Abstract
Polyethylene glycol-phospholipid micelles form a major class of nanocarriers in pharmacy and medicine due to proven capability in drug solubilization, sustained drug release, and evidence for targeted drug delivery in vivo. In this report, we have prepared micelles composed of PEG-block-poly(N-hexyl stearate L-aspartamide) (PEG-b-PHSA), having nine stearic acid side chains and have studied their stability in the presence of serum proteins by Förster resonance energy transfer (FRET) experiments. In the presence of serum albumin, alpha and beta globulins, or gamma globulins, there are minimal changes in FRET over two hours in vitro, indicating integrity of PEG-b-PHSA micelles. In contrast, 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-N-[amino(polyethylene glycol)-5000] (PEG-DSPE) micelles lose FRET over two hours in vitro, especially in the presence of alpha and beta globulins, indicating the disruption of PEG-DSPE micelles and leakage of fluorescent probes. Owing to the aliphatic nature of DSPE and PHSA, both PEG-b-PHSA and PEG-DSPE micelles efficiently solubilize amphotericin B (AmB), a poorly water-soluble antifungal agent used to combat systemic mycoses. However, only PEG-b-PHSA micelles gradually liberate AmB in the presence of alpha and beta globulins, based on time-dependent changes in self-aggregation state of AmB, monitored by UV/VIS spectroscopy. PEG-b-PHSA micelles are remarkably stable in the presence of serum proteins and a more stable alternative for poorly water soluble drugs, which have been solubilized by PEG-DSPE micelles.
Keywords: Amphotericin B, Drug solubilization, FRET, micelle stability, PEG-DSPE, Polymeric micelle
Polymeric micelles have emerged as a major class of nano drug-carriers in pharmacy and medicine due to proven functionality in drug solubilization, controlled drug release, and prospects for drug targeting. Several polymeric micelles have entered clinical trials, primarily in the cancer arena, attesting their promise and safety.1 For drug targeting, polymeric micelles must retain their integrity in blood, entrapping drugs inside their core regions long enough to access targeted tissues, for example through the enhanced permeability and retention effect at solid tumors. However, while polymeric micelles are thermodynamically more stable than surfactant micelles (very low (< μM) critical micelle concentrations (CMCs)), there are questions regarding their kinetic stability in blood and whether they can really function as long-circulating nano drug-carriers for drug targeting. Recent in vitro and in vivo studies have shown that poly(ethylene glycol-block-poly(caprolactone) (PEG-b-PCL) and poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) micelles lose their integrity in serum mimicking conditions,2, 3 lowering their appeal for drug targeting. There are however also reports providing evidence of prolonged circulation of PEG-b-PCL micelles in mice over 24 hours.4 PEG-phospholipid micelles have circulation half-lives of 1 to 2 hours, sufficient to increase the tumor accumulation of paclitaxel.5 Nevertheless, it is reasonable to suspect that enhancing the physical stability of polymeric micelles by increasing hydrophobicity or core cross-linking would increase circulation half-lives and the likelihood of successful drug targeting.
In this work, we show that PEG-block-poly(N-hexyl stearate L-aspartamide) (PEG-b-PHSA) micelles are uniquely stable in the presence of serum proteins, contrasting with the stability of 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-N-[amino(polyethylene glycol)-5000] (PEG-DSPE) micelles, which is readily compromised. While both PEG-b-PHSA and PEG-DSPE each contain stearic acid as a hydrophobic moiety, we can control the level of stearate substitution on PEG-b-PHSA, allowing for enhanced hydrophobicity relative to PEG-DSPE, which has 2 stearic acid moieties. Figure 1 depicts the chemical structures of PEG-b-PHSA and PEG-DSPE. The synthesis of PEG-b-PHSA has been described elsewhere.6–9 Details are also provided in the Supporting Information. The core forming block of PEG-b-PHSA consists of 11 repeating units, 9 of which have been substituted with stearic acid, and the molecular weight of PEG is 12,000 g/mol. Previous reports have looked at the effects of PHSA block length and degree of stearic acid substitution on the thermodynamic stability of PEG-b-PHSA micelles.9 For this study, a high number of stearic acid side chains on a relatively short core forming PHSA block of only 11 repeating units has been achieved, aiming to attain maximum core hydrophobicity.
