Abstract
Airway hyperresponsiveness (AHR) is a clinical feature of asthma and is often in proportion to the underlying severity of the disease. To understand AHR and the mechanisms that contribute to these processes, it is helpful to divide the airway components that affect this feature of asthma into “persistent” and “variable” categories. The persistent component of AHR represents structural changes in the airway, whereas the variable feature relates to inflammatory events. Insight into how these interrelated components of AHR can contribute to asthma is gained by studying treatment effects and models of asthma provocation.
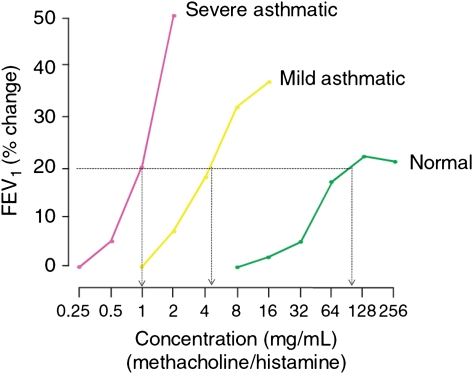
Airway hyperresponsiveness (AHR) is a characteristic feature of asthma and is found in virtually every patient with this disease. However, there is considerable variability in the intensity of AHR in patients with asthma, and the level of AHR is variable both among patients with asthma and within individuals themselves. This level of AHR variability provides insight into mechanisms that regulate this process. For example, the degree of AHR is usually in proportion to the severity of the underlying asthma1; those patients with more severe airway disease often have a greater degree of AHR (Fig 1). In addition, intercurrent provokers, such as viral-induced exacerbations, allergen exposure, and occupational exposures, can temporarily enhance the underlying AHR in individual patients. Furthermore, treatment of asthma not only can improve airflow limitations and reduce symptoms but also can modify underlying AHR. These observations have given insight into the factors and mechanisms that affect AHR and, in particular, the role and contribution, if any, of airway inflammation in the variety of processes that determine and possibly direct AHR.
Figure 1.
Dose-response curves to inhaled direct agonists (histamine or methacholine) in normal, mild, or severe asthma. (Reprinted with permission from O’Byrne et al.2)
To more fully appreciate the role of inflammation in AHR, it is helpful to identify the components that likely make up AHR, review studies that have used either therapeutic interventions or provocative models to modify AHR, and, from these observations, attempt to gain a more comprehensive view of the complexity of processes, including inflammation, that ultimately determine AHR.
What Are the Components of AHR?
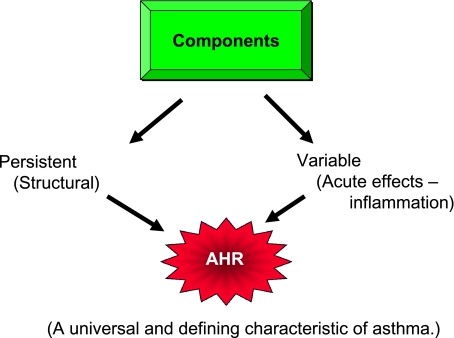
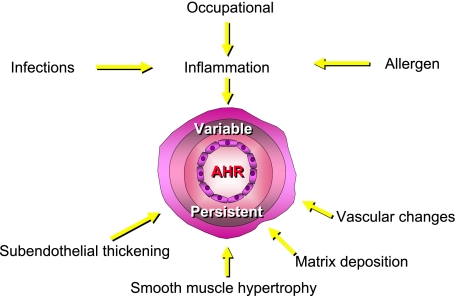
In studying, discussing, and understanding the components that comprise AHR, it is helpful, and possibly informative, to divide the factors that contribute to AHR into two categories: persistent and variable2,3 (Fig 2). Under this concept, which may be an oversimplification of this feature of asthma, the persistent aspects of AHR have been largely attributed to structural changes in the airway, which can include subendothelial thickening, subbasement membrane thickening, smooth muscle hypertrophy, matrix deposition, and altered vascular components (Fig 3). These structural changes are often part of the histopathology found in asthma, particularly in those patients with more severe or longstanding disease; these structural changes alter the architecture of the airways to make them thicker, less compliant, and more narrowed, all features associated with a greater degree of constriction and closure when stimulated by contractile substances. The other component of AHR, the variable aspect, is believed to relate to inflammatory events in the airway, which are variable and influenced by numerous environmental events (ie, allergens, respiratory infections, and treatment).
