Abstract
We investigated patterns of cigarette smoking and Swedish snus (oral smokeless tobacco) use in a population-based sample of 19,073 Swedish twins 20–47 years old who participated in the baseline assessment of a prospective study of tobacco use and cessation in 2005–2006. Age-adjusted prevalence odds ratios (POR) and 95% confidence intervals (CI ) describe the association between tobacco use and sex, after adjustment for non-independence of twin pairs. Kaplan-Meier survival methods produced cumulative incidence curves of age at initiation of tobacco use. Slightly more than half of the baseline population was female (55.2%); the mean age at interview was 33.3 (±7.2) years and did not differ by sex. Having ever smoked daily was less common among males than females (11.9% vs. 15.3%; POR=0.70 [0.64–0.77]), while having ever used snus daily was more common among males than females (31.1% vs. 4.8%; POR 11.7 [95% CI=10.6–13.1]). The median age at initiation of smoking was 15 years for both sexes; median age at onset of snus use was 15 years for males and 18 years for females. Nicotine dependence scores were higher for males than females, and for current than former smokers. Findings from this study are in contrast to our previously published report on tobacco use among 32,123 Swedish twins 42–64 years old who completed a similar survey, and reported lower rates of snus use at later ages. Patterns of tobacco use may be changing in Sweden; snus use appears to be increasing, while daily smoking appears to be decreasing in popularity among the younger Swedish twins.
Introduction
Smoking cessation remains an elusive goal for many smokers as relapse is normative. Although approximately 70% of smokers wish to quit, and more than 46% make an active attempt to quit each year, only 2.3% achieve sustained abstinence despite available smoking cessation methods (Silagy, Lancaster, Stead, Mant, & Fowler, 2004). The extraordinary addictiveness of nicotine is a prominent cause of difficulties in quitting tobacco, but cannot of itself explain the marked interindividual differences in cessation rates. To delineate etiological factors involved in tobacco use, relapse and cessation, we conducted a population-based, prospective study among Swedish twins, 20–47 years old at baseline, from the Screening Twin Adults: Genes and Environment (STAGE) study of the Swedish Twin Registry (Lichtenstein et al., 2006).
Sweden is an ideal place for study of smoking cessation because the proportion of daily smokers has decreased from 35% to 14% among males and from 28% to 18% among females (StatisticsSweden, 2007) over the past 25 years, and the availability of snus (moist, oral smokeless tobacco) which Swedes may be using to help them quit smoking (Foulds, Ramstrom, Burke, & Fagerström, 2003; Furberg et al., 2005; Furberg, Lichtenstein, Pedersen, Bulik, & Sullivan, 2006; Gilljam & Galanti, 2003; Ramstrom, 2000, 2003a, 2003b; Ramstrom & Foulds, 2006; Rodu, Stegmayr, Nasic, & Asplund, 2002; Rodu, Stegmayr, Nasic, Cole, & Asplund, 2003; Stegmayr, Eliasson, & Rodu, 2005). Swedish snus delivers similar levels of nicotine as cigarettes but carries lower risks of cancer than cigarettes (Accortt, Waterbor, Beall, & Howard, 2002; Lagergren, Bergstrom, Lindgren, & Nyren, 2000; Levy et al., 2004; Lewin et al., 1998; Luo et al., 2007; Rodu & Jansson, 2004; Schildt, Eriksson, Hardell, & Magnuson, 1998; Ye, Ekström, Hansson, Bergström, & Nyrén, 1999). Snus is available in Sweden and Norway, but banned in the rest of the European Union. The recent introduction of Swedish-style snus into the American market by RJ Reynolds and Philip Morris has forced the debate over Swedish snus as a harm reduction product from the tobacco control community (Fagerström & Schildt, 2003; Gilljam & Galanti, 2003) to the international health community (Foulds & Kozlowski, 2007; Gartner, Hall, Chapman, & Freeman, 2007; Gray, 2005; Lambe, 2004, 2007; McKee & Gilmore, 2007; Savitz, Meyer, Tanzer, Mirvish, & Lewin, 2006).
Baseline data collection for the STAGE follow-up study of smoking cessation occurred between 2005 and 2006, when we obtained extensive tobacco use data via web and/or telephone interviews from 19,073 male and female twins born between 1959 and 1985. From the baseline population we have identified participants who had ever used cigarettes or snus in their lifetime and are following them prospectively at yearly intervals to identify changes in tobacco use including cessation attempts, relapses, and transitions between cigarette and snus use. The STAGE follow-up study of smoking cessation within the Swedish Twin Registry has the potential to advance significantly our knowledge of cigarettes and snus use patterns, nicotine dependence, cessation attempts, and relapse. The population-based twin design of STAGE allows us to investigate the genetic architecture of tobacco-related outcomes, but also to conduct traditional epidemiological analyses after adjusting for twin correlation (such as the results presented here).
This paper describes tobacco use at the baseline interview for STAGE participants, and contrasts them with those of older Swedish twins from a prior survey. The aim of this paper is threefold: (1) to describe the recruitment and data collection methods of STAGE, (2) to present tobacco use characteristics of the STAGE baseline sample, and (3) to compare tobacco use prevalence between twins from the STAGE sample (N=19,073) with twins who participated in a highly similar survey of older Swedish twins (42–64 years of age) called Screening Across the Lifespan Twin (SALT) study (N=31,213).
Methods
Swedish Twin Registry
The Swedish Twin Registry (http://ki.se/twinreg) is the largest population-based registry of twin births in the world (Pedersen, Lichtenstein, & Svedberg, 2002). The Swedish Twin Registry contains data on current vital status, marital status, address, and place of birth for >99.0% of all ~160,000 Swedish twins born between 1886–2000. The data collection procedures were reviewed and approved by the Swedish Data Inspection Board and the Regional Ethics Committee of the Karolinska Institutet. All subjects provided informed consent during the web questionnaire or through the telephone interview, which was later confirmed by postcard.