Figure 1.

(a) Chemical structure of methoxy poly(ethylene glycol)-block-poly(N-hexyl stearate L-aspartamide) (PEG-b-PHSA). As depicted, approximately 9 L-Asp residues have been modified with stearic acid, determined by 1H NMR spectroscopy. (b) Chemical structure of 1,2-distearoyl-sn-glycero-3-phospho-ethanolamine-N-[amino(polyethylene glycol)-5000] (PEG-DSPE).
The physical characteristics of PEG-b-PHSA and PEG-DSPE micelles are summarized in Table 1. Both PEG-b-PHSA and PEG-DSPE micelles have been prepared using a solvent evaporation method,10, 11 which is similar to one that is used to prepare conventional liposomes. Dynamic light scattering (DLS) measurements show that PEG-b-PHSA micelles have a hydrodynamic diameter of 66.0±5.5 nm with a polydispersity index (PDI) of 0.17±0.08 (n=3). PEG-DSPE micelles are 8.2±5.0 nm in diameter with a PDI of 0.26±0.04 (n=3), consistent with prior studies.5 Fluorescence studies using pyrene, according to methods reported elsewhere,12 show that the CMCs of PEG-b-PHSA micelles are lower than PEG-DSPE micelles (272 versus 603 nM), indicating greater thermodynamic stability. The I1/I3 pyrene peak ratio upon partitioning of the fluorescent probe into micelle cores is a measure of core polarity. Both PEG-b-PHSA and PEG-DSPE micelles show low core polarity consistent with the aliphatic nature of stearic acid. On the other hand, PEG-b-PHSA micelles seem to have more rigid cores (high microviscosity), based upon a higher monomer to intramolecular excimer intensity ratio (IM/IE) of 1,3-(1,1′-dipyrenyl)-propane (P3P)); intramolecular excimer formation occurs within the lifetime of the excited state of pyrene on P3P due to a free rotation of carbon bonds, favored in a low viscosity region, as has been described previously.11, 13 The higher core viscosity and the lower CMC of PEG-b-PHSA micelles likely reflect the higher number of stearic acids on PEG-b-PHSA versus PEG-DSPE.
Table 1.
Physical properties of PEG-b-PHSA and PEG-DSPE micelles
| Polymer | Mn (g/mol) | Dh (nm)c | PDId | CMC (nM)e | I1/I3 | IM/IEf | AmB loading efficiencyg |
|---|---|---|---|---|---|---|---|
| PEG-b-PHSA | 16,700a | 66.0±5.5 | 0.17±0.08 | 272 | 1.08 | 17.2 | 96.6±0.6% |
| PEG-DSPE | 6,081b | 8.2±5.0 | 0.26±0.04 | 603 | 1.14 | 4.92 | 93.9±2.2% |
Based on 1H NMR spectroscopy
From manufacturer
Volume average hydrodynamic diameter ± standard deviation from 3 micelle batches
Polydispersity index ± standard deviation from 3 micelle batches as an indication of relative variance in Z-average size assuming a Gaussian distribution
Determined from pyrene fluorescence emission (ratio of intensities of peak 1 to peak 3) at 22 °C
Determined from P3P intramolecular monomer to excimer emission at 22 ºC
Based on reverse-phase HPLC analysis
To compare the integrity of PEG-b-PHSA and PEG-DSPE micelles, Förster resonance energy transfer (FRET) experiments have been employed with the lipophilic fluorescent energy donor probe (DiOC18(3)) and a lipophilic fluorescent acceptor probe (DiIC18(3)). FRET offers a very precise means to measure changes in the integrity of polymeric micelles because energy transfer efficiency is inversely proportional to r6, where r is the distance between donor and acceptor probes. The excitation wavelength of DiOC18(3) is 484 nm, and it emits fluorescence with maximal intensity at 501 nm. DiIC18(3) is excited at 501 nm and emits fluorescence with a maximal intensity at 565 nm. When the donor probe (DiOC18(3)) is excited and in close proximity to the acceptor probe (DiIC18(3)) FRET occurs. With polymeric micelles core loaded with both DiOC18(3) and DiIC18(3), FRET occurs, and the fluorescence emission from DiIC18(3) can be detected. On the other hand, when the distance between DiOC18(3) and DiIC18(3) increases, there is an increase in the fluorescence emission of DiOC18(3) and a decrease in the fluorescence emission of DiIC18(3) (565 nm).2 If micelles containing only DiOC18(3) are mixed with micelles containing only DiIC18(3), upon excitation at 484 nm, only emission of DiOC18(3) at 501 nm will be detected, unless probe diffusion between micelles occurs. To determine if there is exchange of fluorescent probes between individual micelles during storage at room temperature, a mixture of PEG-b-PHSA micelles containing DiOC18(3) and PEG-b-PHSA micelles containing DiIC18(3) have been incubated for 24 hours in water. The same has been done for PEG-DSPE micelles. Over 24 hours, there is no gain in FRET for PEG-b-PHSA micelles but there is a slight increase of FRET for PEG-DSPE micelles (data not shown). The rigid core environment of the PEG-b-PHSA micelles may prevent diffusion and exchange of probes between micelles while the less rigid core of PEG-DSPE micelles allows some diffusion to occur.
To determine the effects of serum proteins on micellar integrity, time-dependent changes in FRET have been monitored in the presence of serum albumin, alpha and beta globulins, or gamma globulins. When PEG-b-PHSA micelles or PEG-DSPE micelles loaded with DiOC18(3) and DiIC18(3) are exposed to average human blood levels of either serum albumin (40 mg/mL), alpha and beta globulins (14 mg/mL), or gamma globulins (10 mg/mL), differences in energy transfer efficiency are observable between micelles. Figure 2 shows example fluorescence spectra of PEG-DSPE (Figure 2a) and PEG-b-PHSA (Figure 2b) micelles containing DiOC18(3) and DiIC18(3) in the presence alpha and beta globulins over 2 hours at 22 °C. In the case of PEG-DSPE micelles, the fluorescence spectrum changes noticeably: a loss of intensity at 565 nm and a gain fluorescence intensity at 501 nm.
Figure 2.

Time resolved FRET emission spectra of PEG-DSPE micelles (a) and PEG-b-PHSA micelles (b) containing DiOC18(3) and DiIC18(3) in the presence of 14 mg/mL alpha and beta globulins. Spectra for t = 0 min (—), t = 60 min (···), t = 120 min (–··–) are depicted.
We define the FRET ratio as IA/(ID + IA), where IA and ID are the peak fluorescence intensities of DiIC18(3) and DiOC18(3) at 565 and 501 nm, respectively. Figure 3 shows that for PEG-DSPE micelles, IA/(ID + IA) starts at 0.83 and becomes lower over 2 hours in an exponential manner, and the rate is highest for alpha and beta globulins, followed by serum albumin and gamma globulins. Cheng et al., have also shown that PEG-b-PLA micelles containing DiIC18(3) and DiOC18(3) lose FRET most rapidly in the presence of alpha and beta globulins, consistent with the release of DiIC18(3) and DiOC18(3) from PEG-b-PLA micelles due to a loss of the integrity of these polymeric micelles.2 Similarly, PEG-DSPE micelles lose their integrity in the presence of serum proteins, most noticeably with alpha and beta globulins, according to a loss of FRET. We speculate that this disruptive action of serum proteins on PEG-DSPE micelles, particularly alpha and beta globulins is a major factor in the 1 to 2 hour circulation half-lives of PEG-phospholipid micelles in vivo, contributing to a drug loss in plasma circulation and a reduction accumulation at target tissue.
