Abstract
Accumulating evidence indicates that the chemokine receptor CCR5 and the chemokine CCL5 may be involved in the proliferation and metastasis of prostate cancer. Consequently, chemokine receptor CCR5 antagonists could potentially act as anti prostate cancer agents. As the first natural product CCR5 antagonist, anibamine provides a novel chemical structural skeleton compared with other known antagonists identified through high-throughput screening. Our studies demonstrate that anibamine produces significant inhibition of prostate cancer cell proliferation at micromolar to submicromolar concentrations as well as suppressing adhesion and invasion of the highly metastatic M12 prostate cancer cell line. Preliminary in vivo studies indicate that anibamine also inhibits prostate tumor growth in mice. These findings indicate that anibamine may prove to be a novel lead compound for the development of prostate cancer therapeutic agents.
Keywords: chemokine receptor CCR5, antagonist, anibamine, prostate cancer
Prostate cancer is the most common non-cutaneous solid cancer occurring amongst men in the USA, and the second most common malignant cause of male death worldwide1. Current therapies remain limited to surgery, radiation, and/or androgen ablation2. Recent investigations indicate that there is a relationship between some inflammatory processes and cancer, specifically, prostate cancer development3-13. For example, the prostate cancer cell lines PC-3, DU145, and LNCaP express the chemokine CCL5 (RANTES) and the chemokine receptor CCR5. Furthermore, the chemokine receptor CCR5 antagonist, TAK-779 inhibited CCL5-induced proliferation of these prostate cancer cell lines12. Levels of CCL5 and CCR5 are also reported to be greater in prostate cancer specimens than in benign hyperplasia13. Collectively these findings in both patient-derived specimens and prostate cancer cell lines suggest that development of the appropriate chemokine receptor CCR5 antagonists could provide a novel prostate cancer therapy.
Anibamine (Figure 1), a novel pyridine quaternary alkaloid recently isolated from Aniba sp., was found to bind to CCR5 with an IC50 of 1 μM in competition with 125I-gp120, an HIV viral envelop protein14. Thus far, anibamine is the first known natural product acting as a CCR5 antagonist. While the chemokine receptor CCR5 has mainly been targeted in HIV therapies since it was first cloned more than a decade ago15-21, CCR5 antagonists could provide a novel therapeutic approach for prostate cancer treatment through the inhibition of CCL5 induced cell proliferation.
Figure 1.
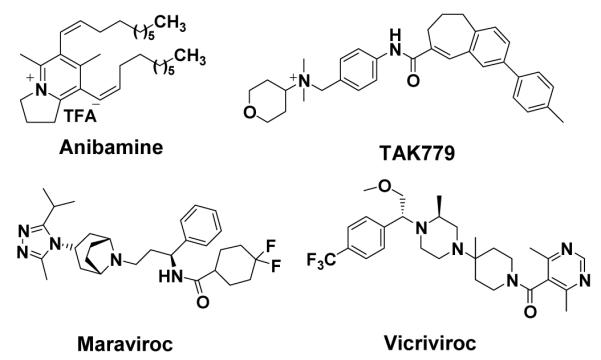
Anibamine and some known CCR5 antagonists
Anibamine has a novel structural skeleton compared to other CCR5 antagonists identified through high-throughput screening. Considering the binding affinity to CCR5 of other original lead compounds22-24, the inhibitory binding affinity of anibamine at 1 μM to CCR5 appears quite promising.
Recently, the total synthesis of anibamine has been reported by one of our laboratories25. The development of this synthetic pathway provides the opportunity for generating anibamine derivatives in order to explore their structure-activity relationships as CCR5 antagonists. The binding of anibamine to the chemokine receptor CCR5 has been characterized and compared with that of other CCR5 antagonists in different homology models of CCR526. The binding pocket of anibamine shares some common features with other high affinity CCR5 antagonists, suggesting binding to similar binding sites. The current studies were designed to explore the utility of developing anibamine as a novel lead compound against prostate cancer.