Figure 2.
Components of airway changes in asthma that contribute to AHR. AHR = airway hyperresponsiveness.
Figure 3.
Factors affecting the variable and persistent components of AHR. See Figure 2 legend for an expansion of the abbreviation.
In arbitrarily dividing the components of AHR into these two principal components, it is understood that these processes are interrelated and likely interdependent. For example, it is presumed that if inflammation is longstanding, the cellular components of the underlying injury become important in and to the development of structural changes in the airway and, thus, further the persistent aspect of AHR. In addition, compartmentalizing the study of AHR into these two arbitrary aspects can be insightful, although perhaps artificial, in dissecting the complexity of this feature of asthma. In the end, the level of AHR represents both collective and synergistic events in the airway and is the result of multiple processes, each with a unique contribution, but not in isolation from other events. The concept of variable and persistent components has undergone revisions. At present, factors altering airway structure appear most related to AHR.
Detection of AHR
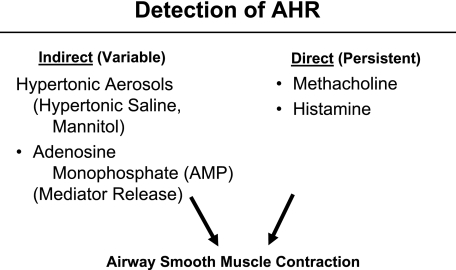
It is important to indicate that detection of AHR can also be arbitrarily divided into two stimuli: direct and indirect (Fig 4). Direct stimulation of the airway to measure AHR is seen principally with methacholine or histamine, both of which act directly on airway smooth muscle to evoke a contractual response.1-3 Because the degree of the airway contractual, or closure, response is enhanced in asthma, smaller concentrations of agonists are needed to decrease the FEV1 by 20% and thus lead to a lower provocative concentration, causing a 20% fall in FEV1 (PC20) value.2 In general, it has been suggested that the actions of methacholine and histamine reflect an effect usually associated with, or attributed to, persistent changes in the airway. This is likely an oversimplification of the interplay between structural and inflammatory events but enables a partial dissection of some of the factors that contribute to AHR.
Figure 4.
Detection of AHR by direct and indirect activators of airway contraction. See Figure 2 legend for expansion of the abbreviation.
It is also important to point out that the presumed direct stimulating factors, particularly to histamine, are not solely the result of actions on airway smooth muscle causing airway closure. Histamine can also activate sensory fibers in the airway and, as a consequence, not only directly contract the bronchial smooth muscle but also lead to a reflex response that can, in turn, amplify bronchospasm. The relative contribution of histamine to direct vs indirect effects is not clearly defined and is likely to be variable and dependent upon existing inflammation or other factors at the time of study. This point is raised both to indicate the crossover that exists with agonists used for measures of AHR and the interweaving of inflammatory processes and structural changes in the airway.
A variety of indirect activators of AHR are also used to detect AHR. The experience with these indirect stimulants is less than that with methacholine or histamine, but they have the potential to add information to measurements with direct agonists. The response to the inhalation of these indirect factors does not appear to occur through a direct action on smooth muscle, but rather as a consequence of existing inflammation and either activation of these cells or as an irritant to the airway smooth muscle. Examples of indirect factors include hypertonic aerosols, such as hypertonic saline or mannitol. In addition, adenosine monophosphate acts by stimulating mast cells to release their mediators, independent of IgE. The degree to which mast cell mediators are released by adenosine monophosphate, in turn, determines the level of AHR and reflects underlying airway inflammation.