Two recent studies have been conducted within the Swedish Twin Registry population to screen for common diseases, and included detailed questions about tobacco use. These studies have been described in detail elsewhere (Lichtenstein et al., 2002; Lichtenstein et al., 2006; Pedersen et al., 2002). The SALT study was conducted between 1998–2002 and focused on twins born prior to 1958. We have published several cross-sectional, tobacco-related analyses among these twins from SALT (N=31,213) (Furberg et al., 2005; Furberg et al., 2006; Furberg et al., in press). As cross-sectional studies have critical limitations (Rothman & Greenland, 1998), we initiated a longitudinal twin study focused on smoking cessation. The Study of Twin Adults: Genes and Environment (STAGE) began in 2005 and recruited a younger cohort of twins born in Sweden between 1959–1985 (Lichtenstein et al., 2006). SALT and STAGE studies have representation from all 25 counties in Sweden, but differ from each other in age-composition and richness of information on tobacco use.
STAGE study
The target population of STAGE was approximately 43,000 eligible twins, with both twins living to at least one year of age. Beginning in May 2005, twins were sent a letter inviting them to participate in the study and were given a personal login to the study webpage which asked approximately 1,300 questions about common complex diseases within 34 sections, including a detailed tobacco use assessment. Twins could also select to complete the interview by phone with a trained interviewer using a computer-based data collection method. The response rate for the baseline tobacco use assessment of STAGE was 47.0% (N=20,117). As 1,044 twins (5.2%) had to be excluded due to missing data, the final sample size of the analyses presented in this paper was 19,073.
Assessment of smoking and snus use in STAGE
Participants answered questions regarding the kind of tobacco they used during their lifetime (cigarettes and/or snus), and the type of tobacco user they considered themselves to be. We classified cigarette smokers as either having smoked cigarettes daily (defined as smoking at least once a day), having smoked cigarettes occasionally (defined as having smoked less than once a day), or having never smoked. Daily smokers and occasional smokers were combined together to form the category “Ever smoked cigarettes.” We classified snus users as either having used snus daily (defined as having used snus at least once a day), having used snus occasionally (defined as having used snus less than once a day), or having never used snus. Daily snus users and occasional snus users were combined together to form the category “Ever used snus.” We created four mutually-exclusive categories of “Lifetime combination tobacco use” which were those who had ever smoked cigarettes and never used snus (exclusive cigarette users), those who had ever used snus but never smoked cigarettes (exclusive snus users), those who ever used both products in their lifetime, and those who never smoked cigarettes or used snus in their lifetime. In addition, respondents provided information about age at initiation of cigarette and snus use, and whether they used either kind of tobacco at the time of interview. Lifetime daily smokers who had not smoked cigarettes in the past month were considered former smokers; all other daily smokers were classified as current smokers. The same classification was used for snus status at the time of interview. The Fagerström Test for Nicotine Dependence (FTND) was used to assess nicotine dependence (Fagerström, 1978; Heatherton, Kozlowski, Frecker, & Fagerström, 1991).
Statistical analyses
Analyses were conducted with SAS 9.1 (SAS Institute, 2004) and were stratified by sex. To examine differences between participants and non-participants of STAGE, we compared proportions of categorical variables using chi-square tests and compared the means of continuous variables using Student t tests. Prevalence odds ratios (POR) and 95% confidence intervals (CI) were obtained from age-adjusted logistic regression models as measures of association between sex and tobacco use. Least squares means and standard errors (SE) were conducted for FTND scores. We used the Kaplan-Meier estimator method to examine age at initiation curves of cigarette smoking and snus use among males and females (Allison, 1995; Cox & Oakes, 1984). For the age at initiation of tobacco use curves, time was defined as chronological age. Ages at initiation of tobacco use were used to define the event (i.e., “Ever smoked cigarettes” and “Ever used snus”), while age at interview was used as the censoring variable for those who never used cigarettes or snus in their lifetime.
Since twin pairs contribute correlated data that can influence the standard errors of the parameter estimates (Liang & Zeger, 1986), we used generalized estimating equations to adjust for the clustering or non-independence of members of a twin pair (Stokes, 1995). Specifically, we utilized the sandwich estimator of the variance and specified the exchangeable correlation structure. To cross-check our results, we randomly selected one member from each twin pair and repeated the aforementioned analyses on this subset of singletons. As expected, the results were nearly identical with the exception of wider confidence intervals due to diminished sample size.
Results
Representativeness of the STAGE study
We conducted analyses to determine the extent of bias between STAGE participants and non-participants with regard to individual-level characteristics including age, sex, birth weight, parents’ birthplace, whether they had ever been convicted of any type of crime, ever been diagnosed with a neurological condition, or ever been diagnosed with a psychiatric condition. STAGE participants and non-participants did not differ by age, birth weight or whether they had been diagnosed with a neurological condition (all p values >.05; data not shown), but significant differences were observed for the remaining variables (p<.0001). Compared with STAGE participants, a higher proportion of non-participants were male, had at least one parent born outside of Sweden, had been convicted of any type of crime, and had been diagnosed with a psychiatric disorder. Non-participants were also less educated and, among males, had lower mean intellectual performance scores at the time of conscription.
Demographic characteristics of the STAGE baseline cohort
Detailed tobacco use information was obtained on 19,073 participants, slightly more than half of whom were female (55.2%). Ages of participants ranged from 20–47 years and mean ages at interview (±SD) for each sex were similar, 33.4 (±7.7) years for males and 33.1 (±7.6) years for females (p>0.05). Nearly half of the STAGE sample had attended university (45.2%), and 63.7% were married or cohabitating. There were 5,466 complete twin pair sets, (N=10,932; 57.3%).
Prevalence of cigarette smoking and snus use
Table 1 presents the lifetime prevalence of cigarette use and snus use for the total STAGE population, and by sex. More than 70% of STAGE participants reported ever using tobacco in their lifetime (71.4%); ever tobacco use was more common among males (76.6%) than females (67.3%; POR=1.57, 95% CI=1.46–1.68).
Table 1.
