Summary
Sublingual immunotherapy with monomeric allergoid, given according to the standard schedule, was reported to be effective and safe in many clinical trials. However, a long period of time may elapse before achievement of a clinical benefit. This study was thus performed using two different shortened (4-day) induction (= up-dosing) schedules, which allowed a rapid achievement of the maintenance dosage. Overall, 86 patients suffering from rhinitis and oculorhinitis have been recruited, none of whom had received immunotherapy before. The study design was prospective, randomized, with three parallel groups receiving, according to a randomization list, one of the three induction (two up-dosing one no-up-dosing) phase schedules under study. A fourth group of patients served as controls, and did not receive any sublingual immunotherapy but only rescue medications if and when necessary. All patients were evaluated to assess their baseline conditions, and thereafter at 3 and 6 months. The evaluation parameters were: Visual Analogue Scale, symptom-medication scores, nasal provocation test. All three induction schedules under study were well accepted by the patients, with very few adverse reactions. The clinical efficacy, evaluated with Visual Analogue Scale (p < 0.001), symptom-medication scores (p < 0.02) and nasal provocation tests (p < 0.01), was found to be significant in all three sublingual immunotherapy-treated groups of 64 (n86) patients, but was not significant in controls 22 (n86). According to the Authors, with this simplified schedule process, sublingual immunotherapy is a therapeutic option that is becoming increasingly well-accepted not only by allergy specialists but also by patients.
Keywords: Rhinitis, Sublingual immunotherapy, Carbamylated allergoid, Up-dosing, Posological schedule
Riassunto
L’immunoterapia sublinguale con allergoide monomerico, somministrata seguendo il consueto schema terapeutico, è risultata efficace e sicura in molti studi clinici. Tuttavia, il raggiungimento di un beneficio clinico richiede un tempo piuttosto lungo. Abbiamo quindi effetuato questo studio utilizzando tre diversi schemi terapeutici con una fase d’induzione abbreviata (up-dosing) (4 giorni), atta a permettere un più rapido raggiungimento della dose di mantenimento. Sono stati reclutati ottantasei pazienti, affetti da rinite e oculorinite allergica e che non erano mai stati sottoposti in precedenza ad alcun tipo di immunoterapia. Lo studio è stato di tipo prospettico, randomizzato, con tre gruppi paralleli sottoposti, in base ad una lista di randomizzazione, ad uno dei tre schemi d’induzione (due up-dosing, uno non-up-dosing) in esame. Ad un quarto gruppo di pazienti di controllo, non è stata somministrata alcuna immunoterapia sublinguale ma solo farmaci sintomatici al bisogno. Tutti i pazienti sono stati valutati per verificare la condizione clinica al tempo zero e dopo 3 e 6 mesi. I parametri di valutazione sono stati: scala sintomatologica analogico visuale, symptom-medication scores, test di provocazione nasale. Tutti e tre gli schemi d’induzione in studio sono stati ben accettati dai pazienti, con pochissimi effetti collaterali. L’efficacia clinica, valutata con scala sintomatologica analogico visuale (p < 0,001), symptom-medication scores (p < 0,02) e test di provocazione nasale (p < 0,01), è risultata significativa in tutti i soggetti trattati con immunoterapia sublinguale appartenenti ai tre gruppi di studio 64 (n86), ma non significativa nei controlli 22 (n86). Secondo gli Autori grazie a questo processo di semplificazione la immunoterapia sublinguale sta diventando una opzione terapeutica che è sempre più ben accettata dai medici e dai pazienti allergici.