Figure 3.
Comparative FRET ratio (IA/(IA+ID)) of PEG-b-PHSA micelles (squares) and PEG-DSPE micelles (triangles) in the presence of: 40 mg/mL serum albumin (a), 14 mg/mL alpha and beta globulins (b), and 10 mg/mL gamma globulins (c). IA/(IA+ID) ratios of DiOC18(3) and DiIC18(3) were monitored over 2 hours.
For PEG-b-PHSA micelles containing DiIC18(3) and DiOC18(3), by contrast, there are only minor changes in the fluorescence spectrum and the IA/(ID + IA) over 2 hours (Figures 3), regardless of the serum protein. For PEG-b-PHSA micelles, IA/(ID + IA) starts at 0.90 and goes slightly lower with no significant differences between serum albumin, alpha and beta globulins, or gamma globulins. Thus, PEG-b-PHSA micelles retain DiIC18(3) and DiOC18(3) in their cores in the presence of serum proteins, contrasting with the behavior of PEG-b-PLA and PEG-DSPE micelles. In comparison with PEG-DSPE micelles, this enhanced stability of PEG-b-PHSA micelles in the presence of serum proteins is probably due to the added number of stearic acid side chains (9 versus 2), which enhances the hydrophobicity of the core-forming block, which in turn lowers the CMC value and increases core viscosity. Notably, it is straightforward to adjust the degree of stearic acid substitution on PEG-b-PHSA, allowing for adjustable stability and perhaps control over the pharmacokinetics of PEG-b-PHSA micelles. In summary, PEG-b-PHSA micelles are stable in the presence of serum proteins according to FRET analysis, and we expect to see major pharmacokinetic differences between PEG-b-PHSA and PEG-DSPE micelles.
To further show that PEG-b-PHSA micelles can retain their integrity in serum mimicking conditions, PEG-b-PHSA micelles have been incubated at 37 °C in alpha and beta globulins, as these proteins appear to be the most destabilizing to micelles. We did not repeat this experiment for PEG-DSPE micelles as they already show a lack of stability at room temperature in the presence of alpha and beta globulins. Figure 4 shows that at physiological temperature, PEG-b-PHSA micelles still do not lose any FRET efficiency at 50 mg/L. When diluted below their CMC (2.5 mg/L), PEG-b-PHSA micelles do appear to lose their integrity at 37 °C under aqueous conditions. This low concentration could not be used in the presence of alpha and beta globulins, as the FRET signal is too weak and heavily overshadowed by fluorescence caused by the proteins. The loss of integrity at such low concentrations is however of little concern, as the very low CMC of PEG-b-PHSA micelles indicate that blood polymer concentrations will stay above the CMC for a while in circulation.
Figure 4.

Comparative FRET ratio (IA/(IA+ID)) of PEG-b-PHSA micelles under different incubation conditions. 50 mg/L polymer solution at 37 °C in water (squares), 50 mg/L polymer solution at 37 °C in alpha and beta globulins (triangles), 2.5 mg/L polymer solution (below CMC) at 22 °C in water (diamonds), 2.5 mg/L polymer solution (below CMC) at 37 °C in water (circles).
While DiIC18(3) and DiOC18(3) can be considered to be poorly water-soluble model drugs, it is important to show that PEG-b-PHSA micelles can be stable in the presence of serum proteins after the incorporation of a poorly water-soluble drug at levels that are therapeutically relevant, as opposed to the tracer levels used for FRET experiments. AmB is a polyene macrolide that is the drug of choice for the treatment of life-threatening systemic fungal diseases, e.g. candidiasis, and a good candidate for polymeric micelle mediated drug delivery, owing to its poor water solubility (ca. 1.0 mg/L) and dose-limiting renal toxicity. PEG-b-PHSA and PEG-DSPE micelles efficiently solubilize AmB in water (Table 1), reaching AmB levels of ca. 1.0 mg/mL (data not shown). AmB is a heptaene chromophore, and its UV/VIS absorption spectrum depends on its self-aggregation state.14 Thus, we have monitored the time resolved UV/VIS absorption spectrum of AmB loaded in PEG-b-PHSA and PEG-DSPE micelles at a 2:1 polymer:AmB molar ratio in the presence of serum proteins as a measure of drug release, informed from the earlier FRET experiments. It must be noted that at this ratio, the concentration of PEG-b-PHSA and PEG-DSPE is above their respective CMCs. This experiment has been done at room temperature under constant stirring on a CARY 50 BIO (Varian, Palo Alto, CA, USA), fitted with a fiber optic dip probe coupler.
