Abstract
A family history of colorectal cancer may increase colorectal cancer risk by influencing adenoma growth or enhancing the formation of new lesions. Data of men from the prospective Health Professionals Follow-Up Study who underwent an endoscopy between 1986 and 2004 were used to evaluate whether a family history of colorectal cancer is associated with adenoma multiplicity or advanced adenoma stage (≥1cm, histology with villous component or carcinoma in situ). 21.4% of the 3,881 adenoma patients and 13.9% of the 24,959 adenoma-free men had a first-degree relative with colorectal cancer. 1,496 men were classified as having advanced and 1,507 as having non-advanced adenomas. 622 men had multiple and 1,985 single adenomas in the distal colon and rectum. A family history of colorectal cancer was similarly associated with advanced and non-advanced adenomas [multivariable odds ratio (OR) (95% confidence interval): advanced versus non-advanced, 0.98 (0.82–1.17), advanced versus adenoma-free: 1.67 (1.47–1.91), non-advanced versus adenoma-free: 1.70 (1.49–1.94)], although potential differences according to adenoma location were seen. A family history of colorectal cancer was more strongly associated with multiple distally located adenomas [odds ratio (95% confidence interval): multiple versus single, 1.35 (1.09–1.68), multiple versus no distally located adenomas: 2.02 (1.67–2.44), single versus no distally located adenomas: 1.49 (1.32–1.68)]. The number of adenomas was also positively associated with a family history of colorectal cancer. Our findings suggest that at the population level heritable factors may be more important in earlier stages of adenoma formation than at stages of adenoma advancement for at least distally located adenomas.
Keywords: family history, cohort study, colorectal neoplasm, colorectal adenomas, colorectal cancer
Introduction
First-degree relatives of individuals with colorectal cancer are approximately twice as likely to get diagnosed with colorectal cancer or their precursor lesions adenomatous polyps as people with unaffected relatives1, 2. The risk of colorectal cancer is four times higher for people who have more than one such relative compared with those without one1, 2. A study on twins suggested that about 35 percent of all colorectal cancer cases can be attributed to heritable factors3. Thus considering that well understood familial syndromes such as the familial adenomatous polyposis (FAP) or hereditary nonpolyposis colorectal cancer syndrome (HPNCC) account for less than five percent of all colorectal cancers4, genetic factors must also be involved in sporadic colorectal carcinogenesis.
There are various ways by which a family history of colorectal cancer may affect colorectal cancer risk. A family history of colorectal cancer may convey a genetic susceptibility that enhances the formation of new lesions or affects the transition from adenomas to carcinomas. Shared behavioral risk factors have also been proposed to underlie part of the association between a family history of colorectal cancer and risk of colorectal neoplasm.
Indications for effects of heritable factors on adenoma growth were found in a study in which small polyps were left in situ for three years before removal5: net adenoma growth was observed in nine out of 14 (64%) patients with a family history of colorectal cancer but only in 22 out of 73 (30%) of the patients without such a family history. Findings from a case-control study on 362 patients suggested a stronger positive association of family history with large adenomas (odds ratio (OR)=2.1, 95% confidence interval (CI)=1.3–3.4) than with small adenomas (OR=1.2, 95% CI=0.7–2.2)6. Although the same impression can be obtained from smaller studies7–10, a pooled analyses on 518 adenoma patients observed a stronger association between a family history of colorectal cancer and adenomas with no, mild, or moderate dysplasia (OR=1.5, 95% CI=1.1–2.1) than with adenomas with severe dysplasia, carcinoma in situ, or intramucosal carcinoma (OR=1.0, 95% CI=0.6–1.9). However, the difference between these associations was not statistically significant11.
The influence of genetic factors on adenoma multiplicity is clearly visible in patients with inherited polyposis syndromes4. Responsible genes such as Adenomatous Polyposis Coli (APC) or human MutY homologue (MYH) could have a more subtle influence in sporadic carcinogenesis4. One study noted a positive association between a sporadic family history of colorectal cancer and adenoma multiplicity6.
We examined associations between family history of colorectal cancer, adenoma multiplicity and adenoma advancement in a large prospective cohort study, which allowed for adjustment for known behavioral risk factors for colorectal neoplasia. Evidence regarding such associations at the population level may yield clues to the search of genetic factors involved in the development of sporadic colorectal adenomas and cancer.
Material and Methods
STUDY POPULATION
The current study comprises a subset of men of the Health Professionals Follow-Up Study (HPFS): an ongoing prospective study among 51,529 male US health professionals who responded to a mailed questionnaire in 1986 when they were between 40 and 75 years old. The Human Subjects Committee of the Harvard School of Public Health approved the HPFS.
At enrolment in 1986, and every two years thereafter, the cohort members were requested to fill out follow-up questionnaires to update information on various risk factors and to identify newly professionally diagnosed cases of various diseases. In 1986, 1990, 1994, 1998 and 2002, participants also completed a semi-quantitative food-frequency questionnaire. Questionnaires were mailed up to four times to non-responders. At the time of the 2002 questionnaire 37,431 men were alive and still participating.
Only men who completed the 1986 questionnaire and who underwent colonoscopy or sigmoidoscopy between 1986 and 2004 were eligible for the present analyses. Participants who reported to have had colorectal polyps, cancer (except non-melanoma skin cancer), ulcerative colitis or Crohn’s disease before 1986 were excluded. We also excluded cohort members who reported implausible caloric intakes, i.e. lower than 800 kcal/day or greater than 4,200 kcal/day) as well as those who left 70 more of the items blank on the food-frequency questionnaire. This resulted in a base population of 29,120 men.
CASE ASCERTAINMENT
For each man who reported to have had an adenoma on a follow-up questionnaire for the first time, we asked for permission to request and review relevant medical records. A study investigator, who was blinded to exposure status, reviewed endoscopy and pathology reports. Only when the self-reported diagnosis was confirmed by a histopathological report, case status was assigned. The self-report of a negative endoscopy was reliable; a review of the medical records obtained from a random sample of 200 patients who reported a negative endoscopic result confirmed the absence of adenomas in all cases.
Adenomas in the cecum, ascending colon, hepatic flexure, transverse colon or splenic flexure were classified as being in the proximal colon. Adenomas in the descending or sigmoid colon were classified as being in the distal colon, and adenomas in the rectum or at the rectosigmoid junction were classified as rectal.
For diagnoses up to 1990, the number of adenomas, the size of the largest adenoma as assessed endoscopically and pathologically, and location of the most proximal adenoma were recorded. For later diagnoses, number and size of the largest adenoma according to endoscopy and pathology reports were recorded for distal, proximal and rectal adenomas separately. In most years, only the most severe histological subtype of adenoma (in ascending order: tubular, tubulovillous, villous, carcinoma in situ) was registered. From 2002 onwards the most severe histopathological adenoma subtype was recorded for each subsite separately.