Introduction
Since its introduction almost a century ago, allergen-specific immunotherapy (SIT) has been shown to be an effective therapeutic tool for the treatment of patients with severe allergic rhinitis and/or asthma. The fact that SIT (traditionally performed by means of injections) was found to be associated with uncommon, but often severe or even fatal systemic reactions, stimulated investigators to explore new and safer therapeutic approaches. Sublingual immunotherapy (SLIT) has been accepted as a new effective and safe administration route, as demonstrated by many meta-analyses 1 2 and confirmed by WHO Position Papers 3 4. A further improvement of SLIT, aimed at offering a better safety profile, has been the development of chemically modified allergens called allergoids, with a lowered recognition by IgE allergen-specific antibodies. SLIT with monomeric allergoid (allergoid-SLIT) has been shown to be clinically effective and safe in many clinical studies 5–11. However, the standard induction phase (called also “up-dosing” or “build-up”) is somewhat time-consuming, requiring from a minimum of 16 days (semi-rush schedule) to a maximum of 14 weeks (traditional schedule). In fact, the induction phase of SLIT has been designed according to the same criteria as those used for injective immunotherapy, where side-effects are frequent, local and systemic, and, in some rare cases, severe and even life-threatening. The safety profile of SLIT and, in particular, of the allergoid-SLIT was found to be much higher compared to injective immunotherapy, and systemic and anaphylactic reactions are virtually absent, as documented by clinical trials and post-marketing surveillance studies 5–7.
The aim of present study was, therefore, to evaluate the possibility of simplifying the initial phase of the allergoid-SLIT by shortening the induction phase (up-dosing) to 4 days by evaluating tolerability as the primary outcome and clinical efficacy as a secondary outcome. In the case of house dust mite-allergic patients, immunotherapy is usually extended through the whole year, while in the present study, the clinical evaluation has been made 6 months after the onset. Efficacy has been evaluated with Visual Analogue Scale (VAS), symptom-medication-scores (SMS) and allergen-specific nasal provocation test (NPT).
Materials and methods
This was a prospective, randomized study, with three parallel groups receiving, according to a randomization list, one of the three induction (two up-dosing, one no-up-dosing) phase schedules under investigation. A fourth group of patients, serving as controls, did not receive any SLIT but only rescue medications if and when necessary. Also the three SLIT groups received rescue medication, in addition to their assigned SLIT, in case of urgent need and, in any case, for a very short period (no more than 3 days). All patients were evaluated upon entry, to assess their baseline conditions, and after 3 and 6 months to assess clinical efficacy. Drug consumption was measured throughout the study.
Patients
Overall 86 patients suffering from rhinitis and oculo-rhinitis, as major symptoms, and had never previously received any form of specific immunotherapy, were enrolled. Patients’ characteristics at baseline are outlined in Table I. Of these, 22 patients (14M, 8F, mean age 26.3 ± 11.1 years) received the allergoid SLIT without up-dosing, starting immediately with the tablet at maintenance 1000 allergenic units (AU) dosage (Group A), 21 patients (12M, 9F, mean age 20.9 ± 8.8 years) received the 4-day 500/1000/1500 AU (Group B), 21 patients (12M, 9F, mean age 27.9 ± 13.5 years) the 4-day 300/600/900/1200 AU up-dosing treatment (Group C), and the remaining 22 patients (12M, 10F, mean age 25.3 ± 10.8 years) served as controls, without receiving any SLIT but only rescue medications at need.
Table I. Characteristics of patients.
| Schedule A | Schedule B | Schedule C | Controls | |
| Patients: | 22 | 21 | 21 | 22 |
| Sex (M/F) | 14/8 | 12/9 | 12/9 | 12/10 |
| Mean age (yrs) ± SD | 26.3 ± 11.1 | 20.9 ± 8.8 | 27.9 ± 13.5 | 25.3 ± 10.8 |
| Allergens SLIT: | ||||
| Parietaria pollen | 4 | 3 | 2 | 2 |
| Dermatophagoides | 8 | 8 | 7 | 9 |
| Grass pollen | 6 | 7 | 9 | 8 |
| Birch pollen | 4 | 3 | 3 | 3 |
SD: standard deviation
The specific sensitizations of all the patients were determined by a positive (> 3 mm) skin prick test response (extracts Lofarma SpA, Milan, Italy) and positive CAP assay results (class 2 or greater) (CAP System EIA, Pharmacia, Uppsala, Sweden) as confirmation of their clinical history.