Baseline tobacco use distributions among 19,073 male and female STAGE participants 20–47 years old.
| All |
Males |
Females |
Age-adjusted |
|
|---|---|---|---|---|
| N=19,073 (%) | n=8,553 (44.8) | n=10,520 (55.2) | POR (95% CI)a | |
| LIFETIME TOBACCO USE (Cigarettes or snus) | ||||
| Ever used cigarettes or snus | 13626 (71.4) | 6547 (76.6) | 7079 (67.3) | 1.57 (1.46–1.68) |
| Never used cigarettes or snus | 5447 (28.6) | 2006 (23.4) | 3441 (32.7) | 1.0 (reference group) |
| LIFETIME CIGARETTE SMOKING | ||||
| Ever smoked cigarettesb | 11844 (62.1) | 5187 (60.7) | 6657 (63.3) | 0.88 (0.83–0.94) |
| Ever smoked cigarettes daily | 2624 (13.7) | 1016 (11.9) | 1608 (15.3) | 0.70 (0.64–0.77) |
| Ever smoked cigarettes occasionally | 9220 (48.4) | 4171 (48.8) | 5049 (48.0) | 0.94 (0.88–1.01) |
| Never smoked cigarettes | 7229 (37.9) | 3366 (39.3) | 3863 (36.7) | 1.0 (reference group) |
| LIFETIME SNUS USE | ||||
| Ever used snusc | 7796 (40.9) | 5123 (59.9) | 2673 (25.4) | 4.46 (4.18–4.76) |
| Ever used snus daily | 3168 (16.6) | 2664 (31.1) | 504 (4.8) | 11.7 (10.6–13.1) |
| Ever used snus occasionally | 4628 (24.3) | 2770 (28.8) | 2169 (20.6) | 2.62 (2.44–2.82) |
| Never used snus | 11277 (59.1) | 3430 (40.1) | 7847 (74.6) | 1.0 (reference group) |
| LIFETIME COMBINATION TOBACCO USEd | ||||
| Ever smoked cigarettes, never used snus | 5830 (30.6) | 1424 (16.7) | 4406 (41.9) | 0.55 (0.51–0.60) |
| Ever used snus, never smoked cigarettes | 1782 (9.3) | 1360 (15.9) | 422 (4.0) | 5.41 (4.81–6.19) |
| Used both cigarettes and snus in lifetime | 6014 (31.5) | 3763 (44.0) | 2251 (21.4) | 2.82 (2.61–3.06) |
| Never smoked cigarettes or used snus | 5447 (28.6) | 2006 (23.4) | 3441 (32.7) | 1.0 (reference group) |
| SMOKING STATUS AT INTERVIEWe | ||||
| Current daily cigarette smoker | 1685 (8.8) | 645 (7.5) | 1040 (9.9) | 0.69 (0.61–0.77) |
| Former daily cigarette smoker | 936 (4.9) | 369 (4.3) | 567 (5.4) | 0.73 (0.63–0.84) |
| Never smoked cigarettes | 7229 (37.9) | 3366 (39.3) | 3863 (36.7) | 1.0 (reference group) |
| SNUS USE STATUS AT INTERVIEWe | ||||
| Current daily snus user | 2581 (13.5) | 2196 (25.7) | 385 (3.7) | 12.6 (11.2–14.2) |
| Former daily snus user | 581 (3.1) | 463 (5.4) | 118 (1.1) | 9.1 (7.3–11.2) |
| Never used snus | 11277 (59.1) | 3430 (40.1) | 7847 (74.6) | 1.0 (reference group) |
Note.
Prevalence Odds Ratios (POR) describe the association between each smoking and snus variable comparing males with females, adjusted for age and twin clustering.
“Ever smoked cigarettes” is the sum of “Ever smoked cigarettes daily” and “Ever smoked cigarettes occasionally.”
“Ever used snus” is the sum of “Ever used snus daily” and “Ever used snus occasionally.”
Lifetime Combination Tobacco Use” shows mutually-exclusive categories of lifetime tobacco use.
Percentages do not add to 100% because occasional tobacco users are not represented.
Although the proportion of participants in STAGE who had ever smoked cigarettes in their lifetime was higher than those who had ever used snus (62.1% vs. 40.9%, respectively), lifetime daily snus use (16.6%) was more common than lifetime daily smoking (13.7%). Type of tobacco used differed significantly by sex. Males were less likely to have ever smoked daily than females (POR=0.70, CI=0.64–0.77), and more likely to have ever used snus daily (POR=11.7, CI=10.6–13.1). Among males, lifetime daily snus use (31.1%) was more common than lifetime daily smoking (11.9%). Among females, the prevalence of lifetime daily snus use was less than 5%, but it was 20.6% for lifetime occasional snus use.
Significant sex differences were observed also for “Lifetime combination tobacco use.” Fewer males than females reported exclusive cigarette use (POR=0.55, CI=0.51–0.60); exclusive snus use, and having used both products in their lifetime was more common in males than females (POR=5.41, CI=4.81–6.19) and (POR=2.82, CI=2.61–3.06, respectively). Among males it was more common to have ever used both products (44.0%) than either cigarettes or snus exclusively (~16%). Among females, only 4% had used snus exclusively, while 21.4% had used both products in their lifetime.
Also shown in Table 1 is the distribution of current and former smoking status at interview among daily tobacco users. The prevalence of current daily smoking in the total STAGE study population was 8.8%; 7.5% for males, 9.9% for females; prevalence of current daily snus use was 13.5%; 25.7% for males, 3.7% for females.
Table 2 presents mean FTND scores by sex and by smoking status at interview. Among people who had ever smoked daily in their lifetime, mean FTND scores were higher among males (mean 3.5±0.09) than females (3.2±0.08; p=.0031). Mean FTND scores were higher for people who were current daily smokers at interview (3.5±0.08) than former daily smokers (2.9±0.10; p=.0001). Males had significantly higher FTND scores than females in all categories of daily smoking, except current daily smoking (3.7±0.15 vs. 3.4±0.14, p=.1389).
Table 2.