Between 1986 and 2004, 3,920 of the participants were diagnosed with adenomas. For classification purposes, we used the adenoma size according to the endoscopy report, but data from the pathology report were used if such information was lacking. Men having at least one adenoma ≥1 cm or with a (tubulo)villous structure or carcinoma in situ were classified as having advanced adenomas (n=1,515); men having only adenomas that were smaller than 1 cm and with no mention of a villous or serrated structure were classified as having non-advanced adenomas (n=1,518). No information on size and histopathology was present in the pathology and endoscopy reports of 638 adenomas, and the sizes of 231 tubular and 18 serrated adenomas were unknown; these adenomas were not classified as being either advanced or non-advanced in the main analyses. Because not all men underwent colonoscopy, the classification according to adenoma multiplicity was based on distal and rectal adenoma only (which we collectedly describe as ‘distally located’), under the assumption of a single adenoma where the number of adenomas was not mentioned in the endoscopy and pathology reports (single distally located adenomas: n=2,003, multiple distally located adenomas: n=629, no distally located adenomas at all: n=26,445). The adenoma location was unknown for 43, and these adenoma patients were excluded from the analyses on multiplicity.
ASSESSMENT OF FAMILY HISTORY OF COLORECTAL CANCER
The 1986 questionnaire included questions on the diagnosis of colorectal cancer and corresponding age (before age 50, age 50 to 59, age 60 to 69, age 70+, age unknown) in the father and mother separately. In 1990 and 1992 the men were asked whether any of the siblings had colorectal cancer, and this was also asked for the father and the mother. The information on the presence or absence of colorectal cancer in the family was updated in 1996 when a question on age of first diagnosis was included; the question referred to any parent, sibling and an additional sibling separately and the same categories were used as in 1986. Later questionnaires did not contain a question on family history of colorectal cancer. A report of colorectal cancer in first-degree relatives appears to be reliable12, 13. Nonetheless, we excluded 250 men (35 of whom were cases) who reported to have a family history of colorectal cancer at only one of the questionnaires but who did not report so on at least two subsequent questionnaires.
The remaining 28,870 men were classified as to whether at least one of their first-degree relatives was known with colorectal cancer and according to age of diagnosis of the youngest affected first-degree relative. For this classification, we used all available information up to 1996 because a family history of colorectal cancer can be regarded as a surrogate or indicator of inherited genetic susceptibility rather than a time-varying factor.
ASSESSMENT OF OTHER EXPOSURE FACTORS
The questionnaires, which have been described in detail elsewhere14, requested information on age, race, height, weight, physical activity, use of aspirin, smoking history and habits, alcohol consumption and whether the men underwent either colonoscopy or sigmoidoscopy in the past two years. The 1996 questionnaire included questions on the numbers of biological brothers and sisters (0, 1, 2, 3, 4, ≥5). The semi-quantitative food frequency questionnaires, which included about 130 items and an open-ended question for unlisted foods, covered more than 90% of the major nutrient intake of participants and inquired after vitamin and mineral supplements14.
Derived nutrients, except alcohol, were adjusted for total energy intake using the residual method15. The average intake up to the date of diagnosis for cases and the date of last endoscopy for adenoma-free men was calculated to best represent long-term exposure and reduce within-person variation16. Subsequently, men were grouped into categories according to the exposure factors of interest. Four adenoma patients and 26 controls did not complete questions on covariates that were taken into account in the final models and were excluded, which resulted in a final study population of 28,840 men (including 3,881 adenoma patients).
STATISTICAL ANALYSIS
To plot the prevalence of adenomas against age on a logarithmic scale, we determined whether an adenoma was present at the first endoscopy that was registered after study entry, and calculated the prevalence across strata of age. Linear regression, weighted by the inverse of the variance of the estimated proportion, was used to estimate the slopes of the resulting curves. In sub-analyses we excluded the men who reported having undergone endoscopy before entering the study and those who underwent their first endoscopy for reasons other than routine screening.
Multinomial logistic regression was used to compare the distributions of a family history of colorectal cancer among patients having advanced adenoma and patients having non-advanced adenoma to evaluate etiological heterogeneity (case-case analyses). Within the same multinomial logistic regression model17, we compared both categories of patients with the people who did not report adenomas during the follow-up period. As a sensitivity analysis, we checked whether the associations remained similar when studying the group with single adenoma only. Whether the association between family history and advanced versus non-advanced adenomas depended on the location of the adenoma was also explored in the group of men with adenoma at only one location. We did so by fitting a model containing two indicator variables for location, the family history indicator variable and two cross-product terms of each of the indicator variables for location and family history. The P-value for evaluating heterogeneity of the odds ratios was obtained by comparing a model with the two cross-product terms and a model without any such term using a likelihood ratio test.
Multinomial logistic regression was also used to compare the distributions of a family history of colorectal cancer among patients with multiple and single distally located adenomas, and men who did not report any adenomas. Data from all adenoma patients were used when studying the association between the number of adenomas in the distal colon or rectum, but data from patients with at least one proximal adenoma were used when studying the association between a family history of colorectal cancer and the number of proximal adenomas because patients with at least one proximal adenoma must have undergone colonoscopy, which is a requirement for the diagnosis of proximal adenomas. However, the latter was not the case for the entire study population.
The main models included total energy intake (quintiles), age (in 5-yr age groups), history of endoscopy prior to study entry (yes/no), routine screening versus other indications for any endoscopy, aspirin use (>2 times/wk versus ≤ 2 times/wk) , use of multivitamins (current, former, never), smoking (never, quit ≤10 yr ago, quit>10 yr ago, current, missing), consumption of red meat (quintiles), alcohol (0, 0–<10, 10–<20, 20–<30, ≥30 g/d) , intake of folate (quintiles), calcium (quintiles), BMI (quintiles), and physical activity (quintiles), in addition to a family history of colorectal cancer. As these presumed risk factors did not appreciably affect the risk estimates corresponding to a family history of colorectal cancer, we did not check whether inclusion of additional dietary and lifestyle factors affected the risk estimates. Because the number of siblings did not importantly affect the risk estimates and because clinical strategies are unlikely to depend on the number of siblings by itself, we have only adjusted the models referring to affected siblings for the number of siblings (0, 1, 2, 3, 4, ≥5).
Additional sensitivity analyses evaluated whether the case-case associations depended on indication of endoscopy (screening versus complaints), race and age (continuous term) by including cross-products term in the model and evaluating them using likelihood ratio tests.
All reported P-values are two-sided. P-values<0.05 were considered statistically significant. The analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
DESCRIPTION OF THE STUDY POPULATION
Up to 1986, 9.0 percent of the men reported a family history of colorectal cancer. This figure increased to 11.3% in 1990, 12.9% in 1992 and 14.9% in 1996, which most likely reflects ageing of the family members of the men.
One type of relative was affected for 92.3% of the 4,286 men with a family history of colorectal cancer (father: n=1,610; mother: n=1,353; sibling: n=663; at least one of the parents, but unknown who: n=328), whereas 7.2% had two types of affected relatives (father+mother: n=132; father+at least one sibling: n=78; mother+ at least one sibling : n=90; at least one of the parents+at least one sibling: n=10) and the mother, father and at least one sibling of 22 men (0.5%) were affected. Multiple siblings of 42 men were diagnosed with colorectal cancer.