In the presence of serum albumin, AmB at 20 mg/L exists initially in a self-aggregated state based on a broad peak at 340 nm, but quickly deaggregates based on the spectral features of monomeric AmB at 368, 392, and 418 nm (Figure 5a). On the other hand, AmB exists in a self-aggregated state, i.e. soluble aggregates, in the presence of alpha and beta globulins, or gamma globulins over ca. 2 hours (Figures 5b and 5c). At a 2:1 polymer:AmB ratio in PEG-DSPE micelles, AmB appears to exist in a monomeric state based on its UV/VIS absorption spectrum at time 0 minutes upon addition to protein solutions, noting the absence of a broad peak at 340 nm and the characteristic peaks of monomeric AmB at 367, 387, and 416 nm (Figure 6).
Figure 5.

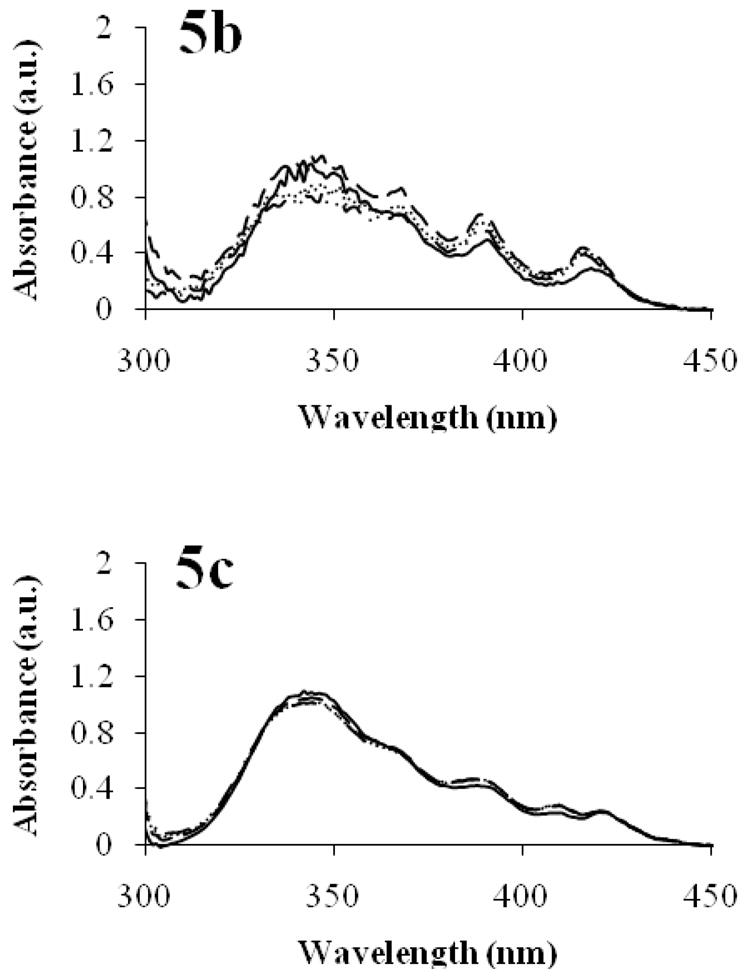
Effects of serum proteins on the UV-absorbance spectrum of AmB. AmB in 40 mg/mL serum albumin (a), AmB in 14 mg/mL alpha and beta globulins (b), and AmB in 10 mg/mL gamma globulins (c). Spectra for t = 0 min (—), t = 15 min (---), t = 60 min (···), t = 120 min (–··–) are depicted. AmB concentration was 20 mg/L.
Figure 6.
Effects of serum proteins on the aggregation state of AmB solubilized by PEG-DSPE micelles. PEG-DSPE/AmB micelles in 40 mg/mL serum albumin (a), PEG-DSPE/AmB micelles in 14 mg/mL alpha and beta globulins (b), and PEG-DSPE/AmB micelles in 10 mg/mL gamma globulins (c). Spectra for t = 0 min (—), t = 15 min (---), t = 60 min (···), t = 120 min (–··–) are depicted. AmB concentration was 20 mg/L.
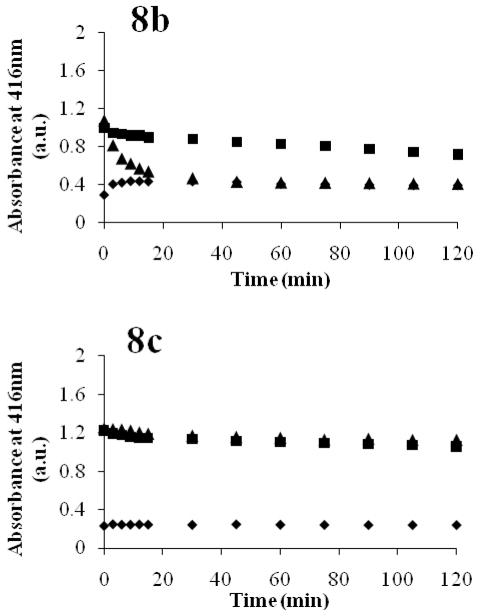
In the presence of serum albumin or gamma globulins, the spectral features of AmB in PEG-DSPE micelles remain largely unchanged. There is a slight increase in monomeric peak intensities of AmB in PEG-DSPE micelles in the presence of serum albumin. As serum albumin is capable of deaggregating AmB,15 the monomeric peak increase indicates dissociation of AmB from PEG-DSPE micelles. This effect of serum albumin on AmB loaded PEG-DSPE micelles has been shown previously.10 The spectral features of AmB in PEG-DSPE micelles in the presence of alpha and beta globulins change dramatically, quickly adopting the spectral features of self-aggregated AmB, characteristic of AmB in the presence of alpha and beta globulins (Figure 6b). By monitoring the absorbance of AmB at the peak around 416 nm, we can see that AmB solubilized by PEG-DSPE micelles aggregates within 15 minutes of exposure to alpha and beta globulins (Figure 8). Thus, PEG-DSPE micelles quickly release AmB in the presence of alpha and beta globulins and slowly release AmB in the presence of serum albumin. These findings are fairly consistent with the earlier FRET experiments, which noted the same trend in the stability of PEG-DSPE micelles in the presence of serum albumin and alpha and beta globulins. AmB loaded PEG-DSPE micelles do not however appear to be disrupted by gamma globulins, while the FRET study does indicate an effect of gamma globulins on PEG-DSPE integrity. It is speculated that this difference in experimental outcome of AmB loaded versus FRET probe loaded PEG-DSPE micelles may be due to the proportionally greater amount of hydrophobic AmB in the micellar core, compared to FRET probe amounts used. This greater hydrophobic core content may be helping to stabilize the PEG-DSPE micelles.
Figure 8.

Comparative UV Absorbance at 416 nm of free AmB (diamonds), PEG-DSPE/AmB micelles (triangles), and PEG-b-PHSA/AmB micelles (squares) in the presence of: 40 mg/mL serum albumin (a), 14 mg/mL alpha and beta globulins (b), and 10 mg/mL gamma globulins (c). Absorbance changes of AmB were monitored over 2 hours.