Fagerström Test for Nicotine Dependence (FTND) scores by sex and smoking status among daily smokers in STAGE.
| MEAN FTND SCORE (SE) | All | Males | Females | p value |
|---|---|---|---|---|
| Ever smoked cigarettes daily | 3.3 (0.09) | 3.5 (0.09) | 3.2 (0.08) | p=.0031a |
| Current daily smoker | 3.5 (0.08) | 3.7 (0.15) | 3.4 (0.14) | p=.1389a |
| Former daily smoker | 2.9 (0.10) | 3.2 (0.14) | 2.6 (0.11) | p=.0017a |
| p=.0101b | p<.001b |
Note.
Least square means, standard errors (SE) and p values obtained from mixed models which examined whether FTND scores differed for males vs. females, among all daily smokers, among current daily smokers and among former daily smokers while adjusting for twin correlation.
Least square means, standard errors (SE) and p values obtained from mixed models which examined whether FTND scores differed for current daily smokers vs. former daily smokers, among males and among females while adjusting for twin correlation.
Ages at initiation of tobacco use
As shown in Figure 1, age at initiation of ever lifetime cigarette smoking occurred almost entirely before age 25 in this population, with an accelerated rate between 8 and 20 years of age. Ages at initiation were similar for male and female cigarette users and male snus users (median age at onset 15 years), while female snus users exhibited a later age at onset at 18 years.
Figure 1.
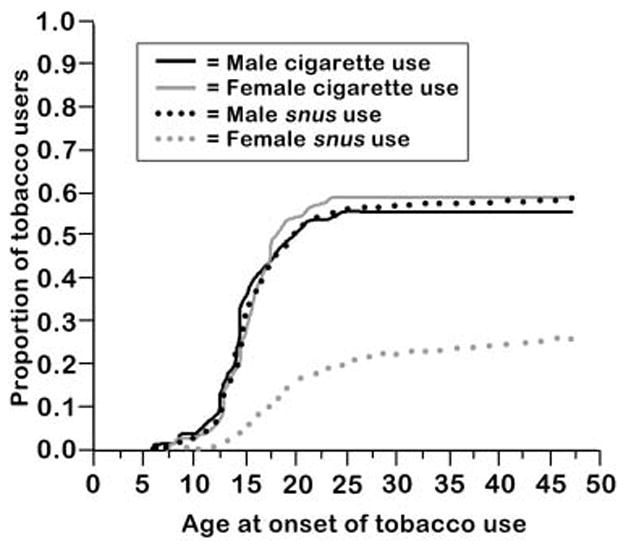
Age at onset of cigarette use and snus use by sex in STAGE.
Comparison of tobacco use prevalence in SALT and STAGE
Details about the SALT study have been previously published (Furberg et al., 2005; Furberg et al., 2006; Furberg et al., in press). That study and STAGE were similar in sex composition and region surveyed, but differed by age. Participants in the SALT study were older and therefore more educated, had higher incomes, and were more often married than the STAGE participants (data not shown).
Figure 2a–b compares the prevalence of tobacco use of the younger twins of STAGE with the older twins of the SALT study, stratified by sex. For all comparisons p<.0001, except “Ever Smoker” among females where p=.0088. The prevalence of ever lifetime tobacco use was slightly higher in STAGE than in SALT study for both males and females. There were striking differences in the prevalence of lifetime daily smoking and snus use between the two studies; having ever smoked daily was less common in STAGE, while having used snus daily was more common in STAGE.
Figure 2.
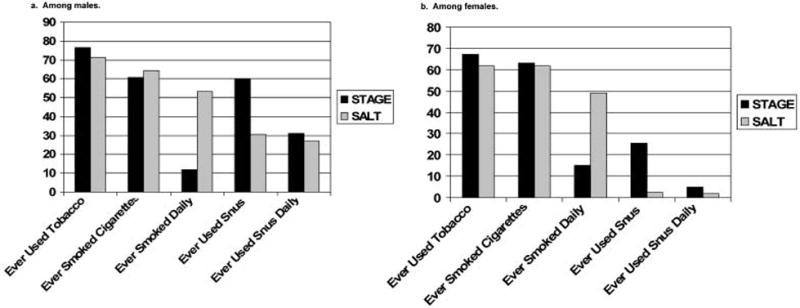
Comparing prevalence of tobacco use in STAGE (twins 20–47 years old) and Screening Across the Lifespan Twin study (SALT) (twins 42–64 years old). Top panel: males; bottom panel: females. Statistically significant p values of logistic regression analyses indicated that tobacco use differ across the two studies, when age and twin correlation are considered. All p<.0001 except for “Ever Smoker” among females, p=.009.
Discussion
Summary
In the baseline assessment of the STAGE cohort, we found that the prevalence of types of tobacco use differed significantly by sex—females tended to smoke cigarettes more than use snus, while males were more or less equally likely to use either product. Among males, the prevalence of daily snus use was higher than that of daily cigarette use. The age at initiation curves for cigarette and snus use overlapped for males, and was similar to the age at onset curve of cigarettes for females. Comparison of prevalences between the younger STAGE and older SALT study cohorts highlighted that snus use appears to be increasing while daily cigarette smoking appears to be decreasing among younger twins.
Comparison to previous reports
Several papers using data from two Swedish cohorts have described prevalence of cigarette smoking and snus use in detail, and findings are generally in line with this report (Ramstrom & Foulds, 2006; Rodu et al., 2002; Stegmayr et al., 2005). Ramstrom and Foulds (2006) conducted a retrospective analysis of data from a cross-sectional survey conducted in 2001–2002 among 6,752 adult Swedes ages 16–79 years. Since they presented tobacco use prevalence rates by age-group we compared our findings among STAGE twins 20–47 years old to their participants who were most similar in age, and our estimates of regular smoking and regular snus use were nearly identical. They also examined how patterns of cigarette and snus impact smoking initiation or smoking cessation, and concluded that snus use is associated with a reduced risk of becoming a smoker and an increased likelihood of quitting smoking. Given our prospective study design, we plan to evaluate over time how transitions between tobacco use in the STAGE cohort are associated with smoking cessation and relapse.