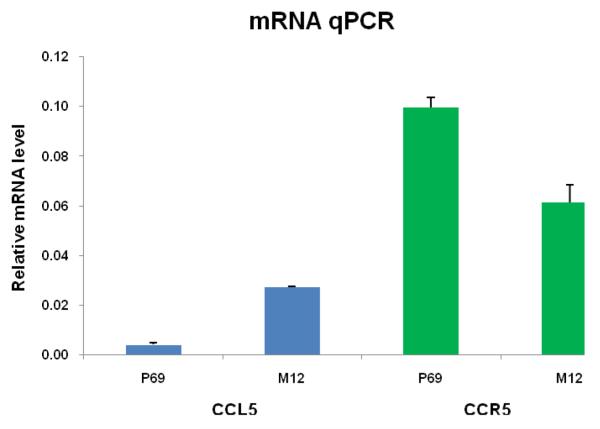
As indicated previously, the expression of CCL5 and CCR5 has been observed in various prostate cancer cell lines, including PC-3, DU145, and LNCaP12,13. Expression of CCR5 and CCL5 mRNA was quantitated via qRT-PCR in the highly metastatic M12 prostate epithelial cell line, as well as in its non tumorigenic parental cell line P6927. The results, shown in Figure 2, indicate that while both genetically related sublines express CCR5, CCL5 expression was evident in the M12 tumorigenic subline but was barely detectable in the parental p69 line. From our results, the relatively elevated levels of CCL5 in the metastatic M12 cell line compared to the nontumorigenic parental p69 line suggest that CCL5 and its receptor CCR5 could be involved in prostate cancer metastatic progression, providing additional support for the potential value of targeting the chemokine receptor CCR5 in prostate cancer.
Figure 2.
Differential expression of CCL5 and CCR5 in isogenic P69 and M12 prostate cancer sublines. SYBR-based qRT-PCR was performed with total RNA extracted from P69 and M12 sublines as described in Materials and Methods. The Y-axis represents the relative mRNA level of CCL5 or CCR5 normalized to RNU48 as an internal control. The standard error of the mean is shown as error bars. Student’s t-test indicates a significant difference with a P-value <0.001 for both CCL5 and CCR5.
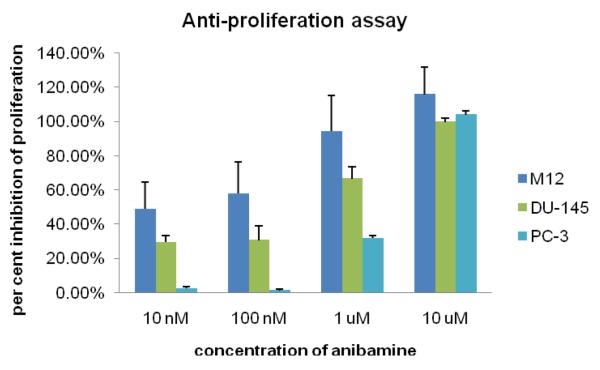
Previously, M12 cells were shown to have a very high invasive ability27. It is also known that adhesion and invasion are important steps that further promote prostate tumorigenesis and metastasis. The growth inhibitory properties of anibamine were evaluated in the prostate cancer cell lines, PC-3, DU145, and M12. Results of these assays are summarized in Figure 3. Anibamine was observed to interfere with prostate cancer cell growth in a dose-dependent manner at micromolar to submicromolar concentrations in all three cell lines. The observations that anibamine can inhibit the invasion and adhesion of M12 cells support the possibility that anibamine may have anti metastatic properties against prostate cancer.
Figure 3.
Inhibition of prostate cancer cell proliferation by anibamine. Three prostate cancer cell lines, M12, PC-3, and DU-145 were exposed to a series of concentrations of anibamine. Proliferation was assessed using the WST-1 Cell Proliferation Reagent (Roche).
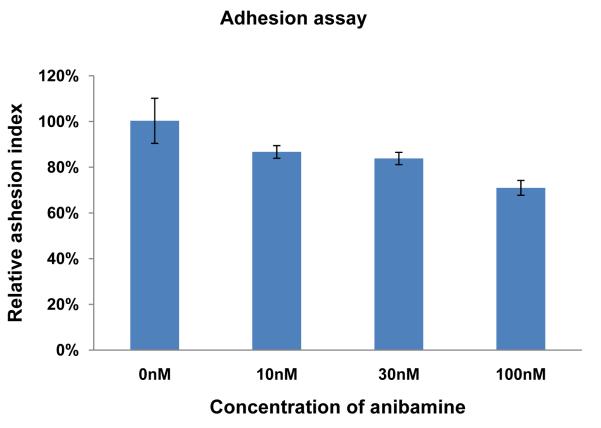
In invasion and adhesion assays, the addition of anibamine inhibited M12 invasive ability by 42 to 65% (Figure 4) depending on the dose and M12 adhesion up to 26% (Figure 5). No additional effects on adhesion were evident at higher concentrations (data not shown).
Figure 4.
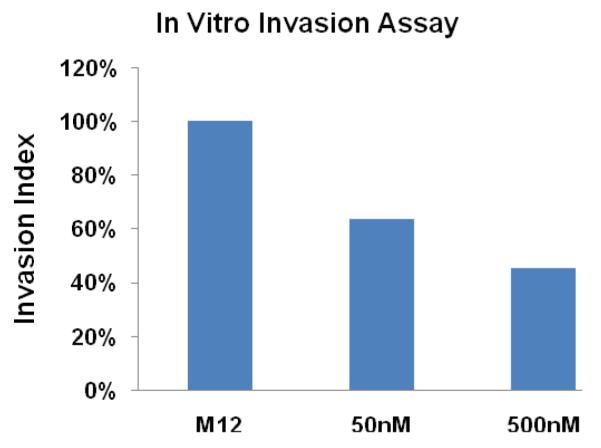
Effect of anibamine on invasive capability of M12 cells. Equal numbers of M12 cells were plated in Transwell chambers −/+ anibamine at the indicated concentrations as described in Materials and Methods. Filters were coated with 1:10 diluted lrECM prior to cell plating. Medium containing 20% FBS, EGF (20 ng/ml) and 5ng/ml CCL5 was added as a chemo-attractant to the lower chamber. Bars indicate standard error. ANOVA testing indicates a significant difference with a P-value <0.001.
Figure 5.
Effect of anibamine on M12 cell adhesion. Equal numbers of M12 cells were pre-treated +/− anibamine at the indicated concentrations for 24 hours and were then plated in 96-well cells coated with diluted lrECM as described in Materials and Methods. The x-axis represents the concentration of anibamine, while the Y-axis represents the relative adhesion index normalized to the M12 control without drug. Bars indicate standard error. ANOVA test indicates a significant difference with a P-value of <0.001 (F=26.8).
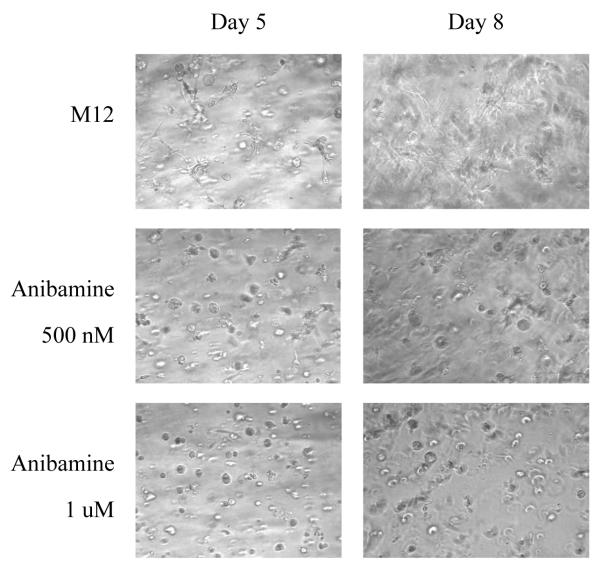
Further, M12 cells embedded in lrECM gels were studied to assess the effect of anibamine on tumor cell morphology. As shown in Figure 6, the M12 subline displayed a disorganized mass of cells when grown in 3D (which is in agreement with its metastatic character); the addition of anibamine reverted M12 cells to spheroid-like structures referred to as acini. These observations further support the premise that anibamine indeed could potentially inhibit prostate tumor metastasis.
Figure 6.
Growth properties of M12 cells +/− anibamine in 3D lrECM gels. M12 cells (1×105) were mixed with 100μl of undiluted lrECM gel +/− 500nM or 1μM anibamine and then plated in 96-well plate as described in Materials and Methods. Light microscopy images were taken from cultures at day 5 and 8 as indicated. Magnification is at 10X.
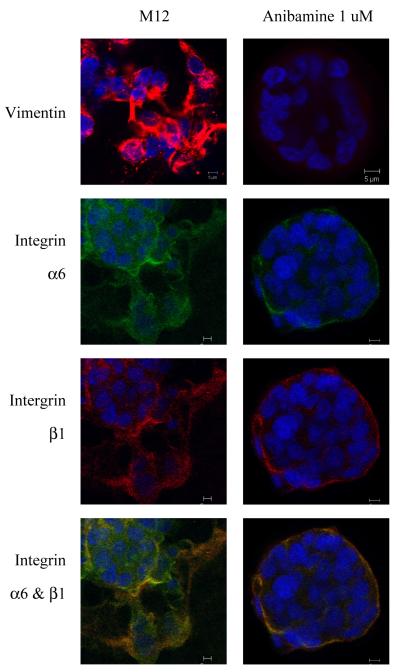
After that, immunohistochemical staining for relevant cell proteins coupled with confocal microscopy was conducted to better examine the morphological differences displayed by these cells when grown embedded in lrECM gels. The M12 subline, which shows high expression of vimentin, spread throughout the lrECM as a disorganized mass, which reflects the highly tumorigenic/metastatic behavior of these cells when injected into male, athymic nude mice28. Interestingly, vimentin gene expression declined with the addition of anibamine (Figure 7). In addition, while the expression of α6- and β1- integrins was quite disorganized in M12 cells. The addition of anibamine reverted the disorganized mass of cells to an acinus with a distinct luman displaying basal polarization of α6- and β1- integrin as shown previously for the parental, benign P69 cells.
Figure 7.
Comparison of content and localization of vimentin and integrin within the morphological structures formed by the M12 prostate sublines +/− anibamine grown embedded in lrECM gels. Confocal immunofluorescence microscopy of structures formed at day 8 stained with antibodies to vimentin (red, top panel), α6-integrin (green) and β1-integrin (red) as indicated. The overlay of α6β1-integrin is shown on the bottom panel. All pictures are taken at a magnification of 63X and nuclei were counterstained with 4′6-diamidino-2-phenylindole (DAPI; blue) as discussed in Materials and Methods.
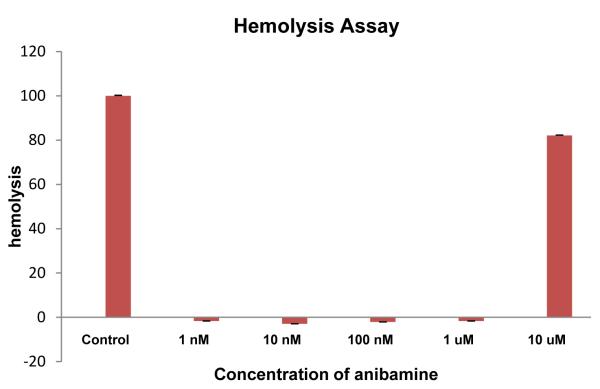
As with the development of any new class of drugs, it was critical to determine whether these compounds could be used at concentrations that are not toxic to normal cells. For our initial screening studies, we examined hemolysis of sheep red blood cells by anibamine, since this was thought to be a possible limitation on the use of this class of agents29. Our result indicates that no toxicity was observed in this assay below or at a concentration of 1 μM (Figure 8), which would support the potential selectivity of this agent.
Figure 8.
Assessment of red blood cell hemolysis by anibamine. Sheep red blood cells were exposed to anibamine in PBS for 60 min. Samples were centrifuged and the absorbance of the supernatant determined at 540nm. Lysis in distilled water was used as a positive control.
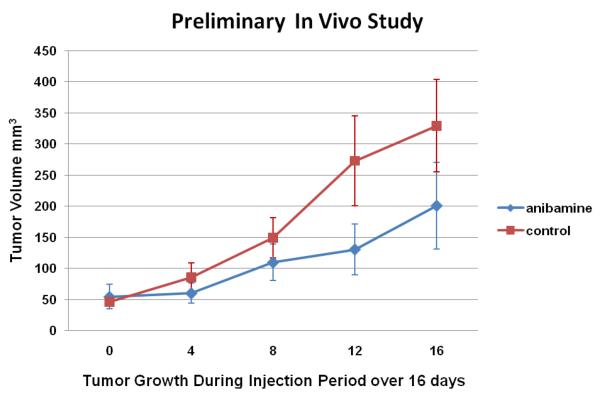
In addition, preliminary data from an on-going in vivo analysis suggests that anibamine can reduce the subcutaneous growth of M12 tumor cells in athymic nude mice. In the three mice with subcutaneous M12 tumors, four days after four injections of anibamine, the size of the tumors was 321.4 mm3, 80.0 mm3, and 202.2 mm3, respectively, averaging 201.2 ± 69.7 mm3. In contrast, the size of the tumors injected with the solvent control was 421.6 mm3, 182.6 mm3, and 384.6 mm3, respectively, averaging 329.6 ± 74.3 mm3. Thus anibamine did appear to reduce the rate of growth of the M12 tumors by roughly 50% (Figure 9). Such observation that anibamine can reduce the subcutaneous growth of M12 tumor cells in athymic nude mice support the premise that anibamine and/or its derivatives could prove to have utility in the treatment of prostate cancer.
Figure 9.
Influence of anibamine on prostate tumor growth in vivo. Six athymic nude mice were injected subcutaneously with 2000 M12 cells. After the tumors became visible, the mice were injected intravenously via a lateral tail vein with 0.3mg/kg of anibamine or with 0.3mg/kg of saline over a 16 day period for four injections (once every fourth day). The tumor size was recorded accordingly as the number of day right after the first injection.
In summary, anibamine showed significant inhibition of prostate cancer cell proliferation at 1 μM and lower concentrations while direct hemolysis was not evident until an approximately 10-fold higher concentration. Anibamine also suppressed the invasive and metastatic properties of M12 cells and compromised the growth of these tumors in vivo. Overall these preclinical studies suggest that anibamine could have a reasonable therapeutic index, supporting the potential utility of this compound as the lead for future drug design and development.
One reservation is that the calculated log Kow for anibamine is 9.129, which indicates that its lipophilicity is significantly higher than the value set forth by “Lipinski’s rule of 5” for drug-like compounds30. In comparing the chemical structure of anibamine with other known CCR5 antagonists (Figure 1), a major difference is that the anibamine side chains are simple, undecorated, aliphatic chains. Therefore, further drug development for CCR5 antagonists based on the chemical structure of anibamine, the first natural product with high binding affinity to the CCR5 chemokine receptor, may lead to a new type of therapeutic agent for metastatic prostate cancer therapy. Further studies of anibamine and its analogs should also serve to clarify the mechanisms by which targeting the chemokine receptor CCR5 may suppress metastatic processes of prostate cancer cells.
Supplementary Material
Acknowledgements
We are grateful to the funding support from US Army Prostate Cancer Research Program PC073739, NIH/NIAID AI69975 and AI074461, and A. D. Williams Multischool Research Award at VCU. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of AIDS and Infectious Diseases or the National Institutes of Health, nor US Army Prostate Cancer Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1(a).The Department of Health and Human Services; US Cancer Statistics: 2002 Incidence and Mortality, the most comprehensive federal report available on state-specific cancer rates. 2005 November;; (b) Miller BA, Ries LAG, Hankey BF. Seer Cancer Statistics Review. 1973 NIH Publ., 1993, No. 93-2789. [Google Scholar]
- 2. http://www.cancer.gov/cancertopics/treatment/prostate.
- 3.Frederick MJ, Gary L. Chemokines in Cancer. Reviews in Molecular Medicine. 2001:1. doi: 10.1017/S1462399401003301. [DOI] [PubMed] [Google Scholar]
- 4.Nasu K, Matsui N, Narahara H, Tanaka Y, Takai N, Miyakawa I, Higuchi YM. A human endometrial stromal sarcoma cell line that constitutely produces interleukin-6, interleukin-8, and monocyte chemoattractant protein-1. Arch. Pathol. Lab. Med. 1998;122:836. [PubMed] [Google Scholar]
- 5.Melani C, Pupa SM, Stoppacciaro A, Menard S, Colnaghi MI, Parmiani G, Colombo MP. An in vivo model to compare human leukocyte infiltration in carcinoma xenografts producing different chemokines. Int. J. Cancer. 1995;62:572. doi: 10.1002/ijc.2910620514. [DOI] [PubMed] [Google Scholar]
- 6.Selvan RS, Butterfield JH, Krangel M,S. Expression of multiple chemokine genes by a human mast cell leukemia. J. Biol. Chem. 1994;269:13893. [PubMed] [Google Scholar]
- 7.Schadendorf D, Moller A, Algermissen B, Worm M, Sticherling M, Czarnetzki BM. IL-8 produced by human malignant melanoma cells in vitro is an essential autocrine growth factor. J. Immunol. 1993;151:2667. [PubMed] [Google Scholar]
- 8.Miyamoto M, Shimizu Y, Okada K, Kashii Y, Higuchi K, Watanabe A. Effect of interleukin-8 on production of tumor-associated substances and autocrine growth of human liver and pancreatic cancer cells. Cancer Immunol. Immunother. 1998;47:47. doi: 10.1007/s002620050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. Origin and function of tumor-associated macrophages. Immunol. Today. 1992;13:265. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 10.Opdenakker G, Van Damme J. Chemotactic Factors, Passive Invasion and Metastasis of Cancer Cells. Immumol. Today. 1992;13:463. doi: 10.1016/0167-5699(92)90079-M. [DOI] [PubMed] [Google Scholar]
- 11.Opdenakker G, Van Damme J. Cytokines and Proteases in Invasive Processes. Cytokine. 1992;4:251. doi: 10.1016/1043-4666(92)90064-x. [DOI] [PubMed] [Google Scholar]
- 12.Vaday GG, Peehl DM, Kadam PA, Lawrence DM. Expression of CCL5(RANTES) and CCR5 in Prostate Cancer. The Prostate. 2006;66:124. doi: 10.1002/pros.20306. [DOI] [PubMed] [Google Scholar]
- 13.Koenig JE, Senge T, Allhoff EP, Koenig W. Analysis of the Inflammatory Network in Benign Prostate Hyperplasia and Prostate Cancer. The Prostate. 2004;58:121. doi: 10.1002/pros.10317. [DOI] [PubMed] [Google Scholar]
- 14.Jayasuriya H, Herath KB, Ondeyka JG, Polishook JD, Bills GF, Dombrowski AW, Springer MS, Siciliano S, Malkowitz L, Sanchez M, Guan Z, Tiwari S, Stevenson DW, Borris RP, Singh SB. Isolation and structure of antagonists of chemokine receptor CCR5. J. Nat. Prod. 2004;67:1036. doi: 10.1021/np049974l. [DOI] [PubMed] [Google Scholar]
- 15.Littman DR. Chemokine Receptors: Keys to AIDS Pathogenesis? Cell. 1998;93:677. doi: 10.1016/s0092-8674(00)81429-4. [DOI] [PubMed] [Google Scholar]
- 16.Chinen J, Shearer WT. Molecular virology and immunology of HIV infection. J. Allergy Clin. Immunol. 2002;110:189. doi: 10.1067/mai.2002.126226. [DOI] [PubMed] [Google Scholar]
- 17.Kedzierska K, Crowe SM, Turville S, Cunningham AL. The influence of cytokines, chemokines and their receptors on HIV-1 replication in monocytes and macrophages. Rev. Med. Virol. 2003;13:39. doi: 10.1002/rmv.369. [DOI] [PubMed] [Google Scholar]
- 18(a).Tagat JR, McCombie SW, Nazareno D, Labroli MA, Xiao Y, Steensma RW, Strizki JM, Baroudy BM, Cox K, Lachowicz J, Varty G, Watkins R. Piperazine-Based CCR5 Antagonists as HIV-1 Inhibitors. IV. Discovery of 1-[(4,6-Dimethyl-5-pyrimidinyl)carbonyl]- 4-[4-{2-methoxy-1(R)-4-(trifluoromethyl)phenyl}ethyl-3(S)-methyl-1-piperazinyl]-4-methylpiperidine (Sch-417690/Sch-D), a Potent, Highly Selective, and Orally Bioavailable CCR5 Antagonist. J. Med Chem. 2004;48:2405. doi: 10.1021/jm0304515. [DOI] [PubMed] [Google Scholar]; (b) Strizki JM, Tremblay C, Xu S, Wojcik L, Wagner N, Gonsiorek W, Hipkin RW, Chou CC, Pugliese-Sivo C, Xiao Y, Tagat JR, Cox K, Priestley T, Sorota S, Huang W, Hirsch M, Reyes GR, Baroudy BM. Discovery and characterization of vicriviroc (SCH 417690), a CCR5 antagonist with potent activity against human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 2005;49:4911. doi: 10.1128/AAC.49.12.4911-4919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a).Lynch CL, Willoughby CA, Hale JJ, Holson EJ, Budhu RJ, Gentry AL, Rosauer KG, Caldwell CG, Chen P, Mills SG, MacCoss M, Berk S, Chen L, Chapman KT, Malkowitz L, Springer MS, Gould SL, DeMartino JA, Siciliano SJ, Cascieri MA, Carella A, Carver G, Holmes K, Schleif WA, Danzeisen R, Hazuda D, Kessler J, Lineberger J, Miller M, Emini EA. 1,3,4-Trisubstituted pyrrolidine CCR5 receptor antagonists: modifications of the arylpropylpiperidine side chains. Bioorg. Med. Chem. Lett. 2003;6:119. doi: 10.1016/s0960-894x(02)00829-6. [DOI] [PubMed] [Google Scholar]; b) Shen DM, Shu M, Mills SG, Chapman KT, Malkowitz L, Springer MS, Gould SL, DeMartino JA, Siciliano SJ, Kwei GY, Carella A, Carver G, Holmes K, Schleif WA, Danzeisen R, Hazuda D, Kessler J, Lineberger J, Miller MD, Emini EA. Antagonists of human CCR5 receptor containing 4-(pyrazolyl)piperidine side chains. Part 1: Discovery and SAR study of 4-pyrazolylpiperidine side chains. Bioorg. Med. Chem. Lett. 2004;23:935. doi: 10.1016/j.bmcl.2003.12.004. [DOI] [PubMed] [Google Scholar]; c) Shen DM, Shu M, Willoughby CA, Shah S, Lynch CL, Hale JJ, Mills SG, Chapman KT, Malkowitz L, Springer MS, Gould SL, DeMartino JA, Siciliano SJ, Lyons K, Pivnichny JV, Kwei GY, Carella A, Carver G, Holmes K, Schleif WA, Danzeisen R, Hazuda D, Kessler J, Lineberger J, Miller MD, Emini EA. Antagonists of human CCR5 receptor containing 4-(pyrazolyl)piperidine side chains. Part 2: Discovery of potent, selective, and orally bioavailable compounds. Bioorg. Med. Chem. Lett. 2004;23:941. doi: 10.1016/j.bmcl.2003.12.005. [DOI] [PubMed] [Google Scholar]; d) Shu M, Loebach JL, Parker KA, Mills SG, Chapman KT, Shen DM, Malkowitz L, Springer MS, Gould SL, DeMartino JA, Siciliano SJ, Salvo JD, Lyons K, Pivnichny JV, Kwei GY, Carella A, Carver G, Holmes K, Schleif WA, Danzeisen R, Hazuda D, Kessler J, Lineberger, Miller MD, Emini EA. Antagonists of human CCR5 receptor containing 4-(pyrazolyl)piperidine side chains. Part 3: SAR studies on the benzylpyrazole segment. Bioorg. Med. Chem. Lett. 2004;14:947. doi: 10.1016/j.bmcl.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Maeda K, Nakata H, Koh Y, Miyakawa T, Ogata H, Takaoka Y, Shibayama S, Sagawa K, Fukushima D, Moravek J, Koyanagi Y, Mitsuya H. Spirodiketopiperazine-based CCR5 inhibitor which preserves CC-chemokine/CCR5 interactions and exerts potent activity against R5 human immunodeficiency virus type 1 in vitro. J. Virology. 2004;78:8654. doi: 10.1128/JVI.78.16.8654-8662.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a).Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, Mori J, Rickett G, Smith-Burchnell C, Napier C, Webster R, Armour D, Price D, Stammen B, Wood A, Perros M. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob. Agents Chemother. 2005;49:4721. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) FDA Panel Backs HIV Drug, Pfizer Treatment Blocks Pathway Used to Infect Cells. Wall Street Journal. 2007 April 25; [Google Scholar]; c) FDA Voices Concerns Over New HIV Drug Class. Wall Street Journal. 2007 April 21; [Google Scholar]
- 22.Dorn CP, Finke PE, Oates B, Budhu RJ, Mills SG, MacCoss M, Malkowitz L, Springer MS, Daugherty BL, Gould SL, DeMartino JA, Siciliano SJ, Carella A, Carver G, Holmes K, Danzeisen R, Hazuda D, Kessler J, Lineberger J, Miller M, Schleif WA, Emini EA. Antagonists of the human CCR5 receptor as anti-HIV-1 agents. part 1: discovery and initial structure-activity relationships for 1 -amino-2-phenyl-4-(piperidin-1-yl)butanes. Bioorg. Med. Chem. Lett. 2001;11:259. doi: 10.1016/s0960-894x(00)00637-5. [DOI] [PubMed] [Google Scholar]
- 23.Palani A, Shapiro S, Clader JW, Greenlee WJ, Cox K, Strizki J, Endres M, Baroudy BM. Discovery of 4-[(Z)-(4-bromophenyl)- (ethoxyimino)methyl]-1′-[(2,4-dimethyl-3- pyridinyl)carbonyl]-4′-methyl-1,4′- bipiperidine N-oxide (SCH 351125): an orally bioavailable human CCR5 antagonist for the treatment of HIV infection. J. Med. Chem. 2001;44:3339. doi: 10.1021/jm015526o. [DOI] [PubMed] [Google Scholar]
- 24.Shiraishi M, Aramaki Y, Seto M, Imoto H, Nishikawa Y, Kanzaki N, Okamoto M, Sawada H, Nishimura O, Baba M, Fujino M. Discovery of novel, potent, and selective small-molecule CCR5 antagonists as anti-HIV-1 agents: synthesis and biological evaluation of anilide derivatives with a quaternary ammonium moiety. J. Med. Chem. 2000;43:2049. doi: 10.1021/jm9906264. [DOI] [PubMed] [Google Scholar]
- 25.Li G, Watson K, Buckheit RW, Zhang Y. Total Synthesis of Anibamine, a Novel Natural Product as a Chemokine Receptor CCR5 Antagonist. Org. Lett. 2007;9:2043. doi: 10.1021/ol070748n. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Haney KM, Kellogg GE, Zhang Y. A Comparative Docking Study of Anibamine as the First Natural Product CCR5 Antagonist in CCR5 Homology Models. J. Chem. Inf. Model. 2009;49:120. doi: 10.1021/ci800356a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bae VL, Jackson-Cook CK, Maygarden SJ, Plymate SR, Chen J, Ware JL. Metastatic Sublines of an SV40 Large T Antigen Immortalized Human Prostate Epithelial Cell line. The Prostate. 1998;34:275. doi: 10.1002/(sici)1097-0045(19980301)34:4<275::aid-pros5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Fournier MV, Ware JL, Bissell MJ, Yacoub A, Zehner ZE. Inhibition of vimentin or beta1 integrin reverts morphology of prostate tumor cells grown in laminin-rich extracellular matrix gels and reduces tumor growth in vivo. Mol Cancer Ther. 2009;8:499. doi: 10.1158/1535-7163.MCT-08-0544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klausmeyer P, Chmurny GN, McCloud TG, Tucker KD, Shoemaker RH. A Novel Antimicrobial Indolizinium Alkaloid from Aniba panurensis. J. Nat. Prod. 2004;67:1732. doi: 10.1021/np040114e. [DOI] [PubMed] [Google Scholar]
- 30.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Del. Rev. 2001;46:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.