Table 1 illustrates that dietary and lifestyle characteristics were comparable for men with and without a family history of colorectal cancer. Men with a family history of colorectal cancer however, were more likely to have undergone endoscopy before study entry, but they were less likely to have been examined for routine screening. A similar pattern was observed for the characteristics at study entry (not shown).
Table 1.
Age-adjusted characteristics of the men of the Health Professionals Follow-Up Study according to the presence or absence of a family history of colorectal cancer*
| Characteristic | Family history of colorectal cancer n= 4,286 |
No family history of colorectal cancer n=24,554 |
|---|---|---|
| Age at most recent endoscopy (mean ± sd, yr) | 66.7 ± 9.2 | 66.1 ± 9.1 |
| Race (%) | ||
| Southern European | 22.9 | 22.5 |
| Northern European | 71.1 | 72.6 |
| Other | 6.0 | 4.9 |
| History of smoking (%) | 52.4 | 52.8 |
| Body mass index (mean, kg/m2) | 25.8 | 25.8 |
| Physical activity (mean, met-h/w)† | 31.3 | 31.5 |
| Total energy intake (mean, kcal/day) | 1,965 | 1,957 |
| Alcohol intake (mean, g/d) | 10.8 | 10.9 |
| Mean dietary intake | ||
| Protein (g/d) | 90.8 | 90.9 |
| Carbohydrates (g/d) | 247 | 247 |
| Fat (g/d) | 68.2 | 68.2 |
| Folate (µg/d)‡ | 545 | 543 |
| Methionine (g/d) | 2.12 | 2.13 |
| Calcium (mg/d)‡ | 922 | 935 |
| Vitamin D (IU/d)‡ | 446 | 445 |
| Dietary fiber (g/d) | 22.6 | 22.4 |
| Red meat (servings/d) | 0.55 | 0.55 |
| Use of multivitamins (%) | 54.3 | 55.4 |
| Regular use of aspirin (%)§ | 40.6 | 42.1 |
| History of endoscopy before study entry (%) | 18.0 | 14.8 |
Updated variables are used for time-varying exposures (see method section). Mean values are presented Missing values are excluded.
Met-h: metabolic equivalent task hours: the ratio of the metabolic rate during activity to the resting metabolic rate39.
Includes usage of supplements.
Usage of ≥ 2 times per week.
DESCRIPTION OF ADENOMA CHARACTERISTICS
Of the study participants, 13.5% had at least one adenoma during the follow-up. A total of 2,193 (71.3%) cases had only adenomas that were classified as tubular, 22 (0.7%) cases had only serrated adenomas, 633 (20.6%) had at least one tubulovillous adenoma but no villous adenoma or carcinoma in situ, and 167 (5.4%) had at least one villous adenoma but no carcinoma in situ, and 60 (2.0%) men had carcinoma in situ. The histopathology was unknown for the remaining 806 adenoma patients. A total of 1,203 (35.1%) adenomas were 10 mm in diameter or larger and 2,227 were smaller than 10 mm. Size was unknown for 451 adenoma patients, and 148 of them also belonged to the group without information on histopathology. A total of 709 adenoma patients had adenomas in the rectum, 2,052 men had adenomas in the distal colon and 1,799 men had adenomas in the proximal colon. No information on location was available for 43 adenoma patients. A total of 622 men had multiple adenomas in the distal colon or rectum combined and 500 men were registered with multiple adenomas in the proximal colon.
Men with either tubulovillous or villous adenomas were more likely to have large adenomas than men with tubular adenomas (OR for tubulovillous vs. tubular=8.04, 95% CI=6.52–9.92; OR for villous vs. tubular=10.0, 95% CI=7.15–14.0). Men with adenomas in the distal colon were 2.29 (95% CI=1.95–2.68) times more likely and men with adenomas in the rectum were 2.01 (95% CI=1.64–2.46) times more likely to have advanced adenomas than men with proximal adenomas.
Men with multiple adenomas in the distal colon or rectum were 1.55 (95% CI=1.26–1.91) times more likely to be diagnosed with advanced adenomas than patients with single adenomas which was also the case when similar patients but without any proximal adenomas were studied (OR=1.62, 95% CI=1.27–2.07).
FAMILY HISTORY AND PREVALENCE OF ADENOMAS
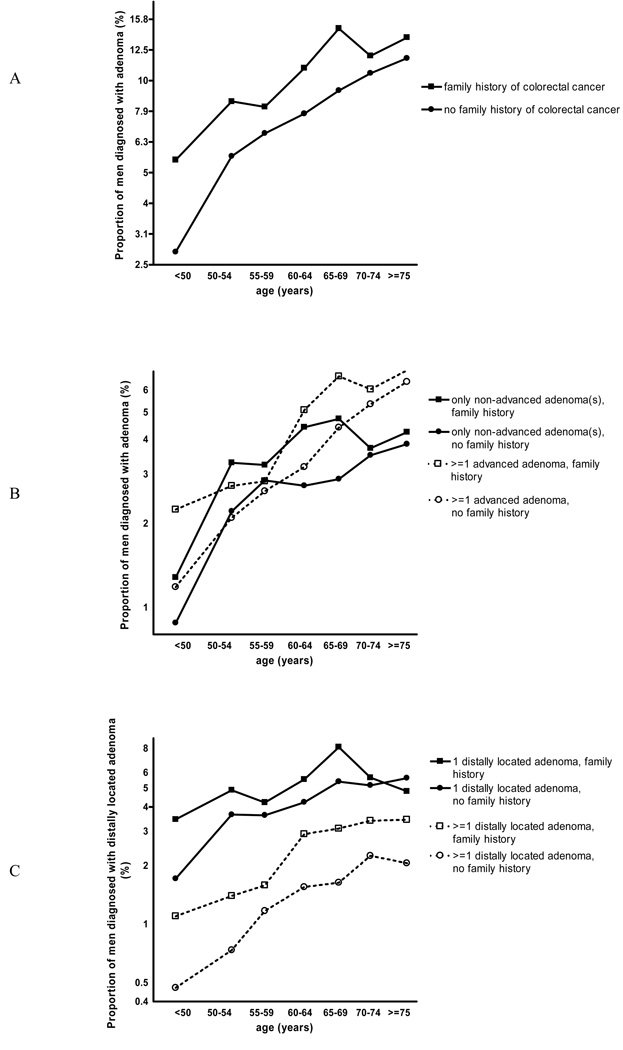
Adenomas occurred more frequently among men who underwent a first endoscopy at older age than among men who were younger at first endoscopy (Figure 1a). Adenomas were more common in men with a family history of colorectal cancer than in men without such a history in all age categories, but the relatively higher prevalence in men with a family history was notably visible in the younger age categories. Apparent straight lines could be drawn independently in this plot for men with and without a family history of colorectal cancer, which suggests that adenoma prevalence increases as a power function of age. The slope of the weighted regression line was 1.8 for men with a family history of colorectal cancer and 2.9 for men without (p=0.039), which suggests that one of the rate-limiting steps in adenoma development has already occurred in men with a family history of colorectal cancer18. Therefore, the association between family history and adenomas appears stronger in the younger age categories. However, this difference could at least partly be attributed to the youngest age group. Although the difference in slopes was still visible after exclusion of people who underwent an endoscopy prior to age 50, the difference in slopes was smaller and not longer statistically significant (slope among those with a family history of colorectal cancer: 1.4; slope among those without such a history: 1.9; p=0.38). A similar plot was obtained when we restricted the analyses to men who underwent their first endoscopy because of routine screening (not shown).