For AmB solubilized by PEG-b-PHSA micelles at a 2:1 ratio, there are only minor changes in its spectral features in the presence of serum proteins (Figure 7). AmB exists in a monomeric state and stays that way, indicating that the drug stays predominately inside PEG-b-PHSA micelles for 2 hours as opposed to being released and adopting a self-aggregated state in the presence of alpha and beta globulins. Similarly, there are only minor changes in the UV/VIS spectra of AmB in the presence of serum albumin or gamma globulins, indicating that there is little release of AmB from PEG-b-PHSA micelles over 2 hours. While this experiment has serum mimicking conditions, that is, the presence of major serum proteins, we note one limitation that the experiment is done above their respective CMCs. Thus, this experiment does not fully mirror in vivo conditions where at some time point the concentrations of PEG-b-PHSA and PEG-DSPE micelles will fall below their respective CMCs. Nonetheless, the results are encouraging and suggest sustained release of AmB from PEG-b-PHSA micelles in the presence of serum proteins instead of dose dumping.
Figure 7.
Effects of serum proteins on the aggregation state of AmB solubilized by PEG-b-PHSA micelles. PEG-b-PHSA/AmB micelles in 40 mg/mL serum albumin (a), PEG-b-PHSA/AmB micelles in 14 mg/mL alpha and beta globulins (b), and PEG-b-PHSA/AmB micelles in 10 mg/mL gamma globulins (c). Spectra for t = 0 min (—), t = 15 min (---), t = 60 min (···), t = 120 min (–··–) are depicted. AmB concentration was 20 mg/L.
In summary, photophysical studies on PEG-b-PHSA micelles suggest that they are remarkably stable in the presence of serum proteins. Minimal changes in FRET occur for PEG-b-PHSA micelles in the presence of serum proteins, even in the presence of alpha and beta globulins, which can disrupt PEG-b-PLA and PEG-DSPE micelles in vitro 2 and presumably in vivo. Even after the incorporation of AmB at levels required for antifungal drug therapy, PEG-b-PHSA micelles are stable, evidenced from the minimal changes observed in the UV/VIS spectrum of AmB in the presence of serum proteins. By contrast, AmB incorporated in PEG-DSPE micelles quickly takes on the spectral features of self-aggregated species in the presence of alpha and beta globulins, indicating rapid drug release. Thus, while both PEG-b-PHSA and PEG-DSPE micelles can solubilize AmB in water in a monomeric state, only PEG-b-PHSA micelles are fairly stable in the presence of serum proteins for 2 hours, pointing to prospects as a long-circulating nanocarrier for poorly water soluble drugs with a circulation half-life greater than 1 to 2 hours, determined for PEG-DSPE micelles.5 We hypothesize that PEG-b-PHSA micelles will gradually liberate AmB in vivo in a monomeric state, reducing its renal toxicity by sustained drug delivery as well the action of monomeric AmB, which is selective for fungal cell membranes over mammalian cell membranes.16 We also speculate that aliphatic drugs solubilized by PEG-phospholipid micelles can be solubilized by PEG-b-PHSA micelles due to their aliphatic nature of their cores, raising prospects for prolonged circulation in blood and progress in targeted drug delivery.
Supplementary Material
Acknowledgments
We thank Gary Girdaukas for his help with the fluorescence experiments. We thank Dr. Masayuki Yokoyama at Jikei University School of Medicine, Tokyo, Japan for the gift of P3P. We thank Dr. Melgardt De Villiers and Dr. Adam Alani at the School of Pharmacy, University of Wisconsin for thoughtful suggestions on this manuscript. Fluorescence spectra were obtained in the Analytical Instrumentation Center of the School of Pharmacy, University of Wisconsin – Madison. We acknowledge financial support from NIH (R01 AI-43346).
Footnotes
Supporting Information Available
Details of polymer synthesis and experimental methods. This information is available free of charge via the Internet at http://pubs.acs.org/.
References
- 1.Matsumura Y, Kataoka K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009;100:572–579. doi: 10.1111/j.1349-7006.2009.01103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen H, Kim S, He W, Wang H, Low PS, Park K, Cheng JX. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo Forster resonance energy transfer imaging. Langmuir. 2008;24:5213–5217. doi: 10.1021/la703570m. [DOI] [PubMed] [Google Scholar]
- 3.Savic R, Azzam T, Eisenberg A, Maysinger D. Assessment of the integrity of poly(caprolactone)-b-poly(ethylene oxide) micelles under biological conditions: A fluorogenic-based approach. Langmuir. 2006;22:3570–3578. doi: 10.1021/la0531998. [DOI] [PubMed] [Google Scholar]
- 4.Liu JB, Zeng FQ, Allen C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur J Pharm and Biopharm. 2007;65:309–319. doi: 10.1016/j.ejpb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliver Rev. 2004;56:1273–1289. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Daly WH, Poche D. The preapration of N-carboxyanhydrides of alpha-amino-acids using bis(trichloromethyl)carbonate. Tetrahedron Lett. 1988;29:5859–5862. [Google Scholar]
- 7.Adams ML, Kwon GS. The effects of acyl chain length on the micelle properties of poly(ethylene oxide)-block-poly(N-hexyl-L-aspartamide)-acyl conjugates. J Biomat Sci-Polym E. 2002;13:991–1006. doi: 10.1163/156856202760319144. [DOI] [PubMed] [Google Scholar]
- 8.Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K. Preparation of micelle-forming polymer drug conjugates. Bioconjugate Chem. 1992;3:295–301. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 9.Lavasanifar A, Samuel J, Kwon GS. The effect of fatty acid substitution on the in vitro release of amphotericin B from micelles composed of poly(ethylene oxide)-block-poly(N-hexyl stearate-L-aspartamide) J Control Release. 2002;79:165–172. doi: 10.1016/s0168-3659(01)00537-5. [DOI] [PubMed] [Google Scholar]
- 10.Vakil R, Kwon GS. Effect of cholesterol on the release of amphotericin B from PEG-phospholipid micelles. Mol Pharm. 2008;5:98–104. doi: 10.1021/mp700081v. [DOI] [PubMed] [Google Scholar]
- 11.Lavasanifar A, Samuel J, Kwon GS. The effect of alkyl core structure on micellar properties of poly(ethylene oxide)-block-poly(L-aspartamide) derivatives. Colloid Surfaces B. 2001;22:115–126. doi: 10.1016/s0927-7765(01)00147-3. [DOI] [PubMed] [Google Scholar]
- 12.Wolszczak M, Miller J. Characterization of non-ionic surfactant aggregates by fluorometric techniques. J Photoch Photobio A. 2002;147:45–54. [Google Scholar]
- 13.Winnik FM. Photophysics of preassociated Pyrenes in Aqueous Polymer-Solutions and in other Organized Media. Chem Rev. 1993;93:587–614. [Google Scholar]
- 14.Lavasanifar A, Samuel J, Sattari S, Kwon GS. Block copolymer micelles for the encapsulation and delivery of amphotericin B. Pharmaceut Res. 2002;19:418–422. doi: 10.1023/a:1015127225021. [DOI] [PubMed] [Google Scholar]
- 15.Aramwit P, Yu BG, Lavasanifar A, Samuel J, Kwon GS. The effect of serum albumin on the aggregation state and toxicity of amphotericin B. J Pharm Sci. 2000;89:1589–1593. doi: 10.1002/1520-6017(200012)89:12<1589::aid-jps10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Bolard J, Legrand P, Heitz F, Cybulska B. One-sided action of amphotericin B on cholesterol-containing membranes is determined by its self-association in the medium. Biochemistry. 1991;30:5707–15. doi: 10.1021/bi00237a011. [DOI] [PubMed] [Google Scholar]
- 17.Vakil R, Kwon GS. Poly(ethylene glycol)-b-poly(epsilon-caprolactone) and PEG-phospholipid form stable mixed micelles in aqueous media. Langmuir. 2006;22:9723–9729. doi: 10.1021/la061408y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.