Findings from two other papers (Rodu et al., 2002; Stegmayr et al., 2005), based on data from the Northern Sweden component of the World Health Organization (WHO) Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases, are also supportive of our conclusions. The WHO study collected detailed tobacco use characteristics from five separate population-based surveys between 1986–2004 (Rodu et al., 2002). Conversion of our sex-specific prevalences into population prevalences shows that our findings are similar to Rodu et al. (2002), with the exception of exclusive snus use, where they found a higher prevalence in males (13% vs. about 8% in STAGE). It is possible this difference is attributable to study location, since snus consumption appears to vary by region in Sweden and is higher among males in northern Sweden (Lindstrom & Isacsson, 2002).
An additional year of follow-up of the WHO cohort was recently reported by Stegmayr et al. (2005), who found that the prevalence of cigarette smoking decreased and snus use increased between previous survey years and 2004. Similar to our report, daily snus use was more popular than cigarette smoking among males, particularly those 25–34 and 35–44 years old. Among females, the prevalence of snus use was highest in 2004 than prior survey years.
Stegmayr et al.(2005) and Ramstrom and Foulds (2006) reported that 0%–2% of Swedes are current daily dual users of cigarettes and snus. As we did not assess current daily dual use in this analysis, it was not appropriate to contrast the prevalence of “Lifetime Combination Tobacco Use” in STAGE (31.5%) with estimates of dual use from these reports. However, it is notable that such a large proportion of STAGE participants reported that they had ever used both products in their lifetime. We intend to examine this subgroup more closely in future prospective analyses where we examine their tobacco consumption and cessation patterns, as current daily dual use of cigarettes and snus could potentially reduce the impact of smoking bans on tobacco use and the likelihood for complete cessation (Gartner et al., 2007).
Sweden’s history with snus
Snus use may be more common among the younger twins of STAGE than among the older twins from the SALT study for several reasons. First, as described by Gilljam and Galanti (2003), snus has become more accessible in Sweden. Snus was first produced in Sweden in the early 19th century when it was the preferred tobacco product among working class men. Sales of snus decreased during the 20th century when cigarettes became popular, but increased following intense marketing to young men and athletes since the 1970s. In 1995, Sweden entered the European Union but was exempted from the oral smokeless tobacco sales ban. Today Swedish snus is sold only in Sweden and Norway, and is being test-marketed in the United States (where it costs less than a pack of cigarettes).
Second, snus is considered by many to be a less harmful alternative to cigarettes since it contains lower concentrations of cancer-causing tobacco-specific nitrosamines than found in other smokeless tobacco products and cigarettes (Curvall, Romert, Norlen, & Enzell, 1987; Foulds et al., 2003; Levy et al., 2004; Osterdahl, Jansson, & Paccou, 2004). Among Swedish men followed-up for 20 years, snus use was associated with increased pancreatic cancer, but not oral or lung cancer (Luo et al., 2007). The high levels of nicotine in snus may contribute to the increased risks in metabolic syndrome (Norberg, Stenlund, Lindahl, Boman, & Weinehall, 2006) and type II diabetes mellitus (Persson et al., 2000), but not all studies support this hypothesis (Eliasson, Asplund, Nasic, & Rodu, 2004). Data on the risk of circulatory disease, including cardiovascular disease and stroke, with snus use are conflicting (Asplund, 2003; Asplund, Nasic, Janlert, & Stegmayr, 2003; Bolinder, Alfredsson, Englund, & de Faire, 1994; Haglund, Eliasson, Stenbeck, & Rosen, 2007; Hergens, Ahlbom, Andersson, & Pershagen, 2005; Huhtasaari, Asplund, Lundberg, Stegmayr, & Wester, 1992; Huhtasaari, Lundberg, Eliasson, Janlert, & Asplund, 1999; Johansson, Sundquist, Qvist, & Sundquist, 2005; Lee, 2007). The Swedish population has become more aware of snus as a potential harm reduction product through increased coverage of this topic in scientific journals and in popular media.
Finally, snus may be increasing in popularity among Swedes as the anti-smoking climate becomes stronger. In 2002, the Swedish Parliament decided that drinking and dining establishments should be smoke-free; it went into law in June 2005. It is possible that some smokers turn to snus as a nicotine source when they can’t smoke. In this analysis, we observed that 40% of males reported having used cigarettes and snus in their lifetime. As we did not examine the timing of such use, we cannot reliably say whether snus use is being used as a smoking cessation aid (smoking first, then snus), as a gateway to smoking (snus first, then smoking), or as current daily dual use (cigarettes and snus together). We plan to address these important distinctions in future analyses in our prospective cohort of tobacco users.
Sex differences in tobacco use
Prior studies support that cigarette smoking is more common among Swedish females than males (Ramstrom & Foulds, 2006; Rodu et al., 2002; Stegmayr et al., 2005). Although snus use was more common among females from STAGE than the SALT study, the highest prevalence of tobacco use among females in STAGE was exclusive cigarette use. In addition, STAGE females appear to be initiating cigarette use earlier than snus use, and at a higher rate than males. As all of the females in STAGE are of child-bearing age, this is especially concerning since nicotine is a neuroteratogen, and all nicotine-containing products should be avoided especially during pregnancy (Ginzel et al., 2007).
Differences in FTND scores
We found that FTND scores were higher for males than females, and for current than former smokers. Fagerström et al. (1996) suggested that current smokers might be more nicotine dependent since the less dependent smokers succeed in quitting smoking. Termed the hardening hypothesis, this theory is currently being debated (Hughes & Brandon, 2003; Warner & Burns, 2003). Results from smoking cessation trials suggest that remaining smokers are more dependent and more difficult to treat (Irvin, Hendricks, & Brandon, 2003). However, O’Connor et al. (2006) recently reported that the number of cigarettes smoked and nicotine intake have decreased in two populations of American smokers between 1998 and 2002. The latter data do not support the hypothesis that remaining smokers are becoming more dependent on nicotine over time. In future prospective analyses we will examine how FTND scores are associated with quit attempts and successes and whether they differ by sex.