Subjects suffering from systemic or immunological diseases, major anatomical disorders of the upper airways, renal insufficiency, coronary heart disease, neurological or psychiatric disorders, receiving chronic corticosteroid or beta-blocking treatments, were not admitted, nor were pregnant women. All patients signed an informed consent form before entering the study.
Investigational treatment and schedules (Table II)
Table II. Administration schedules for induction (up-dosing) phase.
| Schedule A No-up-dosing |
| patients started immediately taking the 1000 AU tablets. |
| Schedule B 4-day 500/1000 AU |
| 1st day: ½ 1000 AU tablet |
| 2nd day: ½ 1000 AU tablet (in the morning) + ½ 1000 AU tablet (in the evening) |
| 3rd day: ½ 1000 AU tablet (in the morning) + one 1000 AU tablet (in the evening) |
| 4th day: one 1000 AU tablet (in the morning) + one 1000 AU tablet (in the evening) |
| Schedule C 4-day 300/600/900/1200 AU |
| 1st day: one 300 AU tablet |
| 2nd day: two 300 AU tablets |
| 3rd day: three 300 AU tablets |
| 4th day: four 300 AU tablets |
Controls: patients receiving no SLIT but only rescue pharmacological medications; AU: allergenic units.
SLIT has been performed using a monomeric carbamylated allergoid (allergoid-SLIT) biologically standardized in allergenic units (AU) and prepared as orosoluble tablets (Lais®, Lofarma SpA, Milan, Italy) 12. The tablets had to be taken in the morning on an empty stomach and kept under the tongue for 1-2 minutes until dissolved before swallowing.
The patients in Group A (no-updosing) started directly with the maintenance 1000 AU dose, taken regularly twice a week for house-dust mites and 5-7 times a week for pollens.
The patients in Group B (4-day 500/1500/2000 AU) had an up-dosing phase lasting 4 days, for which half tablets were prepared, for the first 3 days, by cutting them with a small blade. Therefore, both the Group A and Group B patients used only the immunotherapy set containing 1000 AU, corresponding to the so-called “maintenance” set.
The patients in Group C (4-day 300/600/900/1200 AU) took during the first 4 days progressive quantities of tablets (300 AU). Therefore, they used a new immunotherapy set at 300/1000 AU.
All patients in the three groups continued the therapy with 1000 AU tablets, taken 2 (house-dust mite) or 5-7 times a week (maintenance phase) for the 6-month period of the present study.
The group of controls did not receive any immunotherapy treatment, but only pharmacological rescue medications.
Nasal Provocation Test (NPT)
Allergen-specific NPT has been performed by administering allergens in powder form for specific nasal challenge, titrated at the dosages of 20 and 40 AU and contained in gelatin hard capsules, using lactose (25 mg) as excipient (Allerkin® Test, Lofarma, Milan, Italy). The capsules were administered by means of a special nasal insufflator included in the manufacturer’s package.
Previous studies, performed by the manufacturer, have shown that 40 AU is the mean provocative dose, at nasal challenge, in patients with allergic rhinitis. Progressive dosages were administered in the present study, in a rapid sequence, summing different doses, e.g. 20 + 40 + 20 AU. The test was considered positive (and administration of progressive dosages was therefore, stopped) when specific allergic symptoms were observed: sneezing, itching, rhinorrhoea, nasal obstruction, lacrimation. The cumulative dose was recorded and considered inversely correlated to allergen-specific nasal reactivity.
Pharmacological rescue therapy
The standard pharmacological therapy consisted of antihistamines (cetirizine or loratadine tablets 10 mg, once daily) and long-term intranasal steroids (fluticasone propionate, 125 μg, 2 sprays per nostril/day) in association with long-acting bronchodilators (salmeterol, 100 μg/day) for patients presenting also asthmatic symptoms.
