Abstract
Circadian rhythms in physiology and behavior are temporally synchronized to the day:night cycle through the action of light on the circadian clock. In mammals, transduction of the photic signal reaching the circadian oscillator in the suprachiasmatic nucleus (SCN) occurs through the release of glutamate and pituitary adenylate cyclase-activating peptide (PACAP). Our study aimed at clarifying the role played by PACAP in photic resetting and entrainment. We investigated the circadian response to light of PACAP-null mice lacking the 5th exon of the PACAP coding sequence. Specifically, we examined free-running rhythms, entrainment to 12-h light:12-h dark (LD) cycles, the phase response curve (PRC) to single light pulses, entrainment to a 23-h T-cycle, re-entrainment to 6-h phase shifts in LD cycles, and light-induced c-Fos expression. PACAP-null and wild-type mice show similar free-running periods and similar entrainment to 12:12 LD cycles. However, the PRC of PACAP-null mice lacks a phase-advance portion. Surprisingly, despite the absence of phase advance to single light pulses, PACAP-null mice are able to entrain to a 23-h T-cycle, but with a significantly longer phase angle of entrainment than wild types. In addition, PACAP-null mice re-entrain more slowly to a 6-h phase advance of the LD cycle. Nevertheless, induction of c-Fos by light in late night is normal. In all experiments, PACAP-null mice show specific behavioral impairments in response to phase-advancing photic stimuli. These results suggest that PACAP is required for the normal integration of the phase-advancing light signal by the SCN.
Keywords: circadian rhythms, glutamate, light signal, phase-resetting, phase response curve (PRC), plasticity, pituitary adenylate cyclase-activating peptide (PACAP), suprachiasmatic nucleus (SCN)
Photic information embedded in the alternation between days and nights directly affects circadian systems, ensuring that organisms are in tune with the period imposed by the light:dark (LD) cycle (Pittendrigh and Daan, 1976). This entrainment is a fundamental property of circadian clocks and of rhythms they regulate. In mammals, the master circadian clock is localized within the cells of the hypothalamic suprachiasmatic nucleus (SCN) (Klein et al., 1991). The SCN receives photic information directly from the retina via a retinohypothalamic tract (RHT) projection that contains glutamate and pituitary adenylate cyclase-activating peptide (PACAP) (Hannibal et al., 2000; Moore and Lenn, 1972). The current model for light-induced clock resetting proposes that glutamate and PACAP are co-released in the SCN upon photic stimulation. Whereas the role of glutamate in clock resetting has received considerable attention (Ding et al., 1994; Ebling, 1996; Gillette and Mitchell, 2002), the contribution of PACAP to the photic response of the SCN is still unclear (Morin and Allen, 2006).
Multiple animal models have been designed to clarify the contribution of PACAP to circadian function. Three independently developed strains of transgenic mice with null mutations for PACAP expression have been created, as well as a knockout of PAC1R, the primary PACAP receptor (reviewed in Hannibal, 2006) (Hannibal et al., 1997; Kopp et al., 1999; von Gall et al., 1998). Circadian behavioral responses to light of two of these mouse models differ, likely due to experimental idiosyncracies and subtleties of genetic backgrounds and lesions. Specifically, a PACAP peptide-null mouse, developed in the Institute of Cancer Research (ICR) mouse eliminating exon 5 of the PACAP gene-coding sequence (Kawaguchi et al., 2003), exhibits significant attenuations of light-induced phase advance at CT 21, but no significant alteration in behavioral responses to light during the early night (Kawaguchi et al., 2003). These PACAP-null mice also express lower light-induced c-Fos at CT15 compared to WT, but, interestingly, show identical light-induced c-Fos at CT 21, where the behavioral deficits are observed. The PACAP peptide-null mouse developed by Colwell and colleagues (Colwell et al., 2004) lacks exons 3, 4, and 5a of the PACAP gene, eliminating not only PACAP, but also the PACAP-related peptide (PRP) encoded by exons 3 and 4. Significant attenuations of light-induced phase resetting are seen in both phase delays and advances, in contrast to the reports of Kawaguchi et al. (2003). However, in spite of significant deficits in the response to the acute effects of light, these PACAP-null mice re-entrain to 8-h shifts in the LD cycle at normal rates (Colwell et al., 2004). The third PACAP-null mouse model has not been studied in a circadian context, and is the subject of the present report (Hamelink et al., 2002).