There are obvious strengths and limitations to each of these approaches, direct or indirect, but by applying them to the study of AHR in asthma and understanding their mechanisms of effect, it is becoming possible to more comprehensively explain altered airway function in asthma and to ascertain how these alterations occur. From this approach will come a greater understanding of asthma mechanisms and eventually treatment.
What Lessons Have Been Learned About AHR From Treatment of Asthma?
In 1999, Sont and colleagues4 reported on the effects of a treatment strategy directed toward reducing AHR vs guideline-directed care alone on clinical outcomes of asthma and also the underlying features of airway inflammation and histopathology in these patients. All enrolled subjects had AHR as defined by a PC20 to methacholine < 8 mg/mL. The enrolled patients were divided into two groups: reference treatment (the level of step care was based on guideline recommendations) and an AHR strategy whereby the treatment dose of inhaled corticosteroids (ICS) was adjusted in an attempt to reduce the underlying AHR. The patients enrolled in both treatment arms underwent bronchoscopy with mucosal biopsy at the beginning and completion of the study; the mucosal biopsies were analyzed for inflammation (primarily, eosinophils) and airway wall thickness.
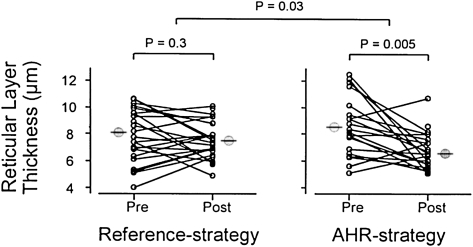
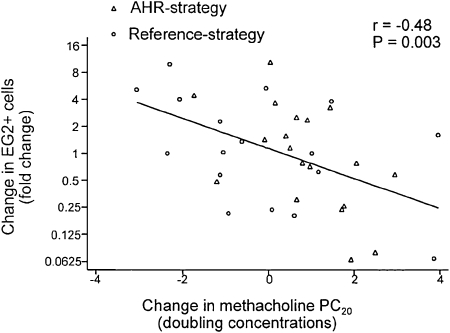
At the completion of the study, the patients in the AHR strategy arm required a larger dose of methacholine to cause a 20% fall in FEV1, probably reflecting the higher dose of ICS given to this group. In addition, those receiving ICS treatment directed by reducing the AHR had a greater improvement in their FEV1 over the 2 years of study. Furthermore, the frequency of mild asthma exacerbations was reduced in the AHR treatment strategy group. Finally, the bronchial mucosal biopsies were analyzed, and the degree of inflammation (ie, eosinophilia) and thickness of the airway were determined. Those patients randomized to the reference group had no change in the airway thickness over the 2 years of treatment (Fig 5). In contrast, subjects in the AHR strategy group had an approximate 40% reduction in airway thickness (reticular layer thickness). Eosinophil numbers in the biopsy specimens also tended to decrease, and interestingly, when the authors evaluated the change in mucosal eosinophilia in relationship to the improvement in methacholine PC20, an inverse statistical correlation emerged: the greater the decrease in eosinophils in the airway, the greater the improvement in methacholine sensitivity (Fig 6).
Figure 5.
Individual changes in reticular layer thickness beneath the epithelium in bronchial biopsies before and after 2 years of treatment in reference and AHR strategy. See Figure 2 legend for expansion of abbreviation. (Reprinted with permission from Sont et al.4)
Figure 6.