Study limitations and strengths
Limitations of the SALT study and STAGE are acknowledged. The response rate of STAGE was 47.0% and may have been due to the lengthy questionnaire. Non-participants of STAGE were more likely than participants to be male, less educated, have at least one parent born outside of Sweden, been convicted of any type of crime, and been diagnosed with a psychiatric disorder. As tobacco use is more prevalent among these groups of people, it is possible that associations observed in this study are underestimated. At this time, the generalizability of our findings is limited to the Swedish population since Swedish snus use not widely available in the rest of the world. However, with the recent introduction of Swedish-style snus into the American tobacco market, insights gained from the prevalence and patterns of tobacco use in Sweden are increasingly relevant.
All tobacco use data from the SALT study and STAGE were obtained through self-report, and use of either tobacco product at interview was not confirmed through biochemical testing. It is possible that recall bias could have affected our findings, particularly among the older Swedish twins of the SALT study. However, there are no reasons to suspect that participants would recall their uses of cigarettes and snus differently. Nor would one expect that males and females recall tobacco use in a different manner. Thus, it is unlikely that differential misclassification error affected our results within each cohort. If non-differential misclassification of tobacco use influenced our findings, they would have biased our results towards the null (Rothman & Greenland, 1998).
Strengths of the STAGE study include its population-based design with representation of a wide age range of males and females, detailed assessment of cigarette and snus use, and excellent statistical power. The most promising feature of STAGE is its prospective design. All lifetime cigarette smokers and/or snus users identified at baseline and who consented to follow-up are being re-contacted and followed-up yearly for cessation attempts (among current tobacco users), or relapses (among former tobacco users). The first follow-up began in 2006. In future analyses we will investigate how snus use impacts cigarette smoking and utilize the twin study design to delineate genetic and environmental effects of tobacco use, nicotine dependence, cessation and relapse.
Acknowledgments
This work was supported by CA085739 from the U.S. National Cancer Institute of the National Institutes of Health, and CA118412. The funding source had no involvement in study design, collection, analysis, interpretation of the data, and was not involved in the decision to submit this paper for publication. None of the authors had a competing interest that could inappropriately bias the work.
Contributor Information
Helena Furberg, Department of Genetics, University of North Carolina at Chapel Hill, NC
Paul Lichtenstein, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden
Nancy L. Pedersen, Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, and Department of Psychology, University of Southern California, Los Angeles, CA
Laura Thornton, Department of Psychiatry, University of North Carolina at Chapel Hill, NC
Cynthia M. Bulik, Department of Nutrition and Department of Psychiatry, University of North Carolina at Chapel Hill, NC
Caryn Lerman, Department of Psychiatry, Transdisciplinary Tobacco Use Research Center, University of Pennsylvania, Philadelphia, PA
Patrick F. Sullivan, Department of Genetics and Department of Psychiatry, University of North Carolina at Chapel Hill, NC.
References
- Accortt NA, Waterbor JW, Beall C, Howard G. Chronic disease mortality in a cohort of smokeless tobacco users. American Journal of Epidemiology. 2002;156:730–737. doi: 10.1093/aje/kwf106. [DOI] [PubMed] [Google Scholar]
- Allison PD. Survival analysis using the SAS system: A practical guide. Cary, NC: SAS Institute, Inc; 1995. [Google Scholar]
- Asplund K. Smokeless tobacco and cardiovascular disease. Progress in Cardiovascular Disease. 2003;45:383–394. doi: 10.1053/pcad.2003.00102. [DOI] [PubMed] [Google Scholar]
- Asplund K, Nasic S, Janlert U, Stegmayr B. Smokeless tobacco as a possible risk factor for stroke in men: A nested case-control study. Stroke. 2003;34:1754–1759. doi: 10.1161/01.STR.0000076011.02935.A1. [DOI] [PubMed] [Google Scholar]
- Bolinder G, Alfredsson L, Englund A, de Faire U. Smokeless tobacco use and increased cardiovascular mortality among Swedish construction workers. American Journal of Public Health. 1994;84:399–404. doi: 10.2105/ajph.84.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR, Oakes D. Analysis of survival data. London: Chapman and Hall; 1984. [Google Scholar]
- Curvall M, Romert L, Norlen E, Enzell CR. Mutagen levels in urine from snuff users, cigarette smokers and non tobacco users—a comparison. Mutation Research. 1987;188:105–110. doi: 10.1016/0165-1218(87)90098-x. [DOI] [PubMed] [Google Scholar]
- Eliasson M, Asplund K, Nasic S, Rodu B. Influence of smoking and snus on the prevalence and incidence of type 2 diabetes amongst men: The northern Sweden MONICA study. Journal of Internal Medicine. 2004;256:101–110. doi: 10.1111/j.1365-2796.2004.01344.x. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. Measuring degree of physical dependence to tobacco smoking with reference to individualization of treatment. Addictive Behaviors. 1978;3:235–241. doi: 10.1016/0306-4603(78)90024-2. [DOI] [PubMed] [Google Scholar]
- Fagerström KO, Kunze M, Schoberberger R, Breslau N, Hughes JR, Hurt RD, et al. Nicotine dependence versus smoking prevalence: Comparisons among countries and categories of smokers. Tobacco Control. 1996;5:52–56. doi: 10.1136/tc.5.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerström KO, Schildt EB. Should the European Union lift the ban on snus? Evidence from the Swedish experience. Addiction. 2003;98:1191–1195. doi: 10.1046/j.1360-0443.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- Foulds J, Kozlowski L. Snus—what should the public-health response be? Lancet. 2007;369:1976–1978. doi: 10.1016/S0140-6736(07)60679-5. [DOI] [PubMed] [Google Scholar]
- Foulds J, Ramstrom L, Burke M, Fagerström KO. Effect of smokeless tobacco (snus) on smoking and public health in Sweden. Tobacco Control. 2003;12:349–359. doi: 10.1136/tc.12.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H, Bulik CM, Lerman C, Lichtenstein P, Pedersen NL, Sullivan PF. Is Swedish snus associated with smoking initiation or smoking cessation? Tobacco Control. 2005;14:422–424. doi: 10.1136/tc.2005.012476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H, Lichtenstein P, Pedersen NL, Bulik C, Sullivan PF. Cigarettes and oral snuff use in Sweden: Prevalence and transitions. Addiction. 2006;101:1509–1515. doi: 10.1111/j.1360-0443.2006.01550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furberg H, Lichtenstein P, Pedersen NL, Bulik CM, Lerman C, Sullivan P. Snus use and other correlates of smoking cessation in the Swedish Twin Registry. Psychological Medicine. doi: 10.1017/S0033291707002346. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner CE, Hall WD, Chapman S, Freeman B. Should the health community promote smokeless tobacco (snus) as a harm reduction measure? PLoS Med. 2007;4:e185. doi: 10.1371/journal.pmed.0040185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilljam H, Galanti MR. Role of snus (oral moist snuff) in smoking cessation and smoking reduction in Sweden. Addiction. 2003;98:1183–1189. doi: 10.1046/j.1360-0443.2003.00379.x. [DOI] [PubMed] [Google Scholar]
- Ginzel KH, Maritz GS, Marks DF, Neuberger M, Pauly JR, Polito JR, et al. Critical review: Nicotine for the fetus, the infant and the adolescent? Journal of Health Psychology. 2007;12:215–224. doi: 10.1177/1359105307074240. [DOI] [PubMed] [Google Scholar]
- Gray N. Mixed feelings on snus. Lancet. 2005;366:966–967. doi: 10.1016/S0140-6736(05)67352-7. [DOI] [PubMed] [Google Scholar]
- Haglund B, Eliasson M, Stenbeck M, Rosen M. Is moist snuff use associated with excess risk of IHD or stroke? A longitudinal follow-up of snuff users in Sweden. Scandinavian Journal of Public Health. 2007;35:618–622. doi: 10.1080/14034940701436949. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hergens MP, Ahlbom A, Andersson T, Pershagen G. Swedish moist snuff and myocardial infarction among men. Epidemiology. 2005;16:12–16. doi: 10.1097/01.ede.0000147108.92895.ba. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Brandon TH. A softer view of hardening. Nicotine & Tobacco Research. 2003;5:961–962. doi: 10.1080/14622200310001615330. [DOI] [PubMed] [Google Scholar]
- Huhtasaari F, Asplund K, Lundberg V, Stegmayr B, Wester PO. Tobacco and myocardial infarction: Is snuff less dangerous than cigarettes? British Medical Journal. 1992;305:1252–1256. doi: 10.1136/bmj.305.6864.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtasaari F, Lundberg V, Eliasson M, Janlert U, Asplund K. Smokeless tobacco as a possible risk factor for myocardial infarction: A population-based study in middle-aged men. Journal of the American College of Cardiology. 1999;34:1784–1790. doi: 10.1016/s0735-1097(99)00409-x. [DOI] [PubMed] [Google Scholar]
- Irvin JE, Hendricks PS, Brandon TH. The increasing recalcitrance of smokers in clinical trials II: Pharmacotherapy trials. Nicotine & Tobacco Research. 2003;5:27–35. doi: 10.1080/1462220031000070534. [DOI] [PubMed] [Google Scholar]
- Johansson SE, Sundquist K, Qvist J, Sundquist J. Smokeless tobacco and coronary heart disease: A 12-year follow-up study. European Journal of Cardiovascular Prevention & Rehabilition. 2005;12:387–392. doi: 10.1097/01.hjr.0000169189.22302.99. [DOI] [PubMed] [Google Scholar]
- Lagergren J, Bergstrom R, Lindgren A, Nyren O. The role of tobacco, snuff and alcohol use in the aetiology of cancer of the oesophagus and gastric cardia. International Journal of Cancer. 2000;85:340–346. [PubMed] [Google Scholar]
- Lambe M. Tobacco control, ethics and Swedish snus. Acta Oncologica. 2004;43:3–4. doi: 10.1080/02841860310022553. [DOI] [PubMed] [Google Scholar]
- Lambe M. Swedish snus for tobacco harm reduction. Lancet. 2007;370:1206. doi: 10.1016/S0140-6736(07)61531-1. [DOI] [PubMed] [Google Scholar]
- Lee PN. Circulatory disease and smokeless tobacco in Western populations: A review of the evidence. International Journal of Epidemiology. 2007;36:789–804. doi: 10.1093/ije/dym039. [DOI] [PubMed] [Google Scholar]
- Levy DT, Mumford EA, Cummings KM, Gilpin EA, Giovino G, Hyland A, et al. The relative risks of a low-nitrosamine smokeless tobacco product compared with smoking cigarettes: Estimates of a panel of experts. Cancer Epidemiology Biomarkers & Prevention. 2004;13:2035–2042. [PubMed] [Google Scholar]
- Lewin F, Norell SE, Johansson H, Gustavsson P, Wennerberg J, Biorklund A, et al. Smoking tobacco, oral snuff, and alcohol in the etiology of squamous cell carcinoma of the head and neck: A population-based case-referent study in Sweden. Cancer. 1998;82:1367–1375. doi: 10.1002/(sici)1097-0142(19980401)82:7<1367::aid-cncr21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Liang KY, Zeger SL. Longitudinal data analysis using general linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL. The Swedish Twin Registry: A unique resource for clinical, epidemiological and genetic studies. Journal of Internal Medicine. 2002;252:184–205. doi: 10.1046/j.1365-2796.2002.01032.x. [DOI] [PubMed] [Google Scholar]
- Lichtenstein P, Sullivan PF, Cnattingius S, Gatz M, Johansson S, Carlstrom E, et al. The Swedish Twin Registry in the third millennium: An update. Twin Research & Human Genetics. 2006;9:875–882. doi: 10.1375/183242706779462444. [DOI] [PubMed] [Google Scholar]
- Lindstrom M, Isacsson SO. Smoking cessation among daily smokers, aged 45–69 years: A longitudinal study in Malmo, Sweden. Addiction. 2002;97:205–215. doi: 10.1046/j.1360-0443.2002.00036.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Ye W, Zendehdel K, Adami J, Adami HO, Boffetta P, et al. Oral use of Swedish moist snuff (snus) and risk for cancer of the mouth, lung, and pancreas in male construction workers: A retrospective cohort study. Lancet. 2007;369:2015–2020. doi: 10.1016/S0140-6736(07)60678-3. [DOI] [PubMed] [Google Scholar]
- McKee M, Gilmore A. Swedish snus for tobacco harm reduction. Lancet. 2007;370:1206. doi: 10.1016/S0140-6736(07)61530-X. [DOI] [PubMed] [Google Scholar]
- Norberg M, Stenlund H, Lindahl B, Boman K, Weinehall L. Contribution of Swedish moist snuff to the metabolic syndrome: A wolf in sheep’s clothing? Scandinavian Journal of Public Health. 2006;34:576–583. doi: 10.1080/14034940600665143. [DOI] [PubMed] [Google Scholar]
- O’Connor RJ, Giovino GA, Kozlowski LT, Shiffman S, Hyland A, Bernert JT, et al. Changes in nicotine intake and cigarette use over time in two nationally representative cross-sectional samples of smokers. American Journal of Epidemiology. 2006;164:750–759. doi: 10.1093/aje/kwj263. [DOI] [PubMed] [Google Scholar]
- Osterdahl BG, Jansson C, Paccou A. Decreased levels of tobacco-specific N-nitrosamines in moist snuff on the Swedish market. Journal of Agricultural Food Chemistry. 2004;52:5085–5088. doi: 10.1021/jf049931a. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Lichtenstein P, Svedberg P. The Swedish Twin Registry in the third millennium. Twin Research. 2002;5:427–432. doi: 10.1375/136905202320906219. [DOI] [PubMed] [Google Scholar]
- Persson PG, Carlsson S, Svanstrom L, Ostenson CG, Efendic S, Grill V. Cigarette smoking, oral moist snuff use and glucose intolerance. Journal of Internal Medicine. 2000;248:103–110. doi: 10.1046/j.1365-2796.2000.00708.x. [DOI] [PubMed] [Google Scholar]
- Ramstrom L. Snuff—an alternative nicotine delivery system. In: Ferrence R, editor. Nicotine and public health. Washington, DC: American Public Health Association; 2000. pp. 159–178. [Google Scholar]
- Ramstrom L. Patterns of use: A gate leading to smoking or a way out? Nicotine & Tobacco Research. 2003a;5:268. [Google Scholar]
- Ramstrom L. Snus: Part of the problem or part of the solution? Addiction. 2003b;98:1198–1199. doi: 10.1046/j.1360-0443.2003.00477.x. discussion 1204–1197. [DOI] [PubMed] [Google Scholar]
- Ramstrom LM, Foulds J. Role of snus in initiation and cessation of tobacco smoking in Sweden. Tobacco Control. 2006;15:210–214. doi: 10.1136/tc.2005.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodu B, Jansson C. Smokeless tobacco and oral cancer: A review of the risks and determinants. Critical Reviews in Oral Biological Medicine. 2004;15:252–263. doi: 10.1177/154411130401500502. [DOI] [PubMed] [Google Scholar]
- Rodu B, Stegmayr B, Nasic S, Asplund K. Impact of smokeless tobacco use on smoking in northern Sweden. Journal of Internal Medicine. 2002;252:398–404. doi: 10.1046/j.1365-2796.2002.01057.x. [DOI] [PubMed] [Google Scholar]
- Rodu B, Stegmayr B, Nasic S, Cole P, Asplund K. Evolving patterns of tobacco use in northern Sweden. Journal of Internal Medicine. 2003;253:660–665. doi: 10.1046/j.1365-2796.2003.01143.x. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S. Modern epidemiology. 2. Philadelphia: Lippincott-Raven; 1998. [Google Scholar]
- SAS Institute, I. SAS/STAT Software: Version 9. Cary, NC: SAS Institute, Inc; 2004. [Google Scholar]
- Savitz DA, Meyer RE, Tanzer JM, Mirvish SS, Lewin F. Public health implications of smokeless tobacco use as a harm reduction strategy. American Journal of Public Health. 2006;96:1934–1939. doi: 10.2105/AJPH.2005.075499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schildt EB, Eriksson M, Hardell L, Magnuson A. Oral snuff, smoking habits and alcohol consumption in relation to oral cancer in a Swedish case-control study. International Journal of Cancer. 1998;77:341–346. doi: 10.1002/(sici)1097-0215(19980729)77:3<341::aid-ijc6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- StatisticsSweden. Living conditions. Report No 114: Use of alcohol and tobacco. Stockholm: Author; 2007. [Google Scholar]
- Stead LF, Perera R, Bullen C, Mant D, Lancater T. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews. 2008;(1) doi: 10. 1002/14651858.CD000146.pub3. Art. No.: CD000146. [DOI] [PubMed] [Google Scholar]
- Stegmayr B, Eliasson M, Rodu B. The decline of smoking in northern Sweden. Scandinavian Journal of Public Health. 2005;33:321–324. doi: 10.1080/14034940510032301. discussion 243. [DOI] [PubMed] [Google Scholar]
- Stokes ME, Davis CS, Koch GG. Categorical data analysis using SAS. Cary, NC: SAS Institute, Inc; 1995. [Google Scholar]
- Warner KE, Burns DM. Hardening and the hard-core smoker: Concepts, evidence, and implications. Nicotine & Tobacco Research. 2003;5:37–48. doi: 10.1080/1462220021000060428. [DOI] [PubMed] [Google Scholar]
- Ye W, Ekström AM, Hansson LE, Bergström R, Nyrén O. Tobacco, alcohol and the risk of gastric cancer by sub-site and histologic type. International Journal of Cancer. 1999;83:223–229. doi: 10.1002/(sici)1097-0215(19991008)83:2<223::aid-ijc13>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]


