Abstract
In situ forming implants (ISFI) have shown promise in delivering adjuvant chemotherapy following minimally invasive cancer therapies such as thermal ablation of tumors. While ISFI systems have been thoroughly investigated for delivery of high molecular weight (Mw) therapeutics, little research has been conducted to optimize their design for delivery of low Mw drugs. This study examined the effect of varying the formulation components on the low Mw drug release profile from a ISFI consisting of poly(D,L-lactide-co-glycolide), fluorescein (model drug), and excipient dissolved in 1-methyl-2-pyrrolidinone (NMP). Effects of varying PLGA Mw, excipient concentration, and drug loading were studied. Additionally, solubility studies were conducted to determine the critical water concentration required for phase inversion. Results demonstrated that PLGA Mw was the most significant factor in modulating low Mw drug release from the ISFI systems. ISFI formulations comprised of a low Mw (16 kDa) PLGA showed a significantly (p<0.05) lower burst release (after 24 hours), 28.2 ± 0.5%, compared to higher Mw PLGA (60 kDa), 55.1 ± 3.1%. Critical water concentration studies also demonstrated that formulations with lower Mw PLGA had increased solubility in water and may thus require more time to phase invert and release the drug.
Keywords: In situ forming implant, Phase inversion, Biodegradable polymers, Cancer chemotherapy, Controlled release
Introduction
With the advent of various biocompatible polymeric materials in recent years, implantable drug delivery systems have been an area of significant research interest1,2. These systems have several advantages over traditional systemic treatments including the ability to deliver high concentrations of a therapeutic substance to a treatment area, maintain therapeutic drug levels for a prolonged time period, and avoid systemic side effects to areas outside of the treatment zone. Because of these features, local drug delivery implants have been of particular interest for delivering toxic chemotherapeutic agents to solid tumors3–5. Numerous chemotherapeutic drug delivery implants have been designed using a variety of biodegradable polymers such as PLGA6,7 polyesters, poly(orthoesters)8, and polyanhydrides9. However, currently only a limited number of drug delivery implants, such as the Gliadel® wafer (MGI, Pharma Inc., Bloomington, MN), have been approved for clinical use10,11. While these implant systems have shown promise in the laboratory setting, issues with drug penetration to the entire treatment area have limited their translation into clinical use12–15.
One strategy that has been developed to overcome diffusional drug penetration limitations is to use multiple implants in strategic locations thereby distributing therapeutic drug dosages to the entire tumor volume16. In addition, combining local drug delivery implants with minimally invasive treatment strategies such as tumor ablation has been proposed17. However placing multiple traditional preformed solid implants in multiple locations using minimally invasive surgical techniques may be technically difficult. For this application, injectable in situ forming, phase inverting implants may be a more attractive solution. Using in situ forming implants (ISFI) to deliver therapeutics to the entire tumor volume should be possible with a single catheter insertion or potentially with a multipronged needle18.
ISFI systems, first developed by Dunn et al19, have made it to market for use as a delivery system for large peptide molecules, such as LH-RH, Eligard®, to treat prostate cancer20. These formulations are usually comprised of a biodegradable polymer such as poly(D,L-lactide-co-glycolide) (PLGA) or poly(ε-caprolactone) dissolved in a water-miscible solvent such as 1-methyl-2-pyrrolidinone (NMP), triacetin, or ethyl benzoate21. The polymer-drug solution undergoes a process of phase inversion upon contact with an aqueous environment, such as extracellular fluid, whereby the previously liquid ISFI polymer solution forms into a solid implant. The rate of drug release from these implants is dependent on many formulation parameters including the type and Mw of polymer22,23, hydrophilicity of the solvent solution24, and any additional excipients added to the solution to modulate release25. While several studies have focused on how the formulation parameters affect delivery of high molecular weight (Mw) peptide molecules20,26, little research has focused on using these injectable depot systems to deliver low Mw drugs, such as most commonly used chemotherapeutic agents.
The present study explored the effects of implant formulation on release of low Mw drugs. A base ISFI formulation with PLGA dissolved in NMP was chosen because of its previously well established protein drug release profile18,24. The Mw of PLGA, drug loading, and percent of excipient added to polymer solution were varied to examine their effects on drug release. In addition, to gain better insight into how polymer Mw and excipient affect the process of phase inversion and subsequent drug diffusion, the critical water concentration required to initiate precipitation of the polymer solution was measured. Finally scanning electron micrographs were obtained to analyze the structure and morphology of the different polymer formulations. Results from this study will lead to improved understanding of ISFI systems and their role in delivery of low Mw drugs.
Materials and Methods
Materials
Polymer formulations were comprised of poly(D,L-lactide-co-glycolide) (PLGA) obtained from Lakeshore Biomaterials (Birmingham, AL), Pluronic P85 (P85, Mw: 4600 Da), a tri-block co-polymer of PEO-PPO-PEO donated by BASF Corp. (Shreveport, LA), and 1-methyl-2-pyrrolidinone (NMP) obtained from Sigma (St. Louis, MO). Sodium fluorescein (Sigma) was used as low Mw mock drug molecule (Mw 376.28).
Polymer solution formulation
A homogenous polymer solution was prepared by dissolving solid components into NMP over 24 hours at 37 °C with frequent mixing. The relative mass percentage of each component, NMP, PLGA, P85, and fluorescein for the base solution was 60/36.5/2.5/1. The type of PLGA used for this base formulation was PLGA 2A (50:50 lactide/glycolide (L/G) ratio and inherent viscosity (i.v.) of 0.20 dL/g). All subsequently tested polymer solutions were formulated by varying the ratio of one component of this base solution (Table 1). In addition to the base formulation with PLGA 2A, formulations using PLGA 3A, 4A, and 4.5A with 50:50 (L/G) ratio and i.v. of 0.30, 0.40, and 0.45 dL/g, respectively, were tested. A formulation comprised of PLGA 7E with a 75:25 (L/G) ratio and an i.v. of 0.70 was also tested. The mean Mw can be approximated from the viscosity of each PLGA polymer and was 18, 33, 50, 60 and 116 kDa for the 2A, 3A, 4A, 4.5A, and 7E polymers, respectively using the following relationship, Mw = 198.24*IV1.5105. To test the effects of varying P85 surfactant excipient on implant properties, the mass percentage of P85 was varied from 0–5%, and adjustments were made to the mass percentage of PLGA accordingly. Similarly, formulations with varying fluorescein loading concentration from 0.05–5% were prepared.
Table I.
Solid Components of Tested Formulations
| Formulations | Polymer Type (%, w/w) |
%P85 w/w |
%Drug Loading Dose w/w |
|---|---|---|---|
| Varying P85 | |||
| 0% P85 | PLGA 2A (39) | 0 | 1 |
| 1% P85 | PLGA 2A (38) | 1 | 1 |
| 2.5% P85 | PLGA 2A (36.5) | 2.5 | 1 |
| 5% P85 | PLGA 2A (34) | 5 | 1 |
| Varying Mw | |||
| PLGA 2A | PLGA 2A (36.5) | 2.5 | 1 |
| PLGA 3A | PLGA 3A (36.5) | 2.5 | 1 |
| PLGA 4A | PLGA 4A (36.5) | 2.5 | 1 |
| PLGA 4.5A | PLGA 4.5A (36.5) | 2.5 | 1 |
| PLGA 7E | PLGA 7E (36.5) | 2.5 | 1 |
| Varying Loading | |||
| 0.5% loading dose | PLGA 2A (37) | 2.5 | 0.5 |
| 1% loading dose | PLGA 2A (36.5) | 2.5 | 1 |
| 2% loading dose | PLGA 2A (35.5) | 2.5 | 2 |
| 5% loading dose | PLGA 2A (32.5) | 2.5 | 5 |
NMP solvent concentration was constant for all formulations at 60% w/w
Drug release studies
To determine the rate of drug release, a standard dissolution study was conducted. For each formulation, a dissolution experiment (n = 4) was initiated by injecting 100 µL of polymer-drug solution into 50 mL of PBS (pH 7.1) that was preheated to 37 °C. Implants were then placed in an orbital incubator shaker (New Brunswick Scientific, C24) set at 37 °C and 80 rpm. Samples of the well mixed bath solution surrounding the implant were taken at 20 sec., 10 min., 30 min, 2 hour, 4 hour, 8 hour, 1 day, 2 day, 4 day, and 7 day time points. Solution was not replenished since the total sample removed from the system was less than 5% of the total volume. In addition, a sample of the bath solution was taken after the implant had been allowed to fully degrade to obtain the initial total drug loading mass in each implant. Standard solutions ranging from 1–100 ng/mL of fluorescein dissolved in PBS were kept under identical conditions and sampled at listed time points to account for any photobleaching of drug molecule during release studies.
Drug released from implants at each time point was quantified using a fluorescence plate reader (Tecan Ltd., Infinite 200 series) using an excitation and emission detection wavelength of 485 and 535 nm, respectively. Fluorescein standards were analyzed in a similar manner and a standard curve was calculated to relate fluorescence of sampled time points to drug concentration. Readings from the fluorescence plate reader were imported into MATLAB to compare them to a standard curve and calculate sample drug concentrations. Then, the percent of initial drug released was calculated and displayed as a function of time. To determine significant differences between the data sets, a one way ANOVA was conducted with Tukey’s honestly significant difference criterion at the 1 hour, 1 day, and 1 week time points.
Critical water concentration
The critical water concentration necessary to initiate phase inversion of the polymer solution was investigated by dissolving formulations of varying Mw PLGA and varying P85 into solutions of NMP and H2O with the H2O in NMP ranging from 10–90%. To quantify precipitation of polymer solution in the H2O:NMP solutions, 100 µL of polymer solution was injected into 10 mL of the H2O:NMP solutions, mixed thoroughly with a spatula, and allowed to dissolve over 1 hour. To separate precipitated polymer from solution, samples were centrifuged at 1200 rpm for 2 minutes and the supernatant was decanted from pellet. Distilled water was added to pellet to ensure full precipitation of remaining polymer and allowed to soak for 24 hours. After 24 hours the excess water was separated from the precipitate, the precipitate was lyophilized for 48 hours and dry mass of lyophilized precipitate was recorded. The dry mass of the polymer precipitate was then compared to the total injected mass of polymer to obtain the mass percent of implant precipitation as a function of % H2O in NMP.
Structural analysis of implants with scanning electron microscopy (SEM)
Structural morphology of each implant formulation was observed with SEM micrographs of pre-formed implants. Implants were prepared by injecting 100 µL of each polymer formulation solution (n=3) into 10 mL of pre-heated PBS, and then placed in an orbital shaker set at 37 °C and 80 rpm for seven days. Implant samples were then removed from their bath solution, freeze fractured in liquid nitrogen, and subsequently lyophilized for 24 hours. Prepared samples were then glued to an aluminum stub and sputter coated with 50 Å of Pd. SEM micrographs were then obtained using a Hitachi Field Emission SEM with an acceleration voltage of 10 kV.
Results
Drug release from implants with varying excipient concentration
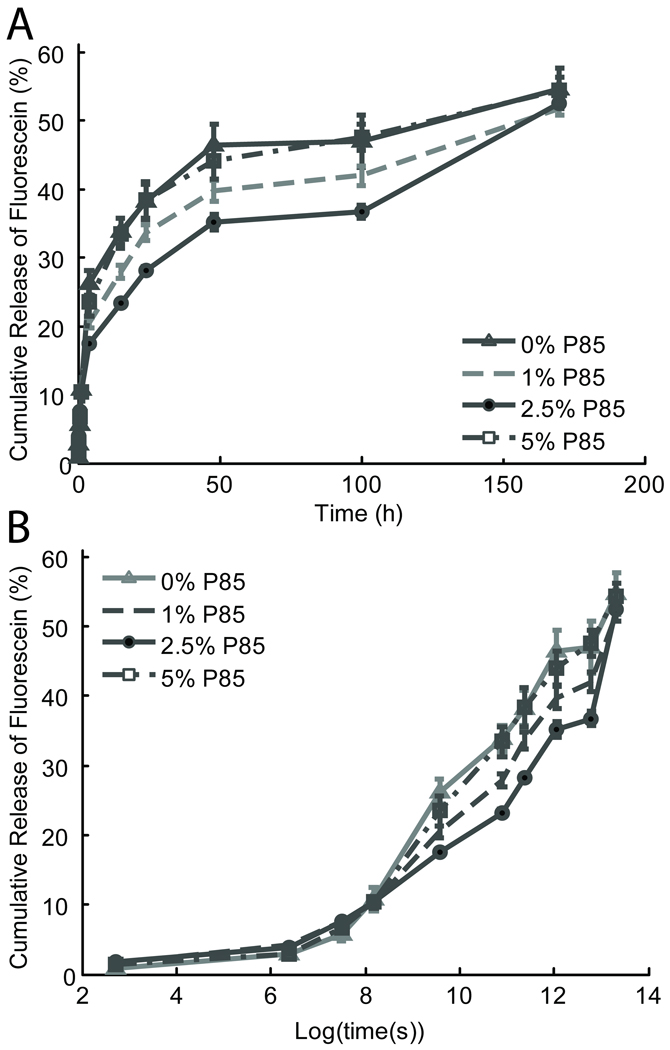
Previous studies examining protein release from ISFI systems have reported that incorporating Pluronic co-block polymers as an excipient molecule can inhibit burst release of protein from these drug delivery systems25. To determine if Pluronic has a similar effect on low Mw compounds, in vitro drug release studies were conducted by measuring fluorescein (Mw 376.28) release from implants with Pluronic P85 concentrations varying from 0–5%. As shown in Figure 1, P85 concentration has a limited effect on the drug release for the first hour after injection. However, during the intermediate 1 hour to 4 day time range, a P85 concentration in the range of 1–2.5% appears to inhibit burst drug release. At the 4 day time point, ISFIs with 1% and 2.5% P85 released a lower mass percent of their drug, 33.6 ± 1.1 and 28.2 ± 0.5 respectively, than formulations without any P85, 38.2 ± 2.5. However increasing P85 beyond this critical concentration range to 5% reversed any inhibition of burst release with these formulations releasing 38.4 ± 2.8 mass percent of their drug. Interestingly, in the day 4 to 7 time range, the previously inhibited 1% and 2.5% P85 formulations released drug at a faster rate and by the day 7 time point all formulations had released a similar mass percent of drug. To determine statistical differences between groups, the cumulative drug release was calculated for each formulation at 1 hour, 1 day, and 1 week time points and a one way ANOVA test was used to determine significant differences (p<0.05) in Table 1. No significant differences between formulation groups were found at the 1 hour and 1 week time points. At the 1 day time point the formulation with 2.5% P85 released a significantly lower percent of drug than the other formulations.
Figure 1.
Fluorescein release from formulations with varying concentrations of P85 over time (h) (A) and Log (time (s)) (B) is displayed to better visualize release over early time points.
Drug release from implants comprised of varying Mw PLGA
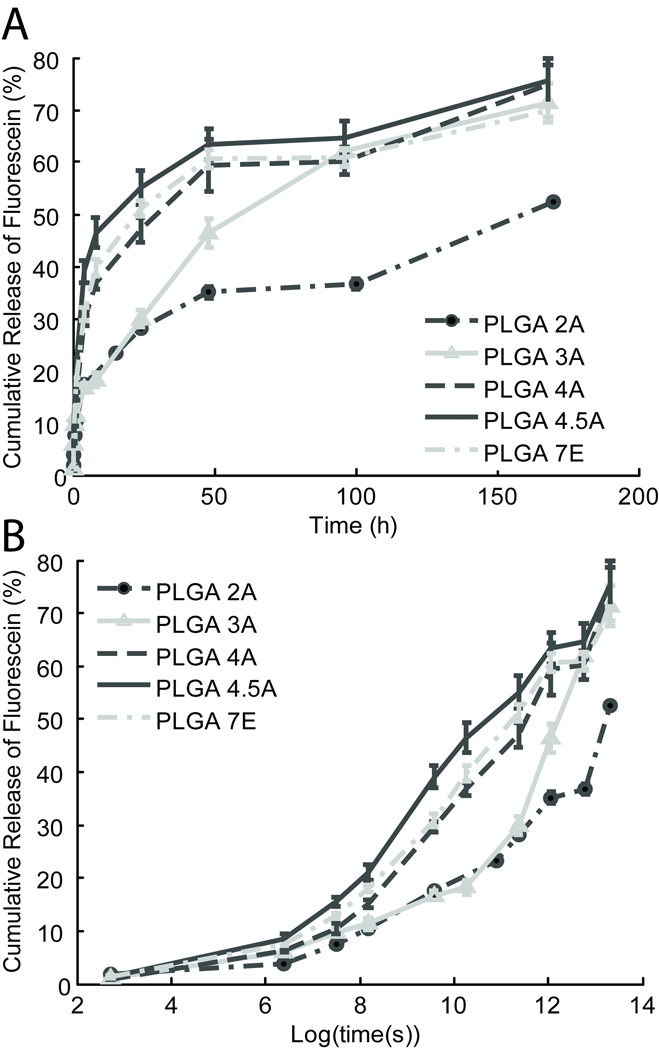
Another factor that may affect drug release from ISFI systems is the Mw of PLGA used to formulate ISFI polymer solution22,23. Formulations comprised of PLGA with Mw ranging from 18–116 kDa were prepared and injected into an in vitro bath solution to examine their drug release over the period of one week. As shown in Figure 2, formulations with lower Mw PLGA (2A and 3A) exhibited a slower drug release than those with the higher Mw PLGA (4A, 4.5A, and 7E). To better visualize the early release, drug release was examined on a log time scale (Figure 2B). Here, it is evident that decreased drug release rate of lower Mw PLGA formulations begins almost immediately after injection. However after the day 4 time point, the lower Mw formulations had an increased release rate, and their total release began to approach that of the higher Mw formulations.
Figure 2.
Fluorescein release from formulations with varying Mw PLGA over time (h) (A) and log (time (s)) (B).
Statistical analysis of the drug release at the 1 hour, 1 day, and 1 week time points is shown in Table 1. While findings were significantly different at a level of p<0.05 between the formulations at the extreme ends of the spectrum, all the 50:50 L/G PLGA polymers displayed a trend whereby lower Mw formulations released drug at a slower rate than their higher Mw counterparts for all time points. However the highest Mw formulation PLGA 7E with a L/G ratio of 75:25 did not follow this trend and released drug at a slower rate than lower Mw PLGA 4.5A formulation. This suggests that in addition to PLGA Mw, the ratio of subunit composition of the PLGA co-polymer also plays a role in affecting drug release from ISFI systems.
Drug release from implants comprised of varying drug loading dosage
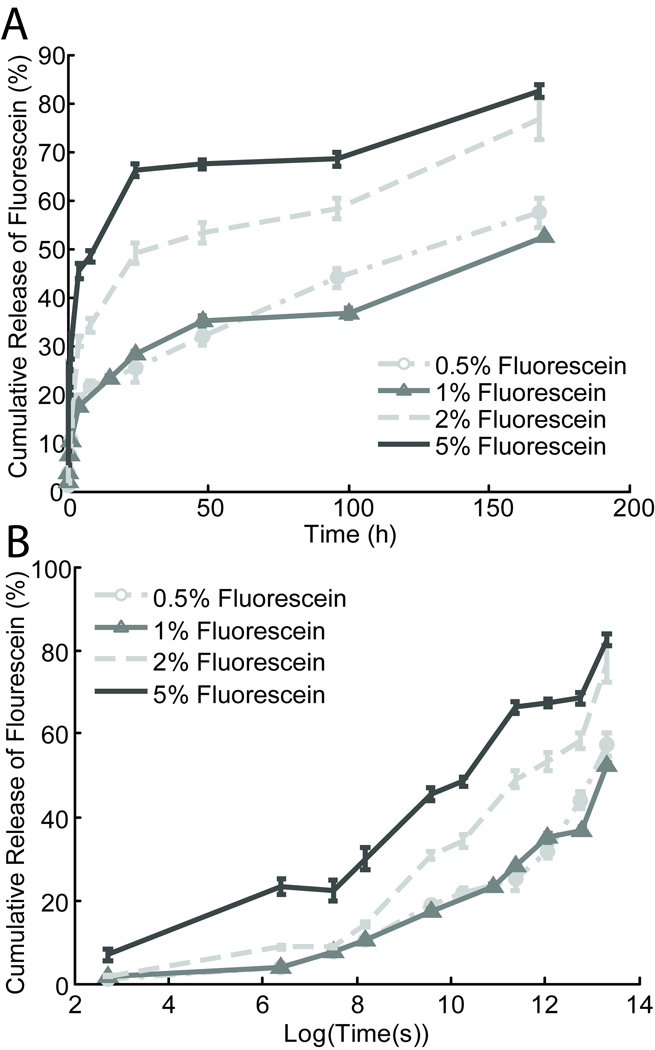
After examining the effects of the PLGA Mw and excipient loading, the effects of varying the drug loading were explored. Because the ISFI systems rely on the process of phase inversion to solidify, increasing the mass percent of drug incorporated into the formulation in exchange for the PLGA component may affect the matrix integrity. Therefore an in vitro drug release study exploring the percent mass of drug released from formulations with varying drug loading dosages from 0.05%–5% was conducted over the time course of one week (Figure 3). Formulations with increased drug loading not only released much higher concentrations of drug as expected, but also had an increased release rate of total drug. The lower 0.05% and 1% drug loading dosages showed a release of drug at 1 hour, 1 day and 1 week, while the higher 2% and 5% dosage formulations showed a significantly higher (p<0.05) total percent of drug released at the corresponding time points (Table 1).
Figure 3.
(A) Fluorescein release from formulations with varying drug loading over time (h), and log (time (s)) (B).
Critical water concentration for phase inversion in varying P85 and MW PLGA polymer formulations
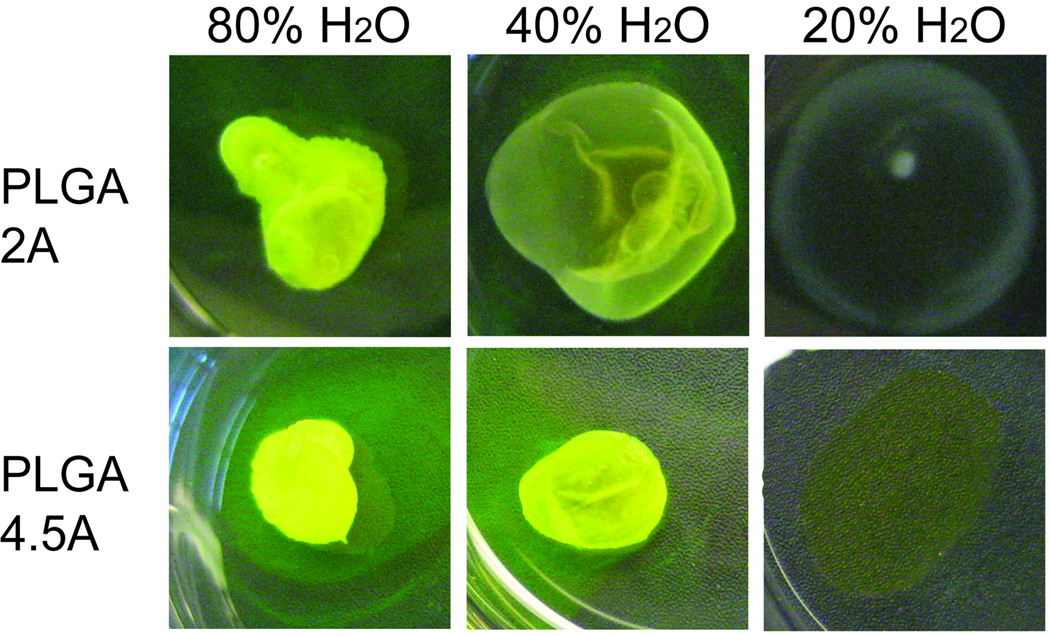
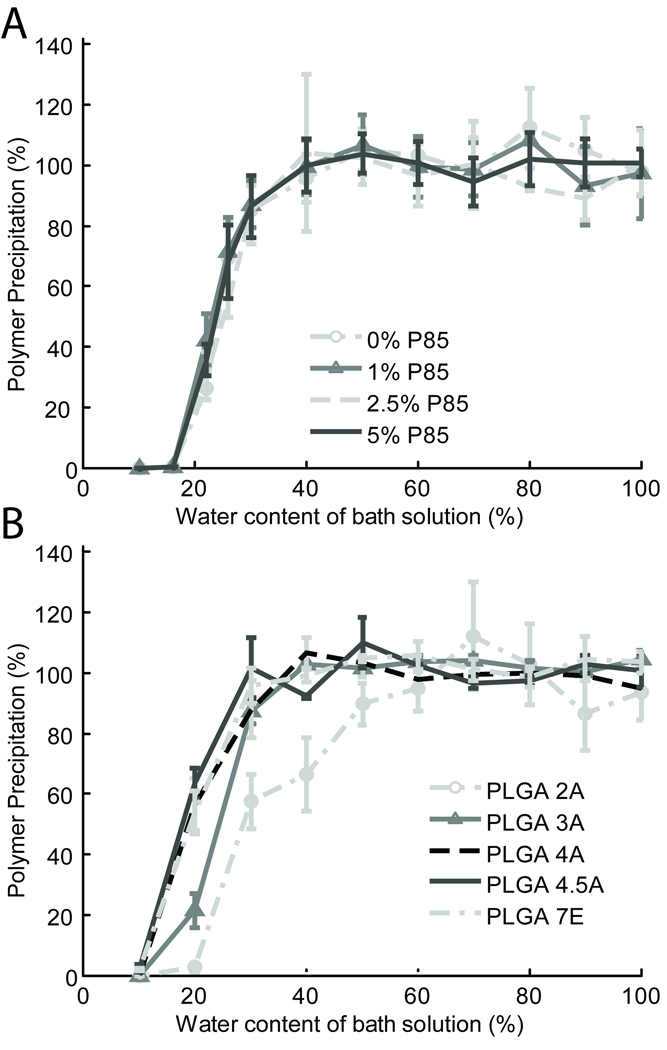
The critical water concentration at which polymer formulations began to precipitate out of solution was investigated by both qualitative and quantitative means. Upon qualitative examination of varying Mw PLGA formulations injected into solutions of differing concentrations of %H2O in NMP, implants comprised of the lowest Mw PLGA, 2A (16 kDa), appeared less dense than implants comprised of the highest Mw PLGA, 4.5A (60 kDa) tested (Figure 4). When both formulations were injected into a solution of 20% H2O in NMP, neither formed an implant. However the higher Mw PLGA 4.5A formulation appears to retain some drug in its matrix, as noted by the yellow color of the film, while the lower Mw PLGA appears as a thin clear film (Figure 4, 20% H2O).
Figure 4.
Representative images of ISFI formulations comprised of PLGA 2A (18 kDa) and PLGA 4.5A (60 kDa) injected into solutions of varying concentrations of H2O (20%, 40%, 80%) in NMP. Lower Mw PLGA 2A implants appear less dense than their counterparts from the higher Mw PLGA 4.5A for all H2O:NMP solutions.
Quantitatively, as shown in Figure 5, lower Mw polymers such as the PLGA 2A and 3A require a greater critical water concentration to precipitate out of solution than the higher Mw 4A, 4.5A, and 75:25 polymers. In contrast, varying Pluronic P85 appeared to have no effect on rate of polymer precipitation. To compare the differences between groups, the water concentration required for 25% and 50% of the initial injected polymer mass to precipitate was interpolated, and a one way ANOVA with a Tukey criterion were used to determine significance (Table 2). Statistical analysis confirmed that the lower Mw formulations 2A and 3A required a significantly (p<0.05) greater critical water concentration than their higher Mw counterparts. No significant differences were seen between formulations with varying P85.
Figure 5.
The percent implant formation or total percent of initial polymer mass that precipitated from solution as a function of water concentration in solution for formulations with varying P85 (A) and varying Mw PLGA (B).
Table II.
Summary of ISFI Drug Release
| Formulations | 1 hour | 1 day | 1 week |
|---|---|---|---|
| Varying P85 | |||
| 0% P85 | 10.8 ± 1.7 | 38.2 ± 2.5 | 54.7 ± 3 |
| 1% P85 | 10.2 ± 0.6 | 33.6 ± 1.1 | 51.8 ± 1.1 |
| 2.5% P85 | 10.4 ± 0.5 | 28.2 ± 0.5† | 52.4 ± 0.4 |
| 5% P85 | 10.4 ± 0.4 | 38.4 ± 2.8 | 54.4 ± 1.9 |
| Varying Mw | |||
| PLGA 2A | 10.4 ± 0.5‡ | 28.2 ± 0.5‡ | 52.4 ± 0.4¥ |
| PLGA 3A | 11.3 ± 1.0Ŧ | 30.0 ± 0.7‡ | 71.4 ± 3.8 |
| PLGA 4A | 15.2 ± 0.7* | 47.0 ± 2.3 | 74.9 ± 3.8 |
| PLGA 4.5A | 21.0 ± 1.4 | 55.1 ± 3.1 | 75.6 ± 4.3 |
| PLGA 7E | 18.0 ± 0.6 | 51.2 ± 1.5 | 69.9 ± 1.3 |
| Varying Loading | |||
| 0.5% loading dose | 10.6 ± 0.2 | 25.3 ± 2.8§ | 57.4 ± 2.9§ |
| 1% loading dose | 10.4 ± 0.5 | 28.2 ± 0.5§ | 52.4 ± 0.4§ |
| 2% loading dose | 14.2 ± 0.6 | 49.0 ± 2.1 | 76.7 ± 4.3 |
| 5% loading dose | 29.9 ± 2.7** | 66.3 ± 1.3 | 82.5 ± 1.4 |
All values shown as ± Standard Error.
Indicates significant difference (p<0.05) from all other varying P85 formulations
Indicates significant difference (p<0.05) from PLGA 4A, 4.5A, and 7E
Indicates significant difference (p<0.05) from PLGA 4.5A, and 7E
Indicates significant difference (p<0.05) from PLGA 2A and 4.5A
Indicates significant difference (p<0.05) from all other Mw formulations
Indicates significant difference (p<0.05) form all other drug loading formulations
Indicates significant difference (p<0.05) from 2% and 5% drug loading
Morphological analysis of polymer implants using scanning electron microscopy
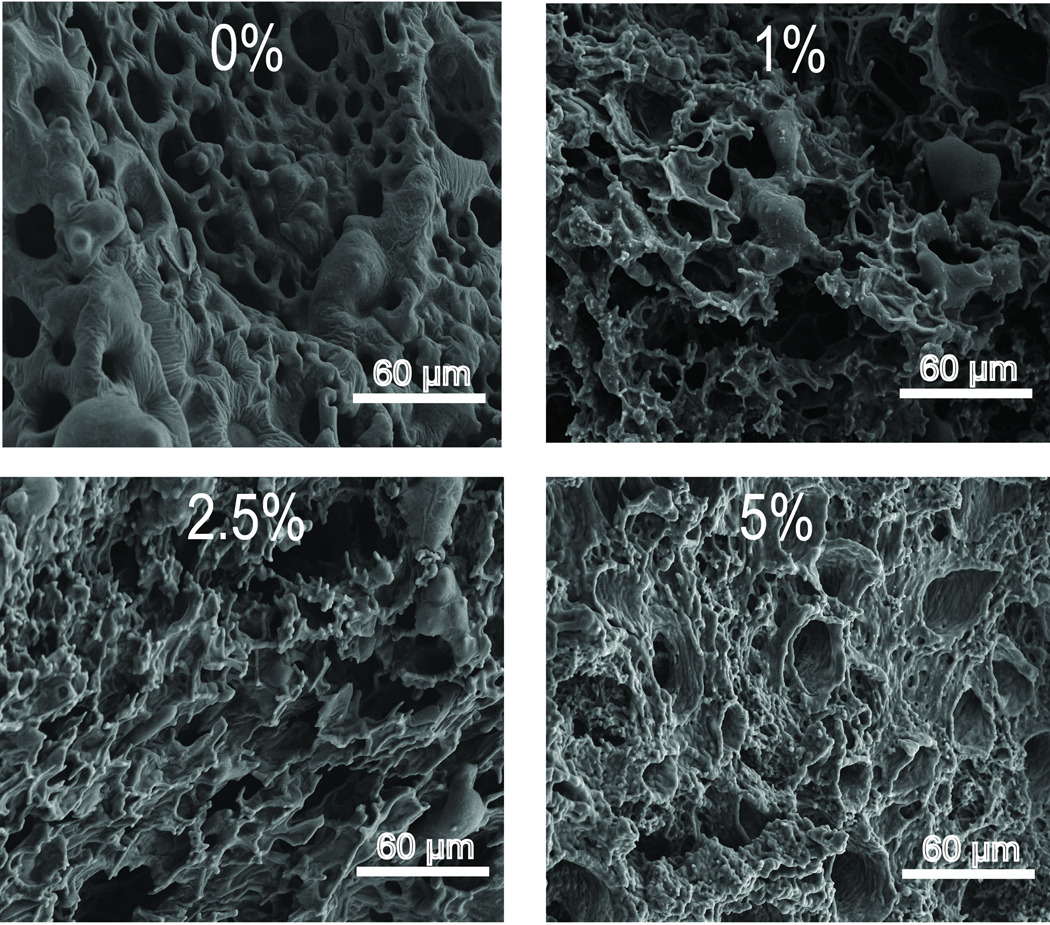
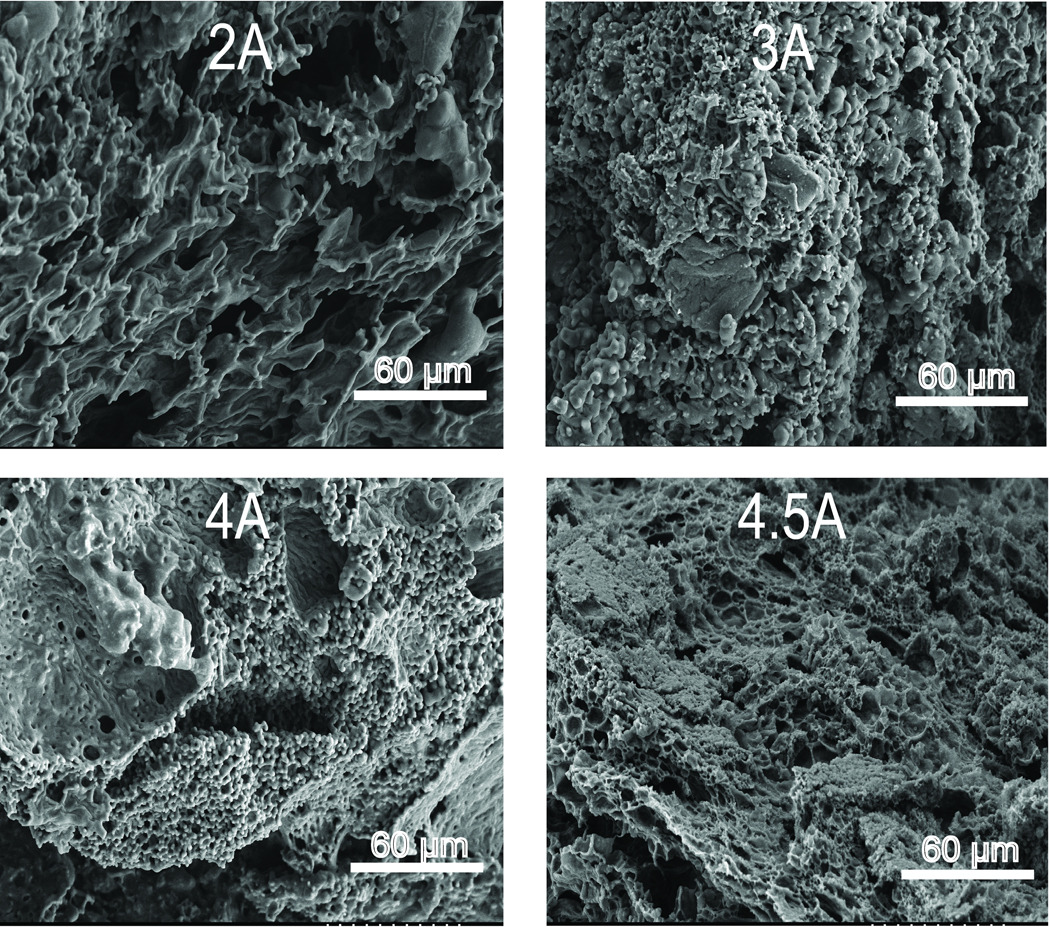
To determine the effects of polymer Mw or use of P85 excipient on implant structure, SEM micrographs of freeze fractured cross-sections of one week old, fully formed implants were acquired. In the micrographs of implants with varying excipient, increased asymmetry and disorganization was seen with increasing concentrations of P85 (Figure 6). Micrographs of implants without excipient (Figure 6, 0%) display a typical honeycomb-like structure with relatively uniform macrovoids and interconnected pores as reported by others27. However micrographs of implants with increasing concentrations of P85 show increasing irregularity with polymer strands attached to the honey-comb like PLGA matrix (Figure 6, 1%, 2.5%, 5%). Qualitative examination of SEM micrographs of implant cross sections with varying Mw PLGA show that implants with lower MW PLGA are more porous than those from higher Mw PLGA (Figure 7). However, while the difference in porosity is readily seen between the cross-section of the lowest Mw PLGA 2A implant and the highest Mw PLGA 4.5A implant, it is difficult to determine any differences in the micrographs of the highest Mw PLGA 4A and 4.5A implant cross-sections.
Figure 6.
SEM micrographs of implant cross sections from formulations with varying concentrations of P85.
Figure 7.
SEM micrographs of implant cross sections from formulations with varying Mw PLGA.
Discussion
The ideal release profile for intratumoral chemotherapeutic drug delivery implants for use after minimally invasive ablative treatments is a short period of burst drug release to quickly reach therapeutic drug levels followed by a sustained constant drug release to maintain those levels over an extended time6. To achieve this type of release, solid drug delivery implants have used a dual layered strategy whereby an outer quick burst release coating is applied to an inner slow release matrix core6. This release profile is very similar to the drug release patterns seen with fast phase inverting ISFI devices. When these ISFI solutions are injected into an aqueous environment, the surface layer quickly phase inverts and the drug contained within is rapidly released28. Then, with continued diffusion of water into the implant, the process of phase inversion continues, and a lower maintenance dosage of drug is released at a slower rate. While this type of release profile is characteristic for fast phase inverting ISFI systems, our current studies show that the burst release and subsequent drug release rates can vary greatly depending on the formulation components. Utilizing this information can enhance the design of implants with optimal levels of burst drug release followed by adequate maintenance dose release.
In ISFI systems designed for protein delivery, the burst drug release was seen as an adverse event, and its elimination has been the focus of many prior studies. Different organic solvents such as ethyl benzoate, or excipient additives such as Pluronic have been utilized to influence the rate of phase inversion leading to a reduction in burst release25. However, for chemotherapeutic implants, a limited burst drug release can be beneficial, thus our goal was to formulate implants by which burst release could be controlled but not entirely eliminated. Therefore slow phase inverting systems that display zero order release profiles, such as those using ethyl benzoate, were not of interest in these studies. In contrast, formulations utilizing Pluronic that had been shown to reduce or limit burst drug release less dramatically were a viable option18.
However while results from our drug release studies did show that P85 displayed some ability to limit drug release in the intermediate time range (1 hour – 2 days), this effect was much less pronounced than that reported in studies examining release of higher Mw drugs25. Qualitative examination of our SEM micrographs corroborated previous findings by Desnoyer et al, which found that Pluronic interacts with the implant pore structure thereby hindering diffusion25. Therefore while transport of higher Mw drugs with larger radii may be hindered by Pluronic, these obstructions have only limited effects on release of low Mw compounds.
Conversely, results from formulations with varying Mw PLGA demonstrated that polymer Mw significantly affects low Mw drug release profiles from ISFI systems. However, contrary to expectation, although ISFIs made utilizing lower Mw PLGA particles have greater porosity, they release drug at a slower rate than their higher Mw counterparts. One explanation for this phenomenon is that the lower Mw formulations have a slower rate of phase inversion; drug is trapped in the non-phase inverted core for a greater period of time in lower Mw ISFI systems. This theory is supported by the critical water concentration studies in which the lower Mw PLGA polymer formulations require a higher critical water concentration to undergo the process of phase inversion. In addition to Mw, the drug release and critical water concentration for the PLGA 7E formulation demonstrates subunit composition also plays a role in drug release. The 75:25 L/G ratio for PLGA 7E contains a greater proportion of the more hydrophobic lactic acid moiety and is therefore less soluble in aqueous solutions. This likely explains why the ISFIs formed from PLGA 7E formulation had a lower critical water concentration and slower drug release rate than the much lower Mw PLGA 4.5A ISFI systems.
Although similar results demonstrating that PLGA Mw plays a significant role in determining drug release from ISFI implants, the effect of polymer Mw on drug release has conflicting reports in the literature. A recent study by Astaneh et el, found medium Mw PLGA (34 kDa) ISFIs having greater burst release than low (12 kDa) and high (48 kDa) Mw PLGA ISFIs of peptide drug molecules22. Another study by Kost et al, found that ISFIs formulated from high Mw PLGA solidify faster and therefore have a slower rate of drug release than low Mw PLGA ISFIs29. While our results conflict with these reports, it is important to note that both of these studies examined release of high Mw peptide drug molecules in contrast to the low Mw drug molecules that were the focus of our experiments. However one of these studies on high Mw drug release by Luan et al, reported similar findings in which drug release from high Mw PLGA particles was faster than low Mw particles in a related system, in situ forming microparticles (ISM)30. However these findings were attributed to the greater porosity seen in particles comprised of high Mw polymer as well as interaction of charged amino acid residues on the drug molecule interacting with polymer carboxylic acid groups30,31. The effect of phase inversion rate on drug release in this microparticle system was likely limited due to the high surface area to volume ratio of these particles. In contrast, examination of SEM micrographs of our implants show lower Mw ISFIs having greater porosity than higher Mw ISFIs, and our drug release findings can be attributed to the faster rate of phase inversion seen in high Mw PLGA ISFIs.
In addition to polymer Mw, another factor that must be considered when formulating ISFIs is the integrity of the PLGA matrix. If too much of the polymer component in the formulation is replaced with either excipient or drug, the interconnectivity between pores in the formed implant may be above the critical percolation threshold, which will increase diffusion of drug out of the implant thereby limiting its ability to release drug in a sustained manner. In the present study experiments with varying P85 and varying drug loading demonstrate this fact when the concentration of these additives reaches a critical threshold. These studies suggest that when the mass percent of PLGA in the polymer formulation decreases below 33% (5% drug loading and 5% P85 trials), the matrix integrity is compromised. However, both formulations for these trials utilize the PLGA 2A, and formulations with higher Mw PLGA or with a greater L/G ratio maybe able to form a stable implant with a lower mass percent of PLGA as is consistent with other ISFI drug release studies.
The results from these studies demonstrate that by varying the polymer formulation of ISFI systems, release can be optimized for delivery of low Mw drug molecules. While these studies with the mock drug molecule, fluorescein, provide a good approximation of release of low Mw hydrophilic drug particles, more hydrophobic drugs likely exhibit a different release profile. Therefore future work should also examine release of a variety of drugs with differing water solubility. Additionally, the effect of PLGA L/G subunit ratio should be examined in greater detail to determine if the results seen with our PLGA 7E trials follow a consistent trend.
Conclusion
In contrast to drug release studies with high Mw peptide drugs, our results demonstrate that excipient Pluronic P85 does not have a large effect on decreasing burst drug release of low Mw drugs from ISFI systems. However, we have found that the burst release of low Mw drugs from ISFI systems can be modulated to a great extent by the polymer Mw. Our findings suggest that formulations comprised of lower Mw PLGA particles have significantly lower burst release than formulations utilizing higher Mw PLGA particles, a phenomenon most likely resulting from increasing affinity of the lower Mw polymer to water. This relationship between polymer Mw and phase inversion can be utilized to modulate burst drug release to optimized levels in ISFI devices for low Mw drug delivery.
Table III.
Summary of water concentration required for polymer precipitation
| Formulations | %H20 for 25% precipitation | %H20 for 50% precipitation |
|---|---|---|
| Varying Pluronic P85 | ||
| 0% P85 | 20.92 ± 1.91 | 24.71 ± 0.79 |
| 1% P85 | 20.03 ± 5.48 | 28.67 ± 0.87 |
| 2.5% P85 | 21.24 ± 2.97 | 27.9 ± 1.05 |
| 5% P85 | 20.49 ± 1.13 | 24.51 ± 0.75 |
| Varying PLGA Mw | ||
| PLGA 2A | 27.05 ± 1.69† | 35.47 ± 2.57† |
| PLGA 3A | 19.9 ± 1.12† | 24.26 ± 0.61 |
| PLGA 4A | 14.8 ± 0.83 | 19.59 ± 1.65 |
| PLGA 4.5A | 13.78 ± 0.42 | 17.92 ± 0.67 |
| PLGA 7E | 14.84 ± 0.93 | 19.36 ± 1.2 |
Sample is significantly different (p<0.05) from all other varying Mw formulations. All values shown as ± Standard Error.
Acknowledgments
This work was supported by NIH grant R01CA1118399 to AAE. RP is also supported in part by the NIH grant T32GM07250 to the Case Western Reserve University Medical Scientist Training Program. The authors would also like to acknowledge Irene Panagopoulos for help with fluorescence analysis and Chris Hoffman for help with the SEM.
Contributor Information
Ravi B. Patel, Department of Biomedical Engineering, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106, phone: 216-983-3011, fax: 216-844-5922, ravi.patel@case.edu
Angela Carlson, Department of Biomedical Engineering, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106, phone: 216-983-3011, fax: 216-844-5922, angela.carlson@case.edu.
Luis Solorio, Department of Biomedical Engineering, Case Western Reserve University, 10900 Euclid Ave, Cleveland, OH 44106, phone: 216-844-0077, fax: 216-844-5922, luis.solorio@case.edu.
Agata A. Exner, Department of Radiology, Case Western Reserve University, 11100 Euclid Ave, Cleveland, OH 44106, phone: 216-844-3544, fax: 216-844-5922, agata.exner@case.edu
References
- 1.Laurencin CT, Langer R. Polymeric controlled release systems: new methods for drug delivery. Clin Lab Med. 1987;7(2):301–323. [PubMed] [Google Scholar]
- 2.Dash AK, Cudworth GC., 2nd Therapeutic applications of implantable drug delivery systems. J Pharmacol Toxicol Methods. 1998;40(1):1–12. doi: 10.1016/s1056-8719(98)00027-6. [DOI] [PubMed] [Google Scholar]
- 3.Exner AA, Saidel GM. Drug-eluting polymer implants in cancer therapy. Expert Opin Drug Deliv. 2008;5(7):775–788. doi: 10.1517/17425247.5.7.775. [DOI] [PubMed] [Google Scholar]
- 4.Weinberg BD, Blanco E, Gao J. Polymer implants for intratumoral drug delivery and cancer therapy. J Pharm Sci. 2008;97(5):1681–1702. doi: 10.1002/jps.21038. [DOI] [PubMed] [Google Scholar]
- 5.Saltzman WM, Fung LK. Polymeric implants for cancer chemotherapy. Adv Drug Deliv Rev. 1997;26(2–3):209–230. doi: 10.1016/s0169-409x(97)00036-7. [DOI] [PubMed] [Google Scholar]
- 6.Qian F, Saidel GM, Sutton DM, Exner A, Gao J. Combined modeling and experimental approach for the development of dual-release polymer millirods. J Control Release. 2002;83(3):427–435. doi: 10.1016/s0168-3659(02)00217-1. [DOI] [PubMed] [Google Scholar]
- 7.Qian F, Nasongkla N, Gao J. Membrane-encased polymer millirods for sustained release of 5-fluorouracil. J Biomed Mater Res. 2002;61(2):203–211. doi: 10.1002/jbm.10156. [DOI] [PubMed] [Google Scholar]
- 8.Bernatchez SF, Merkli A, Minh TL, Tabatabay C, Anderson JM, Gurny R. Biocompatibility of a new semisolid bioerodible poly(ortho ester) intended for the ocular delivery of 5-fluorouracil. J Biomed Mater Res. 1994;28(9):1037–1046. doi: 10.1002/jbm.820280908. [DOI] [PubMed] [Google Scholar]
- 9.Berrada M, Yang Z, Lehnert S. Tumor treatment by sustained intratumoral release of 5-fluorouracil: effects of drug alone and in combined treatments. Int J Radiat Oncol Biol Phys. 2002;54(5):1550–1557. doi: 10.1016/s0360-3016(02)03740-9. [DOI] [PubMed] [Google Scholar]
- 10.Brem H, Gabikian P. Biodegradable polymer implants to treat brain tumors. J Control Release. 2001;74(1–3):63–67. doi: 10.1016/s0168-3659(01)00311-x. [DOI] [PubMed] [Google Scholar]
- 11.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41(6):403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 12.Weinberg BD, Ai H, Blanco E, Anderson JM, Gao J. Antitumor efficacy and local distribution of doxorubicin via intratumoral delivery from polymer millirods. J Biomed Mater Res A. 2007;81(1):161–170. doi: 10.1002/jbm.a.30914. [DOI] [PubMed] [Google Scholar]
- 13.Au JL, Jang SH, Zheng J, Chen CT, Song S, Hu L, Wientjes MG. Determinants of drug delivery and transport to solid tumors. J Control Release. 2001;74(1–3):31–46. doi: 10.1016/s0168-3659(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 14.Strasser JF, Fung LK, Eller S, Grossman SA, Saltzman WM. Distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea and tracers in the rabbit brain after interstitial delivery by biodegradable polymer implants. J Pharmacol Exp Ther. 1995;275(3):1647–1655. [PubMed] [Google Scholar]
- 15.Minchinton AI, Tannock IF. Drug penetration in solid tumours. Nat Rev Cancer. 2006;6(8):583–592. doi: 10.1038/nrc1893. [DOI] [PubMed] [Google Scholar]
- 16.Weinberg BD, Patel RB, Wu H, Blanco E, Barnett CC, Exner AA, Saidel GM, Gao J. Model simulation and experimental validation of intratumoral chemotherapy using multiple polymer implants. Med Biol Eng Comput. 2008;46(10):1039–1049. doi: 10.1007/s11517-008-0354-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weinberg BD, Blanco E, Lempka SF, Anderson JM, Exner AA, Gao J. Combined radiofrequency ablation and doxorubicin-eluting polymer implants for liver cancer treatment. J Biomed Mater Res A. 2007;81(1):205–213. doi: 10.1002/jbm.a.30926. [DOI] [PubMed] [Google Scholar]
- 18.Krupka TM, Weinberg BD, Ziats NP, Haaga JR, Exner AA. Injectable polymer depot combined with radiofrequency ablation for treatment of experimental carcinoma in rat. Invest Radiol. 2006;41(12):890–897. doi: 10.1097/01.rli.0000246102.56801.2f. [DOI] [PubMed] [Google Scholar]
- 19.Dunn RL, English JP, Cowsar DP. Biodegradable in-situ forming implants and methods of producing the same. 1990 July 3; [Google Scholar]
- 20.Sartor O. Eligard: leuprolide acetate in a novel sustained-release delivery system. Urology. 2003;61(2 Suppl 1):25–31. doi: 10.1016/s0090-4295(02)02396-8. [DOI] [PubMed] [Google Scholar]
- 21.McHugh AJ. The role of polymer membrane formation in sustained release drug delivery systems. J Control Release. 2005;109(1–3):211–221. doi: 10.1016/j.jconrel.2005.09.038. [DOI] [PubMed] [Google Scholar]
- 22.Astaneh R, Erfan M, Moghimi H, Mobedi H. Changes in morphology of in situ forming PLGA implant prepared by different polymer molecular weight and its effect on release behavior. J Pharm Sci. 2009;98(1):135–145. doi: 10.1002/jps.21415. [DOI] [PubMed] [Google Scholar]
- 23.Ravivarapu HB, Moyer KL, Dunn RL. Parameters affecting the efficacy of a sustained release polymeric implant of leuprolide. Int J Pharm. 2000;194(2):181–191. doi: 10.1016/s0378-5173(99)00371-3. [DOI] [PubMed] [Google Scholar]
- 24.Brodbeck KJ, DesNoyer JR, McHugh AJ. Phase inversion dynamics of PLGA solutions related to drug delivery. Part II. The role of solution thermodynamics and bath-side mass transfer. J Control Release. 1999;62(3):333–344. doi: 10.1016/s0168-3659(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 25.DesNoyer JR, McHugh AJ. The effect of Pluronic on the protein release kinetics of an injectable drug delivery system. J Control Release. 2003;86(1):15–24. doi: 10.1016/s0168-3659(02)00293-6. [DOI] [PubMed] [Google Scholar]
- 26.Periti P, Mazzei T, Mini E. Clinical pharmacokinetics of depot leuprorelin. Clin Pharmacokinet. 2002;41(7):485–504. doi: 10.2165/00003088-200241070-00003. [DOI] [PubMed] [Google Scholar]
- 27.Pean JM, Venier-Julienne MC, Boury F, Menei P, Denizot B, Benoit JP. NGF release from poly(D,L-lactide-co-glycolide) microspheres. Effect of some formulation parameters on encapsulated NGF stability. J Control Release. 1998;56(1–3):175–187. doi: 10.1016/s0168-3659(98)00086-8. [DOI] [PubMed] [Google Scholar]
- 28.Graham PD, Brodbeck KJ, McHugh AJ. Phase inversion dynamics of PLGA solutions related to drug delivery. J Control Release. 1999;58(2):233–245. doi: 10.1016/s0168-3659(98)00158-8. [DOI] [PubMed] [Google Scholar]
- 29.Eliaz RE, Kost J. Characterization of a polymeric PLGA-injectable implant delivery system for the controlled release of proteins. J Biomed Mater Res. 2000;50(3):388–396. doi: 10.1002/(sici)1097-4636(20000605)50:3<388::aid-jbm13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Luan X, Bodmeier R. Influence of the poly(lactide-co-glycolide) type on the leuprolide release from in situ forming microparticle systems. J Control Release. 2006;110(2):266–272. doi: 10.1016/j.jconrel.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Jaraswekin S, Prakongpan S, Bodmeier R. Effect of poly(lactide-co-glycolide) molecular weight on the release of dexamethasone sodium phosphate from microparticles. J Microencapsul. 2007;24(2):117–128. doi: 10.1080/02652040701233655. [DOI] [PubMed] [Google Scholar]