Relationship between changes in EG2+ eosinophils and changes in methacholine PC20 during 2 years’ treatment in reference and AHR strategy. EG2+ = Monoclonal antibody that binds to eosinophil cationic protein; PC20 = provocative concentration causing a 20% fall in FEV1. See Figure 2 legend for expansion of the other abbreviation. (Reprinted with permission from Sont et al.4)
The authors made a number of conclusions from their observations that are relevant to the discussion of AHR. First, antiinflammatory therapy directed toward reducing AHR can improve lung function. Second, the ICS treatment that induced changes in AHR also reduced changes of airway wall remodeling (ie, reticular layer thickness) and the degree of eosinophilia. Whether the changes in airway wall thickness and fewer eosinophils are linked and/or associated with a reduction in AHR could not be determined. Nonetheless, by adjusting the treatment dose of ICS to reduce AHR, there was a diminished thickness of the airways. Because airway thickness can factor into the degree of AHR, these outcomes appear interrelated. In the schema of the introductory comments, the reduction in AHR with higher doses of ICS appears targeted to the persistent, or structural, components of AHR. This, however, still remains conjectural. These data also raise the question of how much inflammation, reflected by the presence of eosinophils, contributes to AHR.
To further explore the role of cellular and tissue inflammation, Gibson and colleagues5 enrolled 20 patients with asthma in a study. Each subject was given 2,000 μg of beclomethasone per day for 8 weeks. At the completion of this treatment phase, AHR was measured to methacholine and hypertonic saline. These determinants of AHR were followed by a collection of induced sputum. One week later, the subjects under went a bronchoscopy with lavage and mucosal biopsy to evaluate airway lumen and mucosal inflammation. The changes in AHR were compared with the various markers of inflammation, including airway lumen and airway wall (Table 1). Changes in AHR to methacholine, in particular, were associated with reductions in mucosal (bronchial biopsy) metachromatic cells. In contrast, associated changes in eosinophils had less effect on AHR to methacholine or mannitol.
Table 1.
—Relationship Between Cell Counts (%) in Bronchial Biopsies, BAL, and Induced Sputum and Physiologic Measures
| Cell Type and Source | PEF Variability | PD20 Methacholine | MAN | PD20 Saline |
| Metachromatic cells | ||||
| BBB | 0.59* | −0.74* | 0.39* | −0.75* |
| BAL | −0.13 | −0.38 | −0.22 | −0.46 |
| Sputum | 0.23 | −0.35 | 0.14 | −0.20 |
| Eosinophils | ||||
| BBB | −0.09 | 0.29 | −0.18 | 0.13 |
| BAL | 0.26 | −0.31 | 0.19 | −0.43 |
| Sputum | 0.43 | −0.29 | 0.21 | −0.63 |
BBB = bronchial biopsies; MAN = mannitol; PD20 = Provocative dose causing a 20% fall in FEV1; PEF = peak expiratory flow. (Reprinted with permission from Gibson et al.5)
P < .05.
From these observations, the authors5 concluded that the effects of reducing cellular inflammation on AHR were dependent upon many factors, including the cell type (ie, eosinophil vs metachromatic cell) and the location of the inflammatory process (ie, the lumen vs airway wall). Under these experimental conditions, two major messages can be drawn: metachromatic cells and their residence in the airway wall are the dominant factors that are most likely to influence altered airway physiology associated with AHR. Their findings also suggest that long-term treatments with effective doses of ICS are needed to bring about changes in the mucosal tissue and for these changes to translate into functional improvement in AHR. This study also underscores the strength behind combining assessments of inflammatory cell type and their location to unravel which cell and under what conditions it affects AHR. Again, this study suggests that it is the structural status of the airway, not necessarily inflammation, that is important to AHR.
The Effect of an Inhaled Allergen Bronchial Provocation on AHR
Interleukin (IL)-5 is increased in lumen secretions following allergen challenge and correlates with the presence of eosinophils recruited to the lung.6 To dissect these processes (ie, mediator vs cellular aspects), Shi and colleagues7 had patients with asthma inhale IL-5 and evaluated its effects on AHR and recruitment of eosinophils. Twenty-four hours after inhaling IL-5, eosinophils markedly increased in the sputum and paralleled a significant enhancement in responsiveness with methacholine. These effects were not seen with saline or with a solution containing endotoxin, which could be a potential contributor to and confounder of these effects, especially the heightened response to methacholine. At the time of these studies, the Shi et al data7 strongly suggested that the generation of IL-5 and subsequent recruitment of eosinophils are major determinants of AHR. Precisely how the eosinophils contributed to AHR was not defined.