Figure 1.
Prevalence of adenomas at first endoscopy after study entry across strata of age and family history of colorectal cancer. a) all adenomas combined, b) according to adenoma advancement, c) according to adenoma multiplicity
The aforementioned findings were supported by analyses in which we took variation in modifiable risk factors into account. Men with a family history of colorectal cancer were 1.75 times more likely to get colorectal adenomas than men without an affected first-degree relative (95% CI=1.60–1.91); this association was similar among men who underwent all registered endoscopies for routine screening (OR=1.76, 95% CI=1.60–1.94) and men who underwent at least one endoscopy for another reason (OR=1.71, 95% CI=1.41–2.08; Pheterogeneity=0.81). The associations were similar for men with Southern European (OR=1.83, 95% CI=1.53–2.17), Northern European (OR=1.67, 95% CI=1.51–1.85), or other ethnic background (OR=2.38, 95% CI=1.70–3.34; Pheterogeneity=0.63). As also visible in the figure, the strength of the association was stronger for men who were diagnosed at young age than for men who were diagnosed at older age (for those aged ≤55 years: OR=2.37, 95% CI=1.85–3.02; for those aged >55 years: OR=1.67, 95% CI=1.52–1.83; Pheterogeneity=0.048).
The overall risk was similar for individuals with an affected sibling (OR=1.69, 95% CI=1.42–2.02) and for individuals with an affected parent (OR=1.65, 95% CI=1.50–1.81). Men with multiple affected relatives (OR=2.36, 95% CI=1.84–3.04) were more likely to be diagnosed than men with only one affected relative (OR=1.75, 95% CI=1.60–1.92).
FAMILY HISTORY OF COLORECTAL CANCER AND ADVANCED AND NON-ADVANCED ADENOMAS
Figure 1b shows that advanced adenomas were more frequently found than non-advanced adenomas from age 60 onwards in those with and without a family history of colorectal cancer, but the picture was less clear at younger ages. The slopes of the curves referring to advanced and non-advanced adenomas were similar among men with and without a family history of colorectal cancer. In both groups, the proportion of men with advanced and with non-advanced adenomas increased with age.
After adjustment for presumed risk factors, a family history of colorectal cancer was similarly associated with advanced and non-advanced adenomas when the entire study population (Table 2) as well as when the subgroup of men with only one adenoma was studied (advanced versus non-advanced: OR= 0.89, 95% CI=0.71–1.13; advanced versus not known with adenomas: OR=1.42, 95% CI=1.19–1.71; non-advanced versus not known with adenomas: OR=1.59, 95% CI=1.36–1.86). We conducted sensitivity analyses to check whether these associations remained similar when men having adenomas at one location only were studied. These analyses suggested different associations according to adenoma location (Pheterogeneity=0.023): a family history of colorectal cancer tended to be more strongly associated with non-advanced than with advanced adenomas in the distal colon. This also applied to rectal adenomas, but the smaller number of cases resulted in a wide confidence interval for the case-case analysis and an association with family history could only be detected for advanced rectal adenomas. Proximal advanced adenomas, however, were more strongly associated with a family history than were proximal non-advanced adenomas. When we restricted the case subgroup to those with only one proximal adenoma (advanced: n=206, non-advanced: n=480), however, this difference was less convincing (advanced versus non-advanced: OR= 1.18, 95% CI=0.79–1.76; advanced versus not known with adenomas: OR=1.79, 95% CI=1.28–2.49; non-advanced versus not known with adenomas: OR=1.51, 95% CI=1.20–1.91). Neither the main case-case analysis nor the sensitivity case-case analyses, which compared the associations of a family history of colorectal cancer with the occurrence of advanced and with the occurrence of non-advanced adenomas, showed statistically significant differences between the associations.
Table 2.
Risk of advanced and non-advanced colorectal adenomas according to location or strength and type of family history of colorectal cancer (CRC)*
| Category | Number of men† | Odds ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Advanced adenomas |
Non-advanced adenomas |
No reported adenomas |
Advanced vs. non- advanced adenomas |
Advanced adenomas vs. no adenomas |
Non-advanced adenomas vs. no adenomas |
|
| n=1,496 | n=1,507 | n=24,959 | ||||
| Risk among all men | 312/1,184‡ | 313/1,194‡ | 3,457/21,502‡ | 0.98 (0.82–1.17) | 1.67 (1.47–1.91) | 1.70 (1.49–1.94) |
| Risk of having only adenoma in the distal colon§ | 121/497‡ | 121/419‡ | - | 0.82 (0.61–1.09) | 1.57 (1.28–1.93) | 1.92 (1.56–2.36) |
| Risk of having only adenomas in the rectum§ | 25/159‡ | 27/136‡ | - | 0.78 (0.43–1.41) | 1.02 (0.67–1.56) | 1.31 (0.86–1.99) |
| Risk of having only proximal adenomas§ | 87/242‡ | 121/479‡ | - | 1.37 (1.00–1.89) | 2.21 (1.72–2.83) | 1.61 (1.31–1.97) |
| Age at diagnosis of the youngest first-degree relative with CRC (in years) | ||||||
| No family history | 1,184 | 1,194 | 21,502 | 1 (ref) | 1 (ref) | 1 (ref) |
| Before age 50 | 24 | 25 | 290 | 0.95 (0.54–1.68) | 1.48 (0.97–2.26) | 1.56 (1.03–2.36) |
| Age 50–59 | 59 | 48 | 559 | 1.22 (0.83–1.81) | 1.93 (1.47–2.55) | 1.58 (1.17–2.14) |
| Age 60–69 | 92 | 92 | 982 | 1.00 (0.74–1.36) | 1.78 (1.42–2.23) | 1.78 (1.42–2.22) |
| ≥ age 70 | 110 | 120 | 1,222 | 0.90 (0.68–1.18) | 1.68 (1.37–2.06) | 1.87 (1.53–2.28) |
| P for linear trend ** | 0.35 | 0.94 | 0.19 | |||
| Affected family member†† | ||||||
| Parent | ||||||
| No | 1,233 | 1,250 | 22,036 | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 263 | 257 | 2,923 | 1.03 (0.85–1.25) | 1.59 (1.38–1.83) | 1.54 (1.34–1.78) |
| Sibling | ||||||
| No | 1,422 | 1,436 | 24,277 | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 74 | 71 | 682 | 0.95 (0.67–1.34) | 1.71 (1.33–2.21) | 1.80 (1.39–2.33) |
| Number of first-degree relatives with CRC | ||||||
| None | 1,184 | 1,194 | 21,502 | 1 (ref) | 1 (ref) | 1 (ref) |
| 1 | 264 | 268 | 2,913 | 0.97 (0.80–1.17) | 1.68 (1.46–1.94) | 1.74 (1.51–2.00) |
| ≥2 | 33 | 29 | 263 | 1.08 (0.65–1.79) | 2.31 (1.59–3.35) | 2.14 (1.45–3.17) |
| P for linear trend | 0.91 | <.0001 | <.0001 | |||
The multinomial logistic regression models were adjusted for age (in 5-yr age groups), history of endoscopy prior to study entry (yes/no), routine screening versus other indications for any endoscopy, aspirin use (>2 times/wk versus ≤ 2 times/wk), use of multivitamins (current, former, never), smoking (never, quit ≤10 yr ago, quit>10 yr ago, current, missing) consumption of red meat (quintiles), alcohol (0, 0-<10, 10-<20, 20-<30, ≥30 g/d); intake of folate (quintiles), calcium (quintiles), BMI (quintiles), physical activity (quintiles), total energy intake (quintiles).
