Kiyomoto H, Yu Y, Hosomi N, et al. Alteration in left ventricular diastolic filling and accumulation of myocardial collagen at insulin-resistant prediabetic stage of a type II diabetic rat model. Circulation. 2000;101(8):899–907. doi: 10.1161/01.cir.101.8.899. [DOI] [PubMed] [Google Scholar]
- 50.Ban CR, Twigg SM. Fibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markers. Vasc Health Risk Manag. 2008;4(3):575–96. doi: 10.2147/vhrm.s1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, et al. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes. 2002;51(9):2709–18. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- 52.McQueen AP, Zhang D, Hu P, Swenson L, Yang Y, Zaha VG, et al. Contractile dysfunction in hypertrophied hearts with deficient insulin receptor signaling: possible role of reduced capillary density. J Mol Cell Cardiol. 2005;39(6):882–92. doi: 10.1016/j.yjmcc.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 53.Van den Bergh A, Vanderper A, Vangheluwe P, Desjardins F, Nevelsteen I, Verreth W, et al. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res. 2008;77(2):371–9. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 54.Brooks BA, Franjic B, Ban CR, Swaraj K, Yue DK, Celermajer DS, et al. Diastolic dysfunction and abnormalities of the microcirculation in type 2 diabetes. Diabetes Obes Metab. 2008;10(9):739–46. doi: 10.1111/j.1463-1326.2007.00803.x. [DOI] [PubMed] [Google Scholar]
- 55.Shivalkar B, Dhondt D, Goovaerts I, Van Gaal L, Bartunek J, Van Crombrugge P, et al. Flow mediated dilatation and cardiac function in type 1 diabetes mellitus. Am J Cardiol. 2006;97(1):77–82. doi: 10.1016/j.amjcard.2005.07.111. [DOI] [PubMed] [Google Scholar]
- 56.Ozasa N, Furukawa Y, Morimoto T, Tadamura E, Kita T, Kimura T. Relation among left ventricular mass, insulin resistance, and hemodynamic parameters in type 2 diabetes. Hypertens Res. 2008;31(3):425–32. doi: 10.1291/hypres.31.425. [DOI] [PubMed] [Google Scholar]
- 57.Hsueh W, Abel ED, Breslow JL, Maeda N, Davis RC, Fisher EA, et al. Recipes for creating animal models of diabetic cardiovascular disease. Circ Res. 2007;100(10):1415–27. doi: 10.1161/01.RES.0000266449.37396.1f. [DOI] [PubMed] [Google Scholar]
- 58.Russell JC, Proctor SD. Small animal models of cardiovascular disease: tools for the study of the roles of metabolic syndrome, dyslipidemia, and atherosclerosis. Cardiovasc Pathol. 2006;15 (6):318–30. doi: 10.1016/j.carpath.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 59.Semeniuk LM, Kryski AJ, Severson DL. Echocardiographic assessment of cardiac function in diabetic db/db and transgenic db/db-hGLUT4 mice. Am J Physiol Heart Circ Physiol. 2002;283 (3):H976–82. doi: 10.1152/ajpheart.00088.2002. [DOI] [PubMed] [Google Scholar]
- 60.Aasum E, Belke DD, Severson DL, Riemersma RA, Cooper M, Andreassen M, et al. Cardiac function and metabolism in Type 2 diabetic mice after treatment with BM 17.0744, a novel PPAR-alpha activator. Am J Physiol Heart Circ Physiol. 2002;283(3):H949–57. doi: 10.1152/ajpheart.00226.2001. [DOI] [PubMed] [Google Scholar]
- 61.Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function, and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52(2):434–41. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- 62.Christoffersen C, Bollano E, Lindegaard ML, Bartels ED, Goetze JP, Andersen CB, et al. Cardiac lipid accumulation associated with diastolic dysfunction in obese mice. Endocrinology. 2003;144 (8):3483–90. doi: 10.1210/en.2003-0242. [DOI] [PubMed] [Google Scholar]
- 63.Dong F, Zhang X, Yang X, Esberg LB, Yang H, Zhang Z, et al. Impaired cardiac contractile function in ventricular myocytes from leptin-deficient ob/ob obese mice. J Endocrinol. 2006;188(1):25–36. doi: 10.1677/joe.1.06241. [DOI] [PubMed] [Google Scholar]
- 64.Stolen TO, Hoydal MA, Kemi OJ, Catalucci D, Ceci M, Aasum E, et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105(6):527–36. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 65.Fang ZY, Schull-Meade R, Leano R, Mottram PM, Prins JB, Marwick TH. Screening for heart disease in diabetic subjects. Am Heart J. 2005;149(2):349–54. doi: 10.1016/j.ahj.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 66.Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105(4):438–45. doi: 10.1161/hc0402.102623. [DOI] [PubMed] [Google Scholar]
- 67.Yue P, Arai T, Terashima M, Sheikh AY, Cao F, Charo D, et al. Magnetic resonance imaging of progressive cardiomyopathic changes in the db/db mouse. Am J Physiol Heart Circ Physiol. 2007;292(5):H2106–18. doi: 10.1152/ajpheart.00856.2006. [DOI] [PubMed] [Google Scholar]
- 68.Van den Bergh A, Flameng W, Herijgers P. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail. 2006;8(8):777–83. doi: 10.1016/j.ejheart.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 69.Radovits T, Korkmaz S, Loganathan S, Barnucz E, Bomicke T, Arif R, et al. Comparative investigation of the left ventricular pressure-volume relationship in rat models of type 1 and type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol. 2009;297(1):H125–33. doi: 10.1152/ajpheart.00165.2009. [DOI] [PubMed] [Google Scholar]
- 70.Scognamiglio R, Avogaro A, Casara D, Crepaldi C, Marin M, Palisi M, et al. Myocardial dysfunction and adrenergic cardiac innervation in patients with insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1998;31(2):404–12. doi: 10.1016/s0735-1097(97)00516-0. [DOI] [PubMed] [Google Scholar]
- 71.Ha JW, Lee HC, Kang ES, Ahn CM, Kim JM, Ahn JA, et al. Abnormal left ventricular longitudinal functional reserve in patients with diabetes mellitus: implication for detecting subclinical myocardial dysfunction using exercise tissue Doppler echocardiography. Heart. 2007;93(12):1571–6. doi: 10.1136/hrt.2006.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Palmieri V, Capaldo B, Russo C, Iaccarino M, Pezzullo S, Quintavalle G, et al. Uncomplicated type 1 diabetes and preclinical left ventricular myocardial dysfunction: insights from echocardiography and exercise cardiac performance evaluation. Diabetes Res Clin Pract. 2008;79(2):262–8. doi: 10.1016/j.diabres.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 73.Abe T, Ohga Y, Tabayashi N, Kobayashi S, Sakata S, Misawa H, et al. Left ventricular diastolic dysfunction in type 2 diabetes mellitus model rats. Am J Physiol Heart Circ Physiol. 2002;282(1):H138–48. doi: 10.1152/ajpheart.2002.282.1.H138. [DOI] [PubMed] [Google Scholar]
- 74.Boudina S, Sena S, O’Neill BT, Tathireddy P, Young ME, Abel ED. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112(17):2686–95. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 75.Bugger H, Abel ED. Rodent models of diabetic cardiomyopathy. Dis Model Mech. 2009;2(9–10):454–66. doi: 10.1242/dmm.001941. [DOI] [PubMed] [Google Scholar]
- 76.Joshi D, Gupta R, Dubey A, Shiwalkar A, Pathak P, Gupta RC, et al. TRC4186, a novel AGE-breaker, improves diabetic cardiomyopathy and nephropathy in Ob-ZSF1 model of type 2 diabetes. J Cardiovasc Pharmacol. 2009;54(1):72–81. doi: 10.1097/FJC.0b013e3181ac3a34. [DOI] [PubMed] [Google Scholar]
- 77.Lamounier-Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, et al. Adipocyte fatty acid-binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009;105(4):326–34. doi: 10.1161/CIRCRESAHA.109.200501. [DOI] [PubMed] [Google Scholar]
- 78.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008;114(3):195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 79.Wright JJ, Kim J, Buchanan J, Boudina S, Sena S, Bakirtzi K, et al. Mechanisms for increased myocardial fatty acid utilization following short-term high-fat feeding. Cardiovasc Res. 2009;82(2):351–60. doi: 10.1093/cvr/cvp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146(12):5341–9. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 81.Thai MV, Guruswamy S, Cao KT, Pessin JE, Olson AL. Myocyte enhancer factor 2 (MEF2)-binding site is required for GLUT4 gene expression in transgenic mice. Regulation of MEF2 DNA binding activity in insulin-deficient diabetes. J Biol Chem. 1998;273(23):14285–92. doi: 10.1074/jbc.273.23.14285. [DOI] [PubMed] [Google Scholar]
- 82.Razeghi P, Young ME, Cockrill TC, Frazier OH, Taegtmeyer H. Downregulation of myocardial myocyte enhancer factor 2C and myocyte enhancer factor 2C-regulated gene expression in diabetic patients with nonischemic heart failure. Circulation. 2002;106 (4):407–11. doi: 10.1161/01.cir.0000026392.80723.dc. [DOI] [PubMed] [Google Scholar]
- 83.How OJ, Aasum E, Severson DL, Chan WY, Essop MF, Larsen TS. Increased myocardial oxygen consumption reduces cardiac efficiency in diabetic mice. Diabetes. 2006;55(2):466–73. doi: 10.2337/diabetes.55.02.06.db05-1164. [DOI] [PubMed] [Google Scholar]
- 84.Mazumder PK, O’Neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Impaired cardiac efficiency and increased fatty acid oxidation in insulin-resistant ob/ob mouse hearts. Diabetes. 2004;53(9):2366–74. doi: 10.2337/diabetes.53.9.2366. [DOI] [PubMed] [Google Scholar]
- 85.Peterson LR, Herrero P, McGill J, Schechtman KB, Kisrieva-Ware Z, Lesniak D, et al. Fatty acids and insulin modulate myocardial substrate metabolism in humans with type 1 diabetes. Diabetes. 2008;57(1):32–40. doi: 10.2337/db07-1199. [DOI] [PubMed] [Google Scholar]
- 86.Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Circulation. 2004;109(18):2191–6. doi: 10.1161/01.CIR.0000127959.28627.F8. [DOI] [PubMed] [Google Scholar]
- 87.Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–8. doi: 10.1152/physiol.00008.2006. [DOI] [PubMed] [Google Scholar]
- 88.Bugger H, Chen D, Riehle C, Soto J, Theobald HA, Hu XX, et al. Tissue-specific remodeling of the mitochondrial proteome in type 1 diabetic akita mice. Diabetes. 2009;58(9):1986–97. doi: 10.2337/db09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Diamant M, Lamb HJ, Groeneveld Y, Endert EL, Smit JW, Bax JJ, et al. Diastolic dysfunction is associated with altered myocardial metabolism in asymptomatic normotensive patients with well-controlled type 2 diabetes mellitus. J Am Coll Cardiol. 2003;42 (2):328–35. doi: 10.1016/s0735-1097(03)00625-9. [DOI] [PubMed] [Google Scholar]
- 90.Metzler B, Schocke MF, Steinboeck P, Wolf C, Judmaier W, Lechleitner M, et al. Decreased high-energy phosphate ratios in the myocardium of men with diabetes mellitus type I. J Cardiovasc Magn Reson. 2002;4(4):493–502. doi: 10.1081/jcmr-120016387. [DOI] [PubMed] [Google Scholar]
- 91.Scheuermann-Freestone M, Madsen PL, Manners D, Blamire AM, Buckingham RE, Styles P, et al. Abnormal cardiac and skeletal muscle energy metabolism in patients with type 2 diabetes. Circulation. 2003;107(24):3040–6. doi: 10.1161/01.CIR.0000072789.89096.10. [DOI] [PubMed] [Google Scholar]
- 92.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, et al. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54(16):1524–32. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 93.Anderson EJ, Kypson AP, Rodriguez E, Anderson CA, Lehr EJ, Neufer PD. Substrate-specific derangements in mitochondrial metabolism and redox balance in the atrium of the type 2 diabetic human heart. J Am Coll Cardiol. 2009;54(20):1891–8. doi: 10.1016/j.jacc.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hassouna A, Loubani M, Matata BM, Fowler A, Standen NB, Galinanes M. Mitochondrial dysfunction as the cause of the failure to precondition the diabetic human myocardium. Cardiovasc Res. 2006;69(2):450–8. doi: 10.1016/j.cardiores.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 95.Echtay KS, Esteves TC, Pakay JL, Jekabsons MB, Lambert AJ, Portero-Otin M, et al. A signalling role for 4-hydroxy-2-nonenal in regulation of mitochondrial uncoupling. EMBO J. 2003;22 (16):4103–10. doi: 10.1093/emboj/cdg412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Herlein JA, Fink BD, O’Malley Y, Sivitz WI. Superoxide and respiratory coupling in mitochondria of insulin-deficient diabetic rats. Endocrinology. 2009;150(1):46–55. doi: 10.1210/en.2008-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]