This concept, however, has not been substantiated by subsequent work. Leckie and colleagues,8 for example, evaluated the effect of administering a monoclonal antibody to IL-5 (anti-IL-5) on the airway response to inhaled antigen (ie, the development of the late phase response and eosinophil subsequent recruitment). In their study, patients with immediate and late-phase reactions to inhaled antigen were given either placebo or one of two doses of anti-IL-5. Although anti-IL-5 significantly reduced blood and sputum eosinophils, there was no effect on the immediate or late-phase reaction to inhaled antigen. Furthermore, there were no changes in AHR post allergen challenge; however, changes in AHR following the antigen challenge did not occur with placebo either. These studies indicate that although eosinophils parallel the development of the late-phase reaction to inhaled antigen, they do not appear causative of this event and may not contribute to an enhancement of AHR.
Further insight into how eosinophils participate in asthma and AHR are noted in a study by Haldar and colleagues9 in which the effects of anti-IL-5 (mepolizumab) were evaluated in patients who were refractory to large doses of inhaled ICS as indicated by persistent sputum eosinophilia. In this study, treatment with mepolizumab reduced sputum eosinophil concentrations. Despite this reduction in sputum eosinophils, there was no change in pulmonary functions or AHR. However, the diminished number of eosinophils was associated with a 50% reduction in asthma exacerbations. These studies raise the possibility that sputum eosinophils do not necessarily reflect or contribute to the intensity of AHR, but rather other factors, other cells, and/or other mediators need to be considered as the dominant, causative mechanisms in this feature of airway dysfunction in asthma.
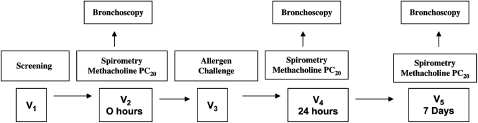
To further address how and under what conditions either structural aspects (persistent AHR) or inflammatory components (variable AHR) modulate AHR, Kariyawasam and colleagues10 conducted a comprehensive study that used antigen bronchoprovocation to induce an allergic inflammatory response and bronchoscopy with biopsy to assess the effects of allergic inflammation on AHR. In this study, patients were seen on five separate occasions (Fig 7). Patients had screening evaluations that included obtaining bronchoscopy with bronchial biopsies after spirometry and a methacholine measurement of PC20. An allergen challenge was then performed and followed by bronchoscopy 24 h later, as well as assessment of the effect of allergen challenge on the methacholine PC20. These studies were then repeated 7 days after the allergen challenge to assess the persistence of the inflammatory reaction and to determine whether the inflammatory changes associated with changes in AHR were in the airway lumen, bronchial mucosa inflammation, or both.
Figure 7.
Summary of the study design from Kariyawasam et al.10 V = visit. See Figures 2 and 6 legends for expansion of other abbreviations. (Reprinted with permission from Kariyawasam et al.10)
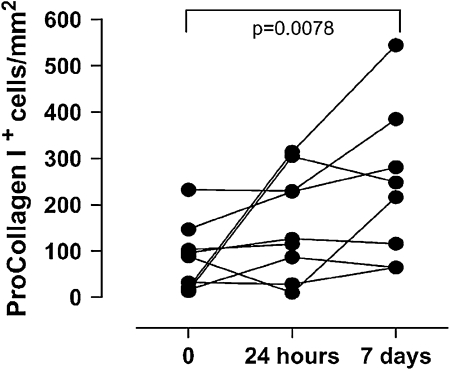
Twenty-four hours after allergen challenge, there was, as expected, a significant decrease in the FEV1 in the majority of patients. Furthermore, AHR to methacholine increased at 24 h post allergen challenge and remained increased at 7 days (Fig 8). When cellular inflammation was determined by BAL eosinophils, a significant increase in this cell was found 24 h after allergen challenge; however, by 7 days post allergen challenge, the eosinophils in the BAL had returned to baseline levels. In contrast, when the investigators measured procollagen-expressing cells in the bronchial biopsy specimen as a marker of airway remodeling, there was an increase in this mucosal marker 7 days after allergen challenge (Fig 9). These data suggest that the changes in AHR following allergen challenge appear to be more closely associated with structural alterations than the early recruitment of luminal eosinophils and ensuing inflammation. Although eosinophils tend to parallel these responses, they did not appear to be the causative process in this response.
Figure 8.
The changes from baseline in FEV1 (A) and methacholine (B) 24 h and 7 days after antigen challenge. ns = not significant. (Reprinted with permission from Kariyawasam et al.10)
Figure 9.
Markers of airway remodeling 24 h and 7 days after antigen challenge. (Reprinted with permission from Kariyawasam et al.10)
Assessing the contribution to airway inflammatory events to AHR is complicated by many factors, including the method of evaluation, the measurement itself, the timing of the readout, and the susceptibility to the inflammatory determinant. For example, detection of airway inflammation is facilitated by noninvasive approaches, such as analysis of induced sputum samples. These determinants measure events in the large to midsize airways. Bronchoscopy with lavage, in contrast, provides a “cleaner” sample but requires an invasive procedure, which by itself can have effects on markers of inflammation. Both sputum and lavage samples reflect inflammation in the airway lumen, which may not fully reflect the histology of the airway wall, a location that is more likely to be a determinant of AHR. Furthermore, it is also likely that the interaction of these inflammatory cells with airway tissues determines the intensity of AHR. At present, quantifying the interactive contribution of cellular elements and resident components to the overall inflammatory reaction is not readily available. Therefore, to identify the contributions of inflammation to AHR, it is important to consider the many aspects of this process, including the location of the cells (ie, lumen vs bronchial wall) and cell type as well as the many mediators associated with the injury to the airway. The present data, however, suggest that it is likely that structural changes of the airway are the greater contributor to AHR than the development of inflammation.
The structural components of the airway previously described include airway smooth muscle. There has been considerable interest in dissecting the contribution of airway remodeling and their relationship to smooth muscle and/or features of airway smooth muscle contractile responses to the presence of AHR in asthma. As noted, the measurement of AHR is characterized by excessive narrowing to contractile substances that cause no effect in normal subjects and a response at a smaller dose. These characteristics represent two changes: an increased sensitivity and an ability of the airway smooth muscle to narrow excessively. Some believe the response to contraction at a lower dose (ie, increased sensitivity) may represent the less significant change, and it is the exaggerated airway narrowing that is more important in asthma and the resulting altered physiology. Efforts are ongoing to sort out that the contribution of airway remodeling and altered smooth muscle to AHR. Paré et al,11 for example, present convincing evidence that airway wall remodeling is a major contributor to the excessive airway narrowing in asthma. These same investigators have pointed out that the excessive deposition of collagen and thickening of the airway wall could protect against excessive airway narrowing.12 Thus, the balance between these changes can lead to opposite effects. In contrast, Oliver et al13 have convincing evidence that the increased muscle mass in asthma is a dominant factor contributing to AHR. Thus, AHR remains a complex component of asthma. Although airway inflammation is likely a contributor to symptoms and airflow limitations in asthma, attention has shifted to structural alteration in the airway as the dominant factor in the regulation of AHR.
Conclusions
Defining the contribution of airway inflammation to AHR depends on many aspects. First, it depends on the component of AHR that is measured, persistent vs variable. Although this is an arbitrary division, it does allow for evaluation of structural changes vs the variable level (ie, inflammation). Second, the measurement of AHR is influenced by the methods used to detect it, whether it is methacholine causing a direct constriction of airway muscle or other substances that are indirect activators and reflect inflammation. Dissecting whether structural or inflammatory changes influence AHR will provide greater insight into this mechanism of asthma. The emerging data suggest that it is the structural changes of the airway that contribute to AHR; included in these structural changes are those of remodeling and smooth muscle hypertrophy.
Acknowledgments
Financial/nonfinancial disclosures: The author has reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Abbreviations
- AHR
airway hyperresponsiveness
- anti-IL
monoclonal antibody to interleukin
- ICS
inhaled corticosteroids
- IL
interleukin
- PC20
provocative concentration causing a 20% fall in FEV1
Funding/Support: This work was supported by the National Institutes of Health, National Heart, Lung and Blood Institute [Grant R01 HL069116] and the National Institute of Allergy and Infectious Diseases [N01-AI-25496].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (http://www.chestpubs.org/site/misc/reprints.xhtml).
References
- 1.Cockcroft DW, Davis BE. Mechanisms of airway hyperresponsiveness. J Allergy Clin Immunol. 2006;118(3):551–559. doi: 10.1016/j.jaci.2006.07.012. quiz 560-561. [DOI] [PubMed] [Google Scholar]
- 2.O’Byrne PM, Gauvreau GM, Brannan JD. Provoked models of asthma: what have we learnt? Clin Exp Allergy. 2009;39(2):181–192. doi: 10.1111/j.1365-2222.2008.03172.x. [DOI] [PubMed] [Google Scholar]
- 3.Covar RA. Bronchoprovocation testing in asthma. Immunol Allergy Clin North Am. 2007;27(4):633–649. doi: 10.1016/j.iac.2007.09.005. vi-vii. [DOI] [PubMed] [Google Scholar]
- 4.Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. The AMPUL Study Group Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1043–1051. doi: 10.1164/ajrccm.159.4.9806052. [DOI] [PubMed] [Google Scholar]
- 5.Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid-treated asthma. J Allergy Clin Immunol. 2000;105(4):752–759. doi: 10.1067/mai.2000.105319. [DOI] [PubMed] [Google Scholar]
- 6.Sedgwick JB, Calhoun WJ, Vrtis RF, Bates ME, McAllister PK, Busse WW. Comparison of airway and blood eosinophil function after in vivo antigen challenge. J Immunol. 1992;149(11):3710–3718. [PubMed] [Google Scholar]
- 7.Shi H-Z, Xiao C-Q, Zhong D, et al. Effect of inhaled interleukin-5 on airway hyperreactivity and eosinophilia in asthmatics. Am J Respir Crit Care Med. 1998;157(1):204–209. doi: 10.1164/ajrccm.157.1.9703027. [DOI] [PubMed] [Google Scholar]
- 8.Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–2148. doi: 10.1016/s0140-6736(00)03496-6. [DOI] [PubMed] [Google Scholar]
- 9.Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi: 10.1056/NEJMoa0808991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kariyawasam HH, Aizen M, Barkans J, Robinson DS, Kay AB. Remodeling and airway hyperresponsiveness but not cellular inflammation persist after allergen challenge in asthma. Am J Respir Crit Care Med. 2007;175(9):896–904. doi: 10.1164/rccm.200609-1260OC. [DOI] [PubMed] [Google Scholar]
- 11.Paré PD, McParland BE, Seow CY. Structural basis for exaggerated airway narrowing. Can J Physiol Pharmacol. 2007;85(7):653–658. doi: 10.1139/Y07-051. [DOI] [PubMed] [Google Scholar]
- 12.Paré PD. Airway hyperresponsiveness in asthma: geometry is not everything! Am J Respir Crit Care Med. 2003;168(8):913–914. doi: 10.1164/rccm.2307005. [DOI] [PubMed] [Google Scholar]
- 13.Oliver MN, Fabry B, Marinkovic A, Mijailovich SM, Butler JP, Fredberg JJ. Airway hyperresponsiveness, remodeling, and smooth muscle mass: right answer, wrong reason? Am J Respir Cell Mol Biol. 2007;37(3):264–272. doi: 10.1165/rcmb.2006-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]