Due to missing values, not all totals add up to 1,496, 1,507 or 24,959.
Men with a family history of CRC / without family history of CRC.
Restricted to men who had only adenomas at one location. Similar conclusions were obtained when we focused on men who had at least one adenoma at the studied location.
This test was based on evaluating an ordinal variable for the age of diagnosis among cases only (4=before age 50, 3=age 50–59, 2= age 60–69, 1=≥ age 70).
Categories are not mutually exclusive. Estimates are mutually adjusted.
The difference between the associations with advanced and non-advanced adenomas did not seem to depend on race (Pheterogeneity=0.65), having had screening endoscopy or not (Pheterogeneity=0.32), or age (Pheterogeneity=0.75). Among the men with adenomas, the distribution of adenoma size seemed comparable among those with (median=6mm, 25th percentile=4mm, 75th percentile=10mm) and without a family history of colorectal cancer (median=7mm, 25th percentile=4mm, 75th percentile=10mm), although a formal statistical test (the Wilcoxon two-sample test) suggested that men without a family history of colorectal cancer were more likely to have larger adenomas (P=0.038). This tendency was also visible when we studied adenomas in the rectum, distal colon and proximal colon separately, but these differences were not statistically significant.
FAMILY HISTORY OF COLORECTAL CANCER AND ADENOMA MULTIPLICITY
Figure 1c shows the distribution of single and multiple adenomas in the distal colon and rectum combined according to age groups and presence or absence of a family history of colorectal cancer. Across all age groups and both among men with and without a family history of colorectal cancer, single distally located adenomas were more prevalent than multiple distally located adenomas. The slope representing the prevalence of single distally located adenomas among men with a family history of colorectal cancer was almost horizontal (suggesting only one rate limiting step), whereas the slope for men without a family history of colorectal cancer was slightly positive, but this was largely due to the younger age groups. A similar pattern was observed regarding multiple distally located adenomas, although the slopes were positive and the parallelism more obvious.
The prevalence of multiple, but also single distally located adenomas was higher among men with a family history of colorectal cancer, which is also reflected in the odds ratios presented in Table 3. The association with family history was stronger for multiple than for single distally located adenomas. The difference in associations between family history and multiple distally located adenomas, and family history and single distally located adenomas, increased in relation to the number of affected relatives. However, the strength of the association did not depend on the age of diagnosis of the family members. The difference between the associations with multiple and single distally located adenomas did not depend on race (Pheterogeneity=0.80), having had screening endoscopy or not (Pheterogeneity=0.67) and age (Pheterogeneity=0.21; see also Figure 1c). The case-case analysis and the two case-control comparisons on family history of colorectal cancer and adenoma multiplicity were very similar when the distal colon and rectum were studied separately (estimates not shown). Associations in the same direction were seen among men only known with non-advanced adenomas (multiple versus single distally located: OR=1.33, 95% CI=0.90–1.96; multiple distal versus no distally located adenomas: OR=2.20, 95% CI=1.56–3.10; single distally located versus no distally located adenomas: OR=1.66, 95% CI=1.37–2.00) and men known with at least one advanced adenoma (multiple versus single distally located: OR= 1.40, 95% CI=1.02–1.91; multiple distally located versus no distally located adenomas: OR=1.90, 95% CI=1.46–2.47; single distally located versus no distally located adenomas: OR=1.36, 95% CI=1.13–1.63).
Table 3.
Risk of multiple distal versus single distally located colorectal adenomas according strength and type of family history of colorectal cancer (CRC)*
| Category2 | Number of men† | Odds ratio (95% CI) | ||||
|---|---|---|---|---|---|---|
| Multiple distally located |
Single distally located |
Not known with distally located adenomas |
Multiple vs. single distally located adenomas |
Multiple distally located vs. no distally located adenomas |
Single distally located vs. no distally located adenomas |
|
| n=622 | n=1,985 | n=26,190 | ||||
| Risk among all men | 152/470‡ | 382/1,603‡ | 3,744/22,446‡ | 1.35 (1.09–1.68) | 2.02 (1.67–2.44) | 1.49 (1.32–1.68) |
| Age at diagnosis of the youngest first-degree relative with CRC (in years) | ||||||
| No family history | 470 | 1,603 | 22,446 | 1 (ref) | 1 (ref) | 1 (ref) |
| Before age 50 | 13 | 29 | 314 | 1.53 (0.79–2.98) | 1.97 (1.12–3.46) | 1.28 (0.87–1.89) |
| Age 50–59 | 25 | 67 | 606 | 1.28 (0.80–2.05) | 2.00 (1.32–3.03) | 1.57 (1.21–2.04) |
| Age 60–69 | 47 | 105 | 1,061 | 1.53 (1.07–2.20) | 2.26 (1.66–3.07) | 1.47 (1.19–1.81) |
| ≥ age 70 | 50 | 150 | 1,339 | 1.14 (0.81–1.59) | 1.88 (1.40–2.54) | 1.65 (1.38–1.98) |
| P for linear trend§ | 0.32 | 0.81 | 0.17 | |||
| Affected family member** | ||||||
| Parent | ||||||
| No | 489 | 1,669 | 23,023 | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 133 | 316 | 3,167 | 1.45 (1.15–1.82) | 1.99 (1.63–2.42) | 1.37 (1.20–1.56) |
| Sibling | ||||||
| No | 590 | 1,899 | 25,446 | 1 (ref) | 1 (ref) | 1 (ref) |
| Yes | 32 | 86 | 744 | 1.02 (0.66–1.57) | 1.65 (1.13–2.40) | 1.61 (1.27–2.04) |
| Number of first-degree relatives with CRC | 1.2347 2.5316 | |||||
| None | 470 | 1,603 | 22,446 | 1 (ref) | 1 (ref) | 1 (ref) |
| 1 | 124 | 326 | 3,155 | 1.29 (1.02–1.63) | 1.95 (1.59–2.39) | 1.52 (1.34–1.72) |
| ≥2 | 18 | 35 | 294 | 1.76 (0.98–3.15) | 3.11(1.91–5.08) | 1.77 (1.23–2.53) |
| P for linear trend | 0.0067 | <0.0001 | <0.0001 | |||
Adjusted for the same factors as listed in Table 2.
Due to missing values, not all totals add up to 622, 1,985 or 26,190.
Men with a family history of CRC / without family history of CRC.
This test was based on evaluating an ordinal variable for the age of diagnosis among cases only (4=before age 50, 3=age 50–59, 2= age 60–69, 1=≥ age 70).
Categories are not mutually exclusive. Estimates are mutually adjusted.
The association between family history and adenoma multiplicity was also visible when we studied the number of adenomas. Of the 1,985 men with one adenoma in the distal colon or rectum 382 (19.2%) had a family history; this also applied to 96 of the 440 (21.8%) men with two distally located adenomas, 32 of the 124 (25.8%) men with three, 13 of the 33 (39.4%) men with four and 11 of the 25 (44.0%) of the men with five or more distally located adenomas.
A similar pattern was visible when the number of proximal adenomas was studied. Of the 1,293 men with one adenoma in the proximal colon 275 (21.3%) had a family history; this applied to 84 of the 313 (26.8%) men with two adenomas in the proximal colon, 29 of the 115 (25.2%) men with three, 13 of the 36 (36.1%) men with four and 11 of the 36 (30.6%) of the men with five or more proximal adenomas.
Discussion
To our knowledge, this study is the first that systematically examined whether a family history of colorectal cancer influences adenoma multiplicity in a large number of asymptomatic and symptomatic men. Previous studies have reported on the prevalence of advanced adenomas in men with and without a family history of colorectal cancer, but these studies were small7–10 and only two of them studied these associations systematically6, 11.
We observed that a family history of colorectal cancer was more strongly associated with multiple distally located adenomas than with single distally located adenomas. A similar indication for proximal adenomas was found. A family history of colorectal cancer was not differently associated with risk of advanced and non-advanced adenomas in the entire population of cases, although potential differences according to subsite were found. The data tended somewhat towards a stronger positive association with non-advanced adenomas than with advanced adenomas in the distal colon and rectum, but towards a stronger association with advanced than with non-advanced proximal adenomas.
By adjusting the associations for presumed modifiable risk factors for colorectal cancer, we largely excluded the explanation that shared environmental factors underlie the observed associations, which is in line with a study that compared incidence rates of colorectal cancer between siblings and spouses, and between parents and their offspring19.
Not all participants underwent colonoscopy, which may have led to some bias in the analyses on advanced and non-advanced adenomas. However, since most polypectomies take place during colonoscopy as recommended20, the impact of bias due to incomplete bowel examinations may be limited with regard to the case-case analyses. Nonetheless, some misclassification of case status has inevitably occurred. As for any adenoma study, some adenomas will have been missed at endoscopy, which are more likely to be small21. The miss rate will probably not affect the case-case analyses importantly because endoscopists are likely to examine the colon thoroughly once an adenoma has been diagnosed. Provided endoscopists do not conduct the examinations more thoroughly when a patient with a family history of colorectal cancer presents, the effect of missed adenomas is likely to be largely diluted by the much larger number of truly adenoma-free men in the comparisons versus men not known with adenomas.
Drawbacks of our study are that different physicians performed the endoscopies and that the classification of size and histopathological characteristics is based on judgment of different community pathologists. It can be argued that the classification of advanced adenomas should incorporate dysplasia as an important determinant of colorectal cancer risk22, but we decided not to do so because the consistency of the classification into high-grade and low-grade dysplasia has been shown to be poor when different community pathologists are involved23, 24. The fair agreement of classification of histopathological types23–25 between pathologists in combination with the strong association between size, histopathological characteristics and dysplasia26–28 further supports our classification. We did not categorize 23% of the adenomas as advanced or non-advanced adenomas, however. As physicians may be less inclined to report observations deemed clinically unimportant than those deemed clinically important, we expect that the non-categorized adenomas are mostly non-advanced adenomas. Reassuringly, our conclusions regarding the case-case analyses are robust given that the OR for advanced versus non-advanced adenomas was 0.93 when we treated the non-classified adenomas as if they were non-advanced adenomas and 1.05 when we treated them as advanced adenomas, which were both not statistically significant.
In line with previous studies, we observed that the younger a person was diagnosed with any type of adenoma, the stronger was the observed association with family history of colorectal cancer. A stronger association was also found among men with young affected family members; hence, the etiology of adenomas and cancer occurring early in life appears to be more strongly determined by heritable factors than for those occurring later. In particular adenomas at younger age may have occurred among men who belong to a family with heritable colorectal cancer syndromes, but we are confident that our findings apply to sporadic carcinogenesis as only a few patients were diagnosed with a large number of adenomas and because fewer than 3% were diagnosed before age 50, whilst the mean age at last endoscopy was 66 years old. Patients with hereditary colorectal cancer syndromes develop the disease at relatively young age, but colorectal cancer patients with a family history also tend to get the disease 10 years earlier than patients without such a history29. Advanced and non-advanced adenomas also occurred at a younger age among men with a family history of colorectal cancer in our study. This could point towards family members being more susceptible to the occurrence of mutations.
The most striking observation of this study was that patients with a family history of adenomas were more likely to have multiple adenomas at diagnosis, which our study design allowed to demonstrate most clearly for distally located adenomas. This corresponds with findings from a small study in which the number of aberrant crypt foci was higher in patients with a family history of sporadic colorectal cancer than in patients without30, and a case-control study also suggested that people with affected relatives were more likely to have multiple adenomas6. A similar indication was found in a study among 992 patients, but this finding has to be interpreted with caution as it is unclear whether the analysis was adjusted for age31. Perhaps the same susceptibility genes32 that modify the severity of the familial adenomatous polyposis coli (FAP) syndrome or other polyposis syndromes may also determine the association between a family history and adenoma multiplicity in sporadic adenoma patients. Cancer genes that were differently expressed in macroscopically normal rectosigmoid mucosa in individuals with a sporadic family history of colon cancer compared with individuals without such history33 could also underlie such an association. Although we could not detect differences in the association of family history of colorectal cancer with adenoma multiplicity according to adenoma location, future studies should tease out the role of family history of colorectal cancer in determining adenoma location further as adenomas at different locations may be determined by different genes or even different mutations within the same gene. Such has already been found for hereditary syndromes: HNPCC, which is caused by mutations in mismatch repair genes, is predominantly associated with proximal tumors, whereas FAP, which is caused by mutations in the APC gene, is more frequently associated with distally located lesions34. Phenotypic variations, including in location, have also been shown for FAP patients with different type of mutations in the APC gene35. It is possible that multiplicity of so called sporadic adenomas may also be caused by different mutational patterns that result in distinct manifestations of the disease, e.g. in adenomas occurring at different locations.