Clinical evaluations
Patients were required to fill in a specific graduated scale, namely the Visual Analogue Scale (VAS), which explores the degree of patient well-being and thus, indirectly, the severity of his/her symptoms during the previous pollen season or during the last 6 months in the case of house-dust mites. In the present study, the maximum level of well-being was 10 and the minimum was 0. The VAS had to be filled in upon entry, and after 6 months.
The severity of symptoms was evaluated according to the following score: 0 indicates the absence of symptoms (no sign or symptom evident); 1, mild symptoms and minimum inconvenience; 2, moderate and troublesome but tolerable symptoms; and 3, severe symptoms interfering with activities of daily life and/or sleeping i.e., conjunctival (itching, tear flow, and redness), nasal (sneezing, itching, runny nose, and obstruction) 13.
The consumption of rescue medications was scored 1 point if no drug was consumed in a month, 2 points if the consumption was rare (i.e., no more than 5 days in which rescue therapy was needed in that month), 3 if this was in the average (i.e., no more than 10 days in which rescue therapy was needed in a month) and 4 if this was elevated, regardless of the kind of drug (i.e., more than 10 days in which rescue therapy was needed in a month). Then, at 3 and 6 months, a cumulative drug intake score was calculated, each kind of drug being scored separately and differently from the others, with systemic steroids having the highest score.
The symptom-medication-scores (SMS) were obtained from the sum of symptom scores (range 0-3) and medication score (range 1-4). Therefore, the range of SMS was 1-7.
All patients were also required to record, on a separate diary, any adverse effect. As far as the allergoid-SLIT is concerned, adverse events (AE) were subdivided into local AE (oral itching, swelling of tongue) and systemic (asthma, rhinitis, urticaria, abdominal pain/diarrhoea, anaphylaxis).
Evaluation of nasal reactivity, by means of NPT, was performed before treatment, and after 3 and 6 months.
Immunotherapy
Some of the patients enrolled in the study were found to have, at diagnosis by means of in vivo and in vitro tests, multiple sensitization, usually to 2-3 different (= not belonging to the same homologous group) allergens. As a rule, in this case, the most relevant allergen was selected for SLIT, considering both clinical and environmental/anamnestic aspects.
Two kinds of immunotherapy sets were used: one containing only the 1000 AU dosage (the same as in the maintenance immunotherapy phase) was used for schedule A and B, and one containing two dosages, 300 and 1000 AU, was used for schedule C (Lais®, Lofarma SpA, Milan, Italy).
Administration schedules for induction (up-dosing) phase
The administration schedules for induction (up-dosing) phase are reported in Table II.
Treatment (maintenance phase)
Regardless of the induction phase, all patients continued the treatment according to the following schedules:
-
Seasonal allergens (pollens): patients continued the treatment taking from 5 to 7 tablets titrated at 1000 AU per week, on different days (e.g. from Monday to Friday or every day, respectively).
Duration of treatment: from 4 (grass pollen) to 6 (Parietaria pollen) months.
-
Perennial allergens (house-dust mite): patients continued the treatment taking 2 tablets titrated at 1000 AU per week, on different days (e.g. Monday and Thursday).
Duration of treatment: 6 months (many patients continued to take SLIT tablets after the end of the study).
The rescue medication, to be administered for symptom control, only in case of urgent need and no longer than 3 days, was as follows, in all three groups: cetirizine or loratadine tablets 10 mg, two or more tablets/day, inhaled salbutamol 100 μg, 2-3 puffs or more/day, intra-nasal fluticasone propionate 250 μg, 2 or more sprays per nostril/day and beclomethasone tablets 1 mg, 1 tablet once or twice daily.
Results
Tolerability
Concerning tolerability, all the patients tolerated all the three dosage schedules under study very well, as also the maintenance treatment. During the up-dosing phase 4 slight side-effects have been recorded in 4 patients, one case of somnolence and one of tiredness, and 2 cases of oral itching. No side-effects were recorded during the maintenance treatment. Furthermore, no patient interrupted the study on account of adverse events.
VAS
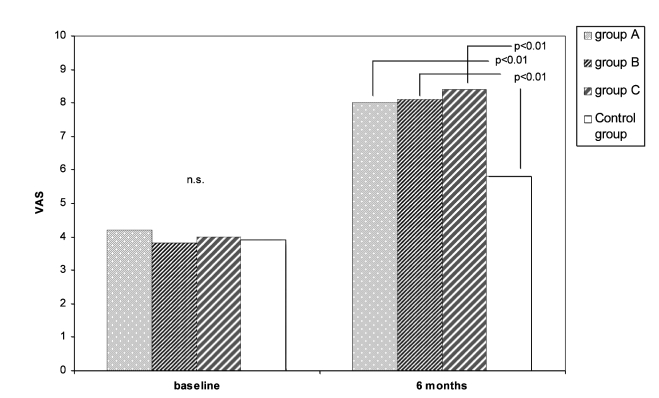
The VAS results are outlined in Figure 1. At baseline, there were no significant differences between the 4 groups. Considering the 3 different groups, it is worth noting that, after 6 months, no statistical differences between the 3 up-dosing schedules (A, B, C) were observed. A significant increase in VAS values has been observed in all 3 study groups, in comparison to the controls (p < 0.001).
Fig. 1.
VAS values in the three SLIT-treated and control groups at baseline and after 6 months.
Symptom-medication scores (SMS)
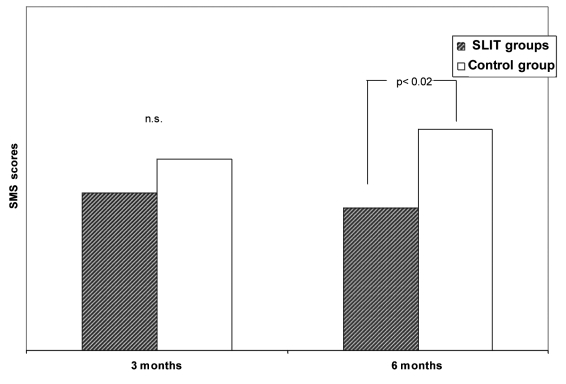
At baseline, no statistically significant difference was observed between the 3 groups of patients submitted to SLIT. Comparing the SMSs, after 3 and after 6 months of SLIT, a difference was observed in 27/32 patients submitted to SLIT for Dermatophagoides (data not presented). However, considering the entire series of patients submitted to SLIT for all kinds of allergens, the difference was no longer statistically significant. In the three groups of patients receiving SLIT (in this case calculated as a single group), there was a statistically significant (p < 0.02) reduction of SMSs in comparison to the control group (Fig. 2).
Fig. 2.
SMS scores in SLIT-treated and control groups after 3 and 6 months.
Nasal provocation test (NPT)
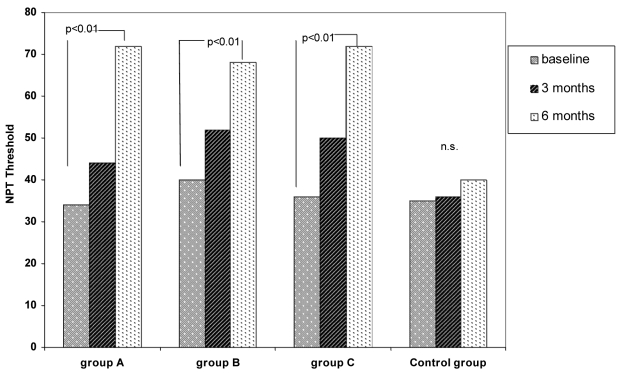
Results of NPT reported in Figure 3 show a clear and significant (p < 0.01) decrease in nasal reactivity (therefore, an increase of the threshold dose) in the three SLIT-treated groups, while the untreated controls remained unchanged (n.s.).
Fig. 3.
NPT in the three SLIT-treated and control groups at baseline, and after 3 and 6 months.
Discussion
In this study, two aspects of SLIT have been examined, the simplified and shortened (4-day) up-dosing phase and the clinical efficacy at short-term (3-6 months). As far as the first aspect, there has been, for some years, a general tendency towards a simplification of the up-dosing phase, used for some decades, in injective (subcutaneous) immunotherapy. Even if some cases of anaphylactic reactions, not fatal, have been recently reported in the literature (5 cases out of several million patients treated), it is generally recognised that the safety of SLIT is very high, consistent with the possibility to shorten the up-dosing phase. Some attempts to dramatically shorten the up-dosing phase with ultra-rush schedules, lasting 20-25 minutes, have been successful, without any relevant adverse reaction and with very good compliance of patients 8 14. Also post-marketing studies showed a very high safety profile of SLIT 7, especially when monomeric allergoids were used 6. Safety, tolerability and efficacy of allergoid-SLIT with a 4-day build-up phase have been evaluated in 39 patients with rhinitis with/without mild asthma due to sensitization to perennial and seasonal allergens, with very encouraging results of both safety and efficacy; in this study, half tablets, corresponding to approx 500 AU, were used for the induction phase 11.
In the present study, the same schedule was adopted in parallel with a schedule without any kind of up-dosing and a schedule using also tablets with a lower dosage (300 AU). It is worthwhile pointing out that in previous studies, SLIT with native allergens (= not allergoid) without up-dosing has been submitted to clinical trials to evaluate efficacy and safety, showing adverse events (mainly local) in a large percentage of treated patients (67%) 15 16. This phenomenon is probably due to the nature of the active substance (native allergen) which maintains the ability to react with allergen-specific IgE antibodies, and consistently increases the serum concentration of these antibodies (approximately 5 times). In the present study, the active substance was a monomeric allergoid, characterized by small molecular size, which allows a significant absorption by the mucosa at buccal level during administration. This allergoid has been obtained by carbamylation at alkaline pH, which ensures a strong reduction in reacting with allergen-specific IgE antibodies and, therefore, a very low allergenic potency, while maintaining the ability to induce protective IgG antibodies 12. This aspect explains the high safety of immunotherapy performed with this product, as observed in post-marketing observational studies 6. It remains to be established which of the three schedules submitted to investigation in the present study is to be considered the most appropriate in clinical use. All three schedules are considered to be appropriate according to the patient’s sensitivity and to practical considerations. Patients without a particularly elevated sensitivity (skin test wheals < 10 mm, CAP class 3 or less, mild/moderate symptoms in clinical history) can be directly advised to use the no-up-dosing schedule (Schedule A). More reactive patients can be addressed to the other two schedules (B and C), bearing in mind that the product of schedule B is simpler (only one dosage, 1000 AU/tablet) but requires the manual procedure of cutting the tablets into two halves, at least in the first phase of treatment, while schedule C is more complex using two different dosages (300 and 1000 AU/tablet) but simpler in administration.
With regard to clinical efficacy, the data obtained, in the present study, are very encouraging, showing a clear and significant improvement in VAS and SMSs. The improvement observed was approximately at the same degree, in the three SLIT-treated groups (while untreated controls remained unchanged), thus indicating that the type of up-dosing schedule does not influence the clinical efficacy. That is obvious considering that 99% of the dosage is administered during the maintenance phase, the up-dosing phase being just a prudential way to progressively introduce the allergen, with the aim to safely reach the maintenance dose. NPT data also showed an increase in the provocative threshold in SLIT-patients (corresponding to a decrease in nasal reactivity), equivalent in the three treated groups and unchanged in controls. This decrease of nasal reactivity is in accordance with the improvement in SMS values observed in the present study. It is worthwhile pointing out that NPT is considered, by European regulatory bodies, as a parameter which is a valid substitute to symptom scores in the clinical evaluation of immunotherapy.
In conclusion, thanks also to this schedule simplification process, SLIT is becoming a therapeutic option that is increasingly well-accepted by allergy doctors and by patients, also in Countries, like Germany and US, where there is a long and well established tradition of injective immunotherapy.
References
- 1.Wilson DR, Torres LI, Durham SR. Sublingual immunotherapy for allergic rhinitis. Cochrane Database Syst Rev 2003;2:CD002893. [DOI] [PubMed] [Google Scholar]
- 2.Penagos M, Compalati E, Tarantini F, et al. Efficacy of sublingual immunotherapy in the treatment of allergic rhinitis in pediatric patients 3 to 18 years of age: a meta-analysis of randomized, placebo-controlled, double-blind trials. Ann Allergy Asthma Immunol 2006;97:141-8. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Malling HJ. WHO position paper. Allergen immunotherapy: therapeutic vaccines for allergic diseases. Allergy 1998;53:1-42. [Google Scholar]
- 4.Bousquet J, Van Cauwenberge P, Khaltaev N. Aria Workshop Group; WHO. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001;108(Suppl):S147-S334. [DOI] [PubMed] [Google Scholar]
- 5.Passalacqua G, Albano M, Fregonese L, et al. Randomised controlled trial of local allergoid immunotherapy on allergic inflammation in mite-induced rhinoconjunctivitis. Lancet 1998;351:629-32. [DOI] [PubMed] [Google Scholar]
- 6.Lombardi C, Gargioni S, Melchiorre A, et al. Safety of sublingual immunotherapy with monomeric allergoid in adults: multicenter post-marketing surveillance study. Allergy 2001;56:989-92. [DOI] [PubMed] [Google Scholar]
- 7.Di Rienzo V, Pagani A, Parmiani S, et al. Post-marketing surveillance study on the safety of sublingual immunotherapy in pediatric patients. Allergy 1999;54:1110-3. [DOI] [PubMed] [Google Scholar]
- 8.Rossi RE, Monasterolo G. A pilot study of feasibility of ultra-rush (20-25 minutes) sublingual-swallow immunotherapy in 679 patients (699 sessions) with allergic rhinitis and/or asthma. Int J Immunopathol Pharmacol 2005;18:277-85. [DOI] [PubMed] [Google Scholar]
- 9.Grosclaude M, Bouillot P, Alt R, et al. Safety of various dosage regimens during induction of sublingual immunotherapy. A preliminary study. Int Arch Allergy Immunol 2002;129:248-53. [DOI] [PubMed] [Google Scholar]
- 10.Passalacqua G, Lombardi C, Guerra L, et al. Sublingual immunotherapy: no more doubts. Eur Ann Allergy Clin Immunol 2005;37:314-20. [PubMed] [Google Scholar]
- 11.Giordano T, Quarta C, Bruno ME, et al. Safety, tolerability and efficacy of sublingual allergoid immunotherapy with a 4-day shortened build-up phase. Eur Ann Allergy Clin Immunol 2006;9:310-2. [PubMed] [Google Scholar]
- 12.Mistrello G, Brenna O, Roncarolo D, et al. Monomeric chemically modified allergens: immunologic and physicochemical characterization. Allergy 1996;51:8-15. [DOI] [PubMed] [Google Scholar]
- 13.Pfaar O, Klimek L; for the Study Group. Efficacy and safety of specific immunotherapy with a high-dose sublingual grass pollen preparation: a double-blind, placebo controlled trial. Ann Allergy Asthma Immunol 2008;100:256-63. [DOI] [PubMed] [Google Scholar]
- 14.Gammeri E, Arena A, D’Anneo R, et al. Safety and tolerability of ultra-rush (20 minutes) sublingual immunotherapy in patients with allergic rhinitis and/or asthma. Allergol Immunopathol 2005;33:142-4. [DOI] [PubMed] [Google Scholar]
- 15.Malling HJ, Lund D, Ipsen H, et al. Safety and immunological changes during sublingual immunotherapy with standardized quality grass allergen tablets. J Investig Allergol Clin Immunol 2006;16:162-8. [PubMed] [Google Scholar]
- 16.Durham SR, Yang WH, Pedersen MR, et al. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol 2006;117:802-9. [DOI] [PubMed] [Google Scholar]