To more thoroughly investigate the role of PACAP in circadian resetting, we evaluated the circadian responses to light of this third PACAP-null animal model. This animal was generated in the C57BL/6 strain with only the PACAP-coding sequence deleted, eliminating a potential source of confound in the results. Details and specificity of the genetic lesion, complete loss of PACAP expression and functional deficits in PACAP-mediated adrenal function have been described previously (Hamelink et al., 2002). We paid particular attention to the phase-advance portion of the circadian cycle, a time where models studied previously displayed common deficits in light-induced resetting.
We report here that mice lacking PACAP show specific impairment in their ability to generate phase advances to a single brief light exposure when housed in darkness, whereas endogenous free-running period and phase-delaying capabilities are intact. PACAP-null mice are able to entrain to a 23-h T-cycle, although with longer phase-angle of entrainment, and with slower rates of entrainment following a 6-h step advance of the LD cycle. These data indicate that PACAP contributes to normal integration of the phase-advancing signal required for acute phase shifts and for entrainment.
MATERIAL AND METHODS
Animals
All manipulations were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee and the Division of Animal Resources at the University of Illinois at Urbana-Champaign. Homozygote PACAP-null mice were derived as described previously (Hamelink et al., 2002) and bred onto a C57Bl/6 background through 12 successive back-crossings (Hamelink et al., 2002). C56BL/6 mice were obtained from the same commercial source as the C57Bl/6 mice used for the backcross line (Charles River), as recommended by the Banbury Conference on Genetic Background in Mice (Mice, 1997).
Apparatus
Mice were housed in clear plastic cages (21cm × 27cm × 14cm) equipped with a running wheel (9 cm in diameter). Cages were placed in a circadian activity monitoring system (CAMS), which consists in a light-tight 1.7 ft3 refrigerator equipped with independent ventilation and a computer-controlled lighting. Two cages were fitted in each CAMS. Food and water were available ad libitum and replenished weekly. Cages were changed every 10 days. Circadian running-wheel behavior was analyzed with Clocklab software (Actimetrics).
Free-running period, entrainment and PRC determinations
WT (n = 37) and PACAP-null male mice (n = 38) first were entrained stably to a 12-h light (100 lux):12-h dark (12:12 LD) cycle. Then, they were allowed to free run in darkness (DD) for at least 10 days to measure free-running circadian periods (τ). Free-running mice were exposed to a 15 min, 100-lux light pulse at one of the following circadian times: CT 0, 1, 2, 3, 4, 6, 12, 14, 16, 18, 20, 22, 23. CT 12 marked the onset of the active phase. At the appropriate CT, the subject was removed from the home CAMS under dim red illumination and placed in an independent illuminated CAMS for the duration of the light pulse. Cage changes were performed at the same time as administration of the light pulse. Animals were allowed to free-run for 10 days before and 10 days after the light pulse in order to measure the magnitude and direction of the phase shift. Each mouse received a maximum of 5 light pulses in a counter-balanced fashion. To control for the effects of handling and cage changes, mice of each genotype were handled identically but were placed in a dark CAMS for 15 min (dark controls). Each animal’s period was used to calculate the time of stimulus onset. Phase shifts were calculated from the difference between lines of best fit marking activity onset for the 5 days prior to and 5 days after the light pulse, with the first two days after the light pulse omitted. Each time point of the PRC comprises phase shift observation from a minimum of 4 and a maximum of 16 mice.
Entrainment to 23-h T-cycles
Free-running male WT (n = 11) and PACAP-null mice (n = 12) were exposed to a 0.25-h light, 22.75-h dark cycle (23-h T-cycle, 100 lux). The first 23-h T-cycle entraining light pulse was presented at ~CT 4, and mice were maintained on this schedule until stably entrained. The phase angle of entrainment was measured as the difference in hours between the onset of activity and the onset of the entraining light pulse. To ascertain entrainment, we calculated the circadian period 5 days prior to and the last 5 days of the 23-h T-cycle.