In spite of the potential etiological heterogeneity of sporadic adenoma multiplicity, the stronger association between family history of colorectal cancer and multiplicity than the one with advanced adenoma stage for at least distally located adenomas in our study suggests that the genetic components involved in sporadic colorectal carcinogenesis mostly drives the occurrence of key mutations rather than enhancing the growth signals in prevalent distally located adenomas in the gut. Indeed, in a cross-sectional study a family history of colorectal cancer was associated with an approximately two-fold higher recurrence rate within three years, whilst no difference in distribution of family history could be detected between patients with and without adenomas at study entry36. These observations suggest that genetic factors may play a more important role in the earlier stages of the adenoma-carcinoma sequence than in later stages. It remains to be determined if this also applies to proximal adenomas, as cancers in the proximal colon develop via different pathways than do distally located cancers34.
However, it cannot be precluded that heritable factors stimulate growth of distally located or proximal minuscule adenomas rather than enhance the occurrence of new lesions. The results from our study suggest that genetic factors may be less likely to stimulate adenoma growth of at least the majority of distally located adenomas importantly because we did not observe a stronger association with advanced than with non-advanced adenomas in the distal colon and rectum; this observation was in line with data from a pooled analysis that compared adenomas according to degree of dysplasia11. On the other hand, a study in which small adenomas were left in situ for up to three years, patients with a first-degree family member with sporadic colorectal cancer were more likely to have adenomas that showed net growth than patients without such a family history5, though this was a small study. In addition, relatively small studies7–10 as well as a larger case-control study6 suggested that larger adenomas were more common among those with relatives with colorectal cancer. In our study a less steep curve was visible among men with a family history of colorectal cancer diagnosed before age 55 in the graphs depicting adenoma prevalence, which supports the existence of a hereditary subgroup of adenomas that develops faster than the majority. In HNPCC kindreds, the ratio of adenomas to cancer is smaller than in the general population, while colorectal cancer is common and occurs at an early age37. This suggests that HNPCC adenomas, which are more likely to be proximally located34, pass quicker through the adenoma-carcinoma sequence than do other adenomas, and this may be the case for other, yet unidentified adenoma subgroups. Likewise, a group of carcinomas may exist with similar properties, which may or may not originate from aggressive adenomas. Nonetheless, in the general population, in which distally located adenomas are more common, a family history of colorectal cancer seems to play a more important role in the early stages of adenoma development than in later stages. Considering that some adenomas might never turn into a more advanced lesion because they regress in size38, we cannot conclude that all adenomas have the potential to develop into a carcinoma provided necessary growth conditions will be fulfilled. The selection process that determines the transition of non-advanced to advanced distally located adenomas, however, does not seem to be substantially influenced by heritable factors strongly related to a family history of colorectal cancer.
In conclusion, our data suggest that adenoma multiplicity seems to have a hereditary basis in sporadic colorectal carcinogenesis, but we could not confirm a role of hereditary factors in adenoma advancement in at least the distal colon and rectum. Additional to confirming our results and aiming to identify the underlying genetic factors, future studies could assess the age distribution of the relatives to be able to construct a family risk index that allows allows studying underlying genetic susceptibility more precisely.
Two brief statements describing the novelty and impact of this paper.
This large prospective cohort study is the first systematic study to show that adenoma multiplicity is positively related to a family history of colorectal cancer in the general population.
At the population level heritable factors may be more important in stages of adenoma formation than at stages of adenoma advancement for at least distally located adenomas.
Acknowledgements
The HPFS is supported by NCI Research Grant CA 55075. Petra Wark’s visit to the Harvard School of Public Health was made possible with support from the Dutch Cancer Society, and she was further supported by the Netherlands Organisation for Health Research and Development and Cancer Research UK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Abbreviations used
- APC
adenomatous polyposis coli
- BMI
body mass index
- CI
confidence interval
- G/d
gram per day
- FAP
familial adenomatous polyposis coli
- HNPCC
hereditary non polyposis colorectal cancer
- HPFS
Health Professionals Follow-Up Study
- MET
metabolic equivalent
- MYH
MutY homologue
- MMR
mismatch repair
- OR
odds ratio
References
- 1.Butterworth AS, Higgins JP, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006;42:216–227. doi: 10.1016/j.ejca.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 2.Baglietto L, Jenkins MA, Severi G, Giles GG, Bishop DT, Boyle P, Hopper JL. Measures of familial aggregation depend on definition of family history: meta-analysis for colorectal cancer. J Clin Epidemiol. 2006;59:114–124. doi: 10.1016/j.jclinepi.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein P, Holm NV, Verkasalo PK, Iliadou A, Kaprio J, Koskenvuo M, Pukkala E, Skytthe A, Hemminki K. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343:78–85. doi: 10.1056/NEJM200007133430201. [DOI] [PubMed] [Google Scholar]
- 4.De la Chapelle A. Genetic predisposition to colorectal cancer. Nat Rev Cancer. 2004;4:769–780. doi: 10.1038/nrc1453. [DOI] [PubMed] [Google Scholar]
- 5.Almendingen K, Hofstad B, Vatn MH. Does a family history of cancer increase the risk of occurrence, growth, and recurrence of colorectal adenomas? Gut. 2003;52:747–751. doi: 10.1136/gut.52.5.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutron MC, Faivre J, Quipourt V, Senesse P, Michiels C. Family history of colorectal tumours and implications for the adenoma-carcinoma sequence: a case control study. Gut. 1995;37:830–834. doi: 10.1136/gut.37.6.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pariente A, Milan C, Lafon J, Faivre J. Colonoscopic screening in first-degree relatives of patients with 'sporadic' colorectal cancer: a case-control study. The Association Nationale des Gastroenterologues des Hopitaux and Registre Bourguignon des Cancers Digestifs (INSERM CRI 9505) Gastroenterology. 1998;115:7–12. doi: 10.1016/s0016-5085(98)70358-0. [DOI] [PubMed] [Google Scholar]
- 8.Guillem JG, Forde KA, Treat MR, Neugut AI, O'Toole KM, Diamond BE. Colonoscopic screening for neoplasms in asymptomatic first-degree relatives of colon cancer patients. A controlled, prospective study. Dis Colon Rectum. 1992;35:523–529. doi: 10.1007/BF02050530. [DOI] [PubMed] [Google Scholar]
- 9.Bazzoli F, Fossi S, Sottili S, Pozzato P, Zagari RM, Morelli MC, Taroni F, Roda E. The risk of adenomatous polyps in asymptomatic first-degree relatives of persons with colon cancer. Gastroenterology. 1995;109:783–788. doi: 10.1016/0016-5085(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 10.Tung SY, Wu CS. Risk factors for colorectal adenomas among immediate family members of patients with colorectal cancer in Taiwan: a case-control study. Am J Gastroenterol. 2000;95:3624–3628. doi: 10.1111/j.1572-0241.2000.03380.x. [DOI] [PubMed] [Google Scholar]
- 11.Terry MB, Neugut AI, Bostick RM, Sandler RS, Haile RW, Jacobson JS, Fenoglio-Preiser CM, Potter JD. Risk factors for advanced colorectal adenomas: a pooled analysis. Cancer Epidemiol Biomarkers Prev. 2002;11:622–629. [PubMed] [Google Scholar]
- 12.Kerber RA, Slattery ML. Comparison of self-reported and database-linked family history of cancer data in a case-control study. Am J Epidemiol. 1997;146:244–248. doi: 10.1093/oxfordjournals.aje.a009259. [DOI] [PubMed] [Google Scholar]
- 13.Murff HJ, Spigel DR, Syngal S. Does this patient have a family history of cancer? An evidence-based analysis of the accuracy of family cancer history. Jama. 2004;292:1480–1489. doi: 10.1001/jama.292.12.1480. [DOI] [PubMed] [Google Scholar]
- 14.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. discussion 27–36. [DOI] [PubMed] [Google Scholar]
- 15.Willett W. Nutritional Epidemiology. 2nd ed. New York, NY: Oxford University Press; 1998. [Google Scholar]
- 16.Hu FB, Manson JE, Liu S, Hunter D, Colditz GA, Michels KB, Speizer FE, Giovannucci E. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–547. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 17.Begg CB, Gray R. Calculation of Polychotomous Logistic-Regression Parameters Using Individualized Regressions. Biometrika. 1984;71:11–18. [Google Scholar]
- 18.Armitage P, Doll R. The age distribution of cancer and a multi-stage theory of carcinogenesis. 1954. Int J Epidemiol. 2004;33:1174–1179. doi: 10.1093/ije/dyh216. [DOI] [PubMed] [Google Scholar]
- 19.Hemminki K, Chen B. Familial risk for colorectal cancers are mainly due to heritable causes. Cancer Epidemiol Biomarkers Prev. 2004;13:1253–1256. [PubMed] [Google Scholar]
- 20.Bond JH. Polyp guideline: diagnosis, treatment, and surveillance for patients with colorectal polyps. Practice Parameters Committee of the American College of Gastroenterology. Am J Gastroenterol. 2000;95:3053–3063. doi: 10.1111/j.1572-0241.2000.03434.x. [DOI] [PubMed] [Google Scholar]
- 21.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, Lehman GA, Mark DG. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 22.Winawer SJ, Zauber AG, Fletcher RH, Stillman JS, O'Brien MJ, Levin B, Smith RA, Lieberman DA, Burt RW, Levin TR, Bond JH, Brooks D, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. CA Cancer J Clin. 2006;56:143–159. doi: 10.3322/canjclin.56.3.143. quiz 84–5. [DOI] [PubMed] [Google Scholar]
- 23.Jensen P, Krogsgaard MR, Christiansen J, Braendstrup O, Johansen A, Olsen J. Observer variability in the assessment of type and dysplasia of colorectal adenomas, analyzed using kappa statistics. Dis Colon Rectum. 1995;38:195–198. doi: 10.1007/BF02052450. [DOI] [PubMed] [Google Scholar]
- 24.Terry MB, Neugut AI, Bostick RM, Potter JD, Haile RW, Fenoglio-Preiser CM. Reliability in the classification of advanced colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 2002;11:660–663. [PubMed] [Google Scholar]
- 25.Costantini M, Sciallero S, Giannini A, Gatteschi B, Rinaldi P, Lanzanova G, Bonelli L, Casetti T, Bertinelli E, Giuliani O, Castiglione G, Mantellini P, et al. Interobserver agreement in the histologic diagnosis of colorectal polyps. the experience of the multicenter adenoma colorectal study (SMAC) J Clin Epidemiol. 2003;56:209–214. doi: 10.1016/s0895-4356(02)00587-5. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien H, Matthew JA, Gee JM, Watson M, Rhodes M, Speakman CT, Stebbings WS, Kennedy HJ, Johnson IT. K-ras mutations, rectal crypt cells proliferation, and meat consumption in patients with left-sided colorectal carcinoma. Eur J Cancer Prev. 2000;9:41–47. doi: 10.1097/00008469-200002000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Gschwantler M, Kriwanek S, Langner E, Goritzer B, Schrutka-Kolbl C, Brownstone E, Feichtinger H, Weiss W. High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol. 2002;14:183–188. doi: 10.1097/00042737-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Jørgensen OD, Kronborg O, Fenger C. The Funen Adenoma Follow-Up Study. Characteristics of patients and initial adenomas in relation to severe dysplasia. Scand J Gastroenterol. 1993;28:239–243. doi: 10.3109/00365529309096079. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs CS, Giovannucci EL, Colditz GA, Hunter DJ, Speizer FE, Willett WC. A prospective study of family history and the risk of colorectal cancer. N Engl J Med. 1994;331:1669–1674. doi: 10.1056/NEJM199412223312501. [DOI] [PubMed] [Google Scholar]
- 30.Stevens RG, Swede H, Heinen CD, Jablonski M, Grupka M, Ross B, Parente M, Tirnauer JS, Giardina C, Rajan TV, Rosenberg DW, Levine J. Aberrant crypt foci in patients with a positive family history of sporadic colorectal cancer. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Church JM. A scoring system for the strength of a family history of colorectal cancer. Dis Colon Rectum. 2005;48:889–896. doi: 10.1007/s10350-004-0880-9. [DOI] [PubMed] [Google Scholar]
- 32.Houlston R, Crabtree M, Phillips R, Crabtree M, Tomlinson I. Explaining differences in the severity of familial adenomatous polyposis and the search for modifier genes. Gut. 2001;48:1–5. doi: 10.1136/gut.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hao CY, Moore DH, Wong P, Bennington JL, Lee NM, Chen LC. Alteration of gene expression in macroscopically normal colonic mucosa from individuals with a family history of sporadic colon cancer. Clin Cancer Res. 2005;11:1400–1407. doi: 10.1158/1078-0432.CCR-04-1942. [DOI] [PubMed] [Google Scholar]
- 34.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 35.Giardiello FM, Brensinger JD, Luce MC, Petersen GM, Cayouette MC, Krush AJ, Bacon JA, Booker SV, Bufill JA, Hamilton SR. Phenotypic expression of disease in families that have mutations in the 5' region of the adenomatous polyposis coli gene. Ann Intern Med. 1997;126:514–519. doi: 10.7326/0003-4819-126-7-199704010-00003. [DOI] [PubMed] [Google Scholar]
- 36.Fossi S, Bazzoli F, Ricciardiello L, Nicolini G, Zagari RM, Pozzato P, Palli D, Roda E. Incidence and recurrence rates of colorectal adenomas in first-degree asymptomatic relatives of patients with colon cancer. Am J Gastroenterol. 2001;96:1601–1604. doi: 10.1111/j.1572-0241.2001.03784.x. [DOI] [PubMed] [Google Scholar]
- 37.Ahlquist DA. Aggressive polyps in hereditary nonpolyposis colorectal cancer: targets for screening. Gastroenterology. 1995;108:1590–1592. doi: 10.1016/0016-5085(95)90711-4. [DOI] [PubMed] [Google Scholar]
- 38.Loeve F, Boer R, Zauber AG, Van Ballegooijen M, Van Oortmarssen GJ, Winawer SJ, Habbema JD. National Polyp Study data: evidence for regression of adenomas. Int J Cancer. 2004;111:633–639. doi: 10.1002/ijc.20277. [DOI] [PubMed] [Google Scholar]
- 39.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, Paffenbarger RS., Jr Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]