6-h phase advances, 6-h phase delays
WT (n = 31, 19 males, 12 females) and PACAP-null mice (n = 21, 16 males, 5 females) stably entrained to a 12:12 LD cycle were subjected to an abrupt, 6-h shift in the LD cycle. The onset of the light portion of the LD cycle was either advanced or delayed by 6 h and mice were allowed to re-entrain to the shifted LD cycle. The rate of re-entrainment, calculated as the number of days required to stably re-entrain to the shifted LD cycle, was determined. Animals were divided into 3 categories based on the number of days required for re-entrainment (≤1, 2–3, ≥4), and the respective percentages of each genotype in each categories were calculated.
Immunocytochemistry
WT and PACAP-null mice (n = 4 each) received a 15 min, 100-lux light pulse at CT 22 and were deeply anesthetized (Euthasol, Henry Schein, 100 mg/kg I.P.) 60 min after light onset. Control mice of both genotypes (n = 4 per genotype) were not exposed to light and anesthetized at the same circadian times under dim red illumination. Mice were perfused transcardially with 50 ml of cold physiological saline followed by 50 ml of cold 4% paraformaldehyde in a 0.1 M phosphate buffer. Brains were extracted and post-fixed overnight at 4°C. Free-floating sections (40 µm) from the SCN were cut on a Vibratome and stored in cryoprotectant at −20°C until staining (Watson et al., 1986). Free-floating sections were stained using a nickel chloride-enhanced diaminobenzidine reaction, as previously described with minor modifications (Beaule and Amir, 2003). Modifications consisted of using a pre-blocking solution containing 5% normal goat serum (NGS) and 5% milk in Tris-buffered saline containing 0.3% Triton X-100 (Triton-TBS). Rabbit polyclonal antibody against c-Fos (Ab5, human c-Fos amino acids 4–17, Calbiochem) was diluted 1:100,000 in Triton-TBS, 2% NGS and 5% milk. c-Fos-positive nuclei per section through the medial SCN were counted.
Statistical analysis
Differences between WT and PACAP-null mice in free-running period and phase angles of entrainment were analyzed using Student’s t-test. Data obtained from the rates of re-entrainment (percentages) were analyzed using Chi-square (X2) analysis. The difference between the PRC of WT and PACAP-null mice was analyzed by a two-way ANOVA with genotype and circadian time as independent variables. Differences in c-Fos expression were evaluated using a two-way ANOVA with light treatment and genoptype as independent variables. Post-hoc differences were explored with the Scheffe test (PRC) or the Tukey test (c-Fos). Alpha was set at 0.05 for all statistical tests.
RESULTS
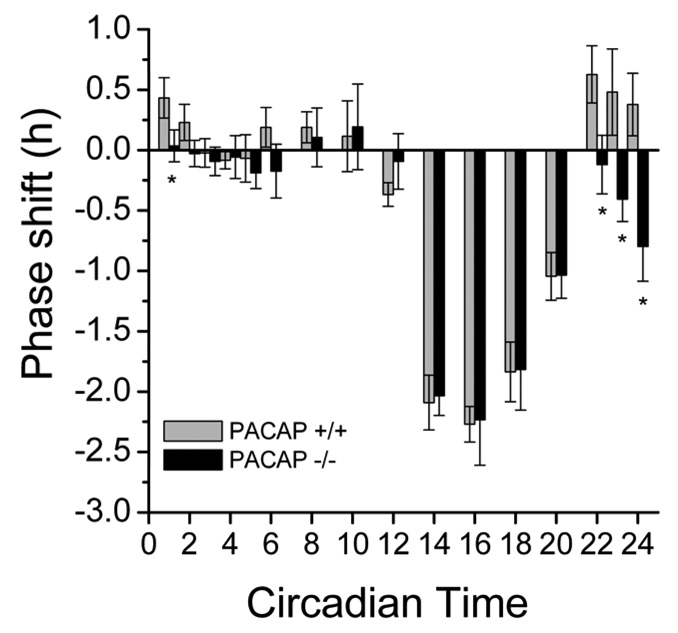
All animals entrained stably to a 12:12 LD cycle. All mice had similar free-running periods in DD (WT: 23.49 ± 0.20 h, n = 37; PACAP-null: 23.48 ± 0.35 h, n = 38, Fig. 1). However, the PRC for 15-min, 100-lux light pulse differs significantly between WT and PACAP-null mice during late subjective night (Fig. 2), consistent with the other animal models. As a group, PACAP-null mice never phase advanced to a single light pulse at any circadian time investigated. Close inspection of the PRC of PACAP-null animals during the predicted phase-advance portion (from CT 20-CT 24/0) revealed small phase delays instead of phase advances (Fig. 2), in agreement with the PRC in PAC1R knockout animals (Hannibal et al, 1997). Although handling procedures caused small phase shifts (less than 30 min) in some dark controls, there was no significant difference between WT and PACAP-null mice (data not shown).
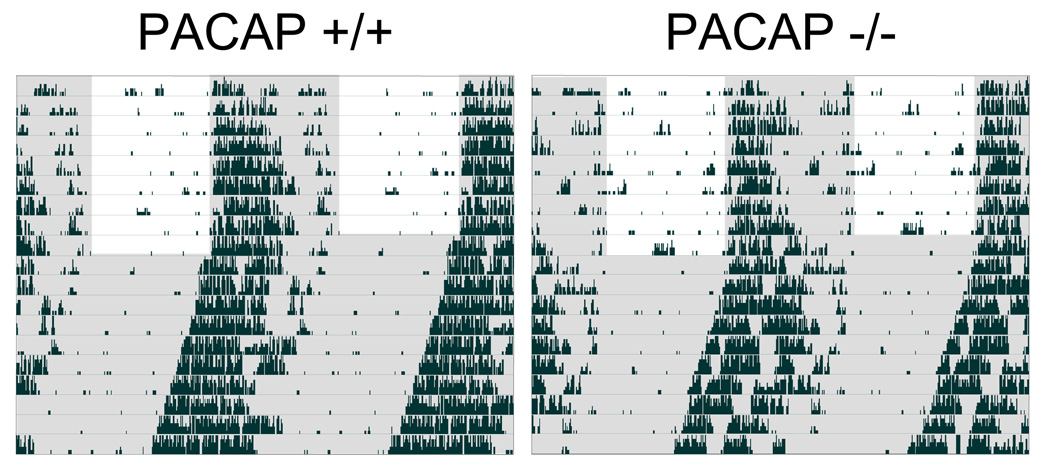
Figure 1.
Loss of PACAP does not alter entrainment or free-running rhythms. Representative double-plotted behavioral actograms of C57Bl/6 WT (n=37) and PACAP-null (n=38) mice demonstrate that entrainment to 12-h light:12-h dark cycles and free-running rhythms are not affected by the lack of PACAP. Shaded area represents darkness. PACAP +/+ (WT), PACAP −/− (PACAP-null).
Figure 2.
Loss of PA CAP specifically alters the phase-advancing response to a pulse of light. Photic phase response curve showing average (+/− s.e.m.) phase shifts to 15-min, 100-lux light pulses. PACAP null mice (PACAP −/−) display deficits in their ability to phase advance compared to wild type controls (PACAP +/+). n ≥ 8, except CT 8–12, where n ≥ 4. * p < 0.0001, Two-Way ANOVA, CT 22-CT 2 = main effect of genotype.
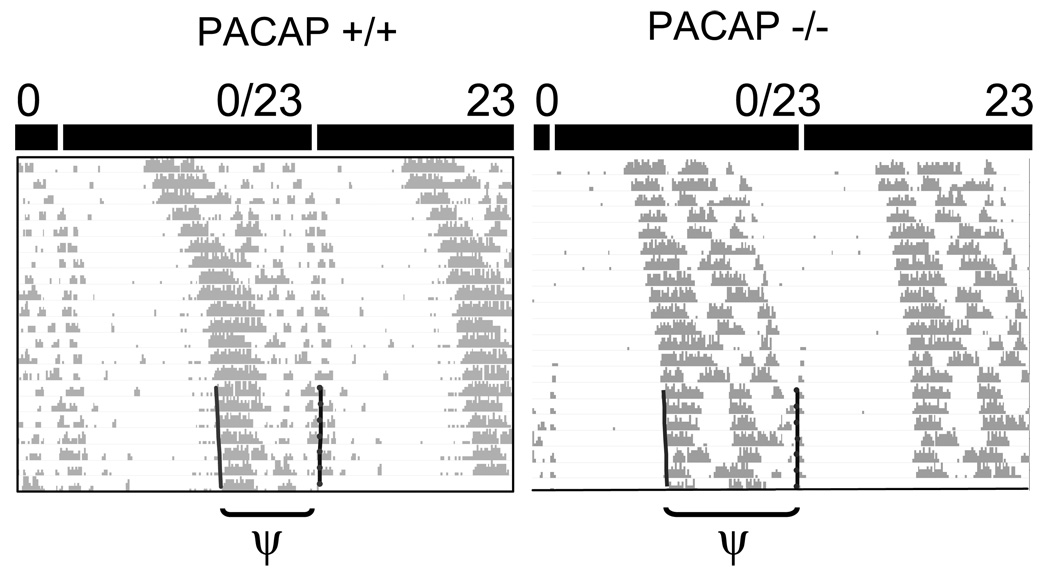
To determine whether PACAP-null mice could respond to a light-pulse regime that repeats at a frequency shorter than their endogenous period, subjects were placed on a 23-h T-cycle (0.25 h light: 22.75 h dark, 100 lux). Although all mice shortened their periods to match the imposed 23-h T-cycle (WT: from 23.63 ±0.05 h in DD to 23.05 ±0.03 h; PACAP-null: from 23.56 ± 0.05 h in DD to 23.02 ±0.01 h, p<0.0001, paired t-test), we found that the ability of PACAP-null mice to entrain to the 23-h T-cycle was different than WT mice (Fig. 3). The phase angle of entrainment, measured in hours between activity onset and entraining light onset, is ~2 h longer in PACAP-null mice than WT mice (phase angles: WT: 10.49 ± 0.33 h, n = 11; PACAP-null: 12.45 ± 0.36 h, n = 12, p < 0.05).
Figure 3.
PACAP-null mice (PACAP −/−) display a longer phase angle of entrainment to a 23-h T-cycle compared with WT mice (PACAP +/+). Representative double-plotted behavioral actograms of mice as they free-run and then become entrained to a 23-h T-cycle. Actograms plotted on a 23-h time scale show stable entrainment. Left vertical line: activity onset. Right vertical line: light onset. ψ = phase angle of entrainment, p < 0.05, Student’s t-test.
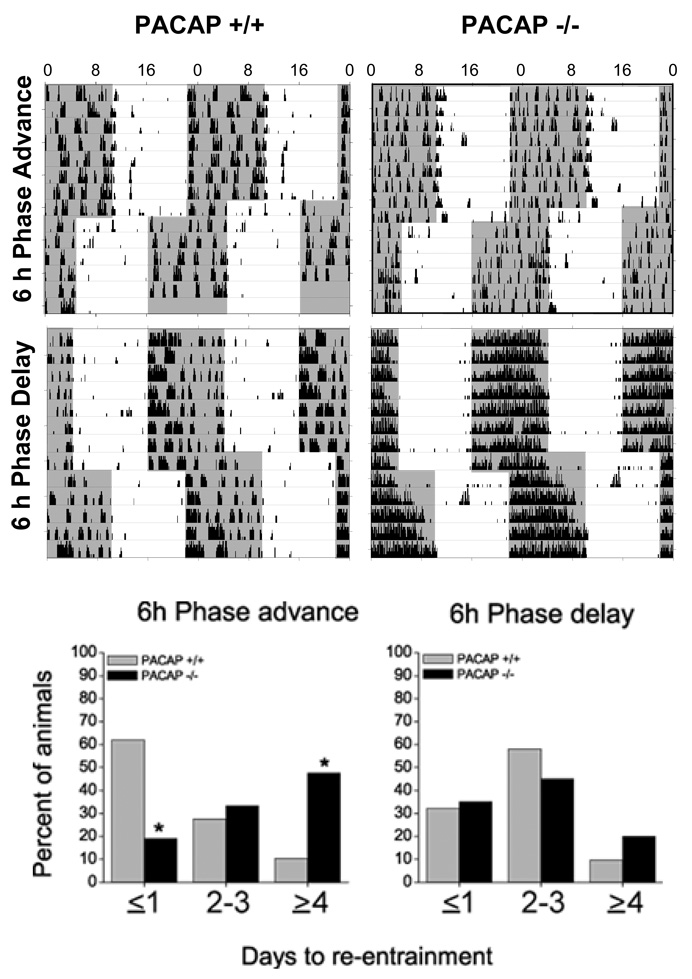
To evaluate entrainment further, we subjected WT and PACAP-null mice to a 6-h advance or delay of the full LD cycle and compared rates of re-entrainment. PACAP-null mice took significantly longer to re-establish stable entrainment to the shifted LD cycle (X2df=2 = 11.77, p < .05, Fig. 4). At ≤1 day, less than 20% of PACAP-null mice had re-entrained compared with >60% of WT. By ≥4 days, >45 % of the PACAP-null mice were not yet re-entrained, whereas >90% of WT had shifted. There is no difference between the genotypes in the rate of re-entrainment to a 6-h delay of the LD cycle (X2df=2 = 1.36, NS). Due to faulty running wheels, the data from two WT mice were omitted from the 6-h phase advance, and the data from one PACAP-null mouse was omitted from the 6-h phase delay analyses.
Figure 4.
PACAP-null mice re-entrain to a phase-advancing lighting schedule, but at a significantly slower rate. Representative double-plotted behavioral actograms for two WT (PACAP +/+) and two PACAP-null mice (PACAP −/−) show patterns of re-entrainment to a new lighting schedule either advanced (top) or delayed (middle) by 6 h. Shaded areas represent darkness. When mean rates are evaluated daily (graph, bottom), significantly fewer PACAP −/− mice (N=21) than PACAP +/+ (N=31) have re-entrained to a 6-h phase advance on day ≤1 and ~45% still are re-entraining at ≥ 4 days, when all WT mice have completed the shift (p < .05, X2). The two genotypes exhibit similar rates of re-entrainment to 6-h phase delays.
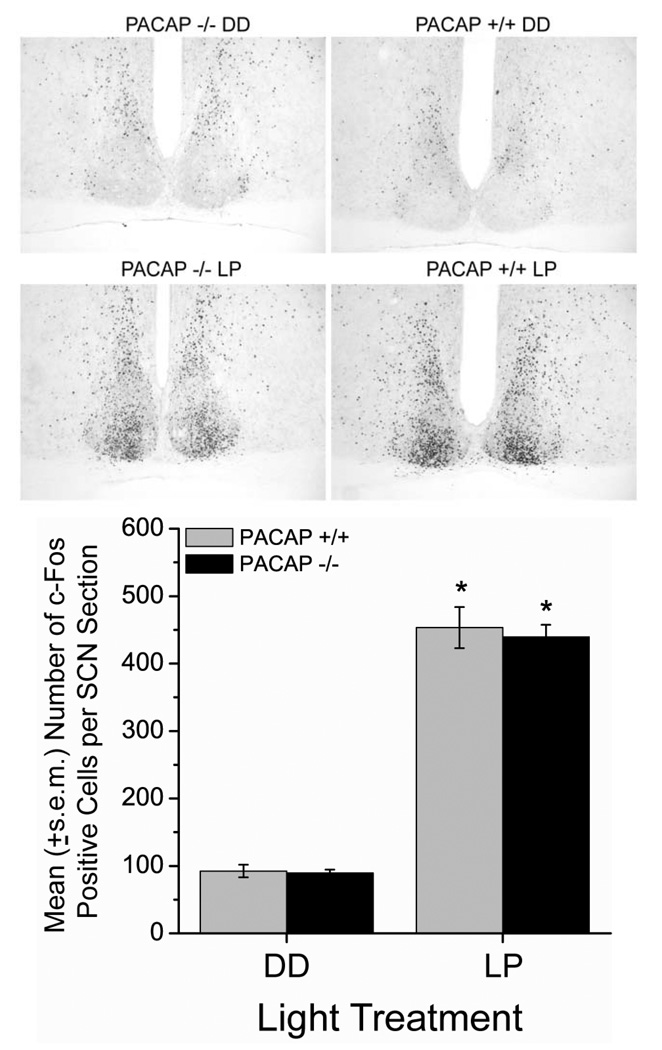
To assess effects of light on photic signaling downstream from receptor activation, we examined induction of c-Fos, an immediate-early gene product whose activation level parallels light intensity and degree of phase shift (Kornhauser et al., 1990). Light significantly increased c-Fos levels in the SCN of both mouse genotypes compared to dark controls (ANOVA, Tukey post-hoc, F(1,12) = 371, p < 0.0001, Fig. 5). However, there are no significant differences in the levels of basal or light-induced c-Fos immunoreactivity between WT and PACAP-null mice and no significant interactions.
Figure 5.
Loss of PACAP does not alter light induction of c-Fos protein in late night. Representative photomicrographs (10× magnification) of the medial SCN show similar patterns and numbers of c-Fos positive cells in PACAP +/+ and −/− mice at CT 22 under basal (top), and light-stimulated (10-min, ~100-lux light pulse, middle) conditions. Graph shows mean (+/− s.e.m.) number of c-Fos immunoreactive cells per unilateral SCN section (40 µm, medial SCN). * p < 0.0001, vs DD, Two-Way ANOVA, Tukey post-hoc.
DISCUSSION
The present set of behavioral experiments found that mice lacking PACAP display specific deficits in their ability to express light-induced phase advances. Instead of phase advances, PACAP-null mice expressed small phase delays following a 15-min light pulse administered during the late subjective night; they displayed a longer phase angle of entrainment to a 23-h T-cycle and showed slower rates of re-entrainment to a 6-h phase advance of the LD cycle. Light-induced phase delays and rates of re-entrainment to a 6-h delay of the LD cycle were unaffected by the lack of PACAP. These results support a necessary role for PACAP in the normal integration of the phase-advancing photic signal.
Present results are consistent with and expand upon previously published studies demonstrating PACAP and its PAC1R receptor are important for normal light-induced phase advances (Colwell et al., 2004; Hannibal et al., 2001; Kawaguchi et al., 2003). However, the present results are inconsistent with the reports that PACAP-null animals show deficits in phase delays and the rate of re-entrainment to an 8-h phase advance is unaffected (Colwell et al., 2004). Because each transgenic animal model is distinct based on genetic background, site of gene insertion, the extent of the genetic lesion of the PACAP-coding sequence (absence or presence of PACAP-related peptide) and subtle differences in methodology, variability between the results with models of different origin is inevitable. However, it is significant that all available models of mice with compromised PACAP signaling display impaired light-induced phase advances.
The circadian system integrates both the intensity and duration of photic information, and both parameters contribute to the resetting response to light (Kornhauser et al., 1996; Kornhauser et al., 1990; Morin and Allen, 2006; Nelson and Takahashi, 1991). Thus, deficits observed in the PACAP-null mice could, potentially, reflect impairments in the animal’s ability to integrate stimulus intensity and/or duration. In fact, while our manuscript was under review, a study was published showing that deficits in the circadian response to light of PAC1R-deficient mice are amplified at light intensities ≤70 lux or lower (Hannibal et al., 2008). However, in our study, we used a light intensity of 100 lux, considered saturating for mice (Sharma et al., 1999), and although durations were varied (from 12-h LD cycles to 15 min LP), only deficits associated with the phase advancing pathway were observed. Although we cannot exclude an effect of the lack of PACAP on photic sensitivity, this seems unlikely since phase delays and c-Fos expression, two measures whose magnitude of expression is positively correlated with light intensity, were not affected.
It is puzzling that PACAP-null mice effectively entrain to a 23-h T-cycle, and re-entrain to a 6-h-phase advance of the LD cycle without a phase advance portion in their photic PRC (Fig. 2). By circadian conventions, the shape of the PRC determines the limits of entrainment to light cycles. Because a nearly complete PRC was generated, the increased phase angle of entrainment exhibited by the PACAP-null animals to a 23-h T-cycle cannot be explained by a simple shift in the advance portion of the PRC towards the subjective day. The shape of the PRC is plastic and, therefore, could have been modified by the exposure to the 23-h T-cycle. However, the mechanism by which 15 min of light presented on a 23-h schedule would reshape the PRC and allow phase advances, in contrast to a single light pulse, is unknown. Discrepancies between the shape of the PRC and the observed capacity for entrainment are not uncommon. In addition to PACAP-null mice (Colwell et al., 2004 and present findings), Dexras1 knockout mice (Cheng et al., 2006) and the cryb mutant Drosophila (Stanewsky et al., 1998) each exhibit large alterations in their PRCs with little effect on entrainment.
On the other hand, the specific deficits we observed in PACAP-null mice could suggest that PACAP plays a role in the integration of photoperiodic information conveyed by the LD cycle. In Drosophila, the peptide pigment-dispersing factor (PDF), which shares similarities with vasoactive intestinal peptide (VIP) and PACAP, has been shown to be critical for the function of the morning oscillator (Nitabach and Taghert, 2008; Renn et al., 1999). It is tempting to propose that in rodents, PACAP is critical for the proper integration of photoperiodic information, or information integrated by a morning oscillator, a hypothesis that to our knowledge has not been tested in any of the PACAP models published to date.
In conclusion, the current findings demonstrate that in the PRC condition PACAP is required for the integration of acute phase-advancing photic signals by the SCN. Such responses would enable immediate behavioral and physiological adjustments, such as effects of day-to-day variations in light onset. In contrast, during entrainment conditions, where photic signals are recurring and predictable, PACAP plays a modulatory role in the phase relationship between the light cycle and the circadian cycle. Together, the deficits observed in the absence of PACAP imply that much of the glutamatergic signaling pathway is intact, but that a parallel PACAP-mediated pathway that is required for phase advances has been altered (Cheng et al., 2006; Gillette and Mitchell, 2002; Tischkau et al., 2003). This interpretation is supported by preliminary data reporting that glutamate-induced phase advances in cell firing rhythms, but not delays, also are absent in SCN brain slices derived from PACAP-null animals, and can be rescued by the re-introduction of PACAP in this system (Lindberg et al., 2004).
In a broader sense, the role of PACAP in photic signals that alter the central circadian timing system is reminiscent of PACAP’s role in long-term plastic changes in hippocampal function (Matsuyama et al., 2003; Otto et al., 2001). The emerging picture in the SCN is one of PACAP acting as a critical modulator of plasticity in circadian clock state, insuring appropriate sensitivity, gain-setting and responsiveness upon the dynamic temporal background of the circadian timing system. Functional advantages of such a system include the integration of multiple signals so as to ensure necessary and temporally appropriate alterations in clock state and timing of the functions it orchestrates.
ACKNOWLEDGEMENTS
The authors recognize with gratitude generous support from Fonds de Recherche sur la Nature et les Technologies (Government of Québec) and Canadian Institutes of Health Research (CIHR) (CB), as well as from the National Institutes of Health (USA): GM07143 (PTL), NS11158 (JWM), in part by NIMH-IRP Project 1 Z01 MH002386-21 (LEE), and HL08670 and NS22155 (MUG).
REFERENCES
- Beaulé C, Amir S. The eyes suppress a circadian rhythm of FOS expression in the suprachiasmatic nucleus in the absence of light. Neuroscience. 2003;121:253–257. doi: 10.1016/s0306-4522(03)00420-2. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Dziema H, Papp J, Mathur DP, Koletar M, Ralph MR, Penninger JM, Obrietan K. The molecular gatekeeper Dexras1 sculpts the photic responsiveness of the mammalian circadian clock. J Neurosci. 2006;26:12984–12995. doi: 10.1523/JNEUROSCI.4253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colwell CS, Michel S, Itri J, Rodriguez W, Tam J, Lelievre V, Hu Z, Waschek JA. Selective deficits in the circadian light response in mice lacking PACAP. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1194–R1201. doi: 10.1152/ajpregu.00268.2004. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Ebling FJ. The role of glutamate in the photic regulation of the suprachiasmatic nucleus. Prog Neurobiol. 1996;50:109–132. doi: 10.1016/s0301-0082(96)00032-9. [DOI] [PubMed] [Google Scholar]
- Gillette MU, Mitchell JW. Signaling in the suprachiasmatic nucleus: selectively responsive and integrative. Cell Tissue Res. 2002;309:99–107. doi: 10.1007/s00441-002-0576-1. [DOI] [PubMed] [Google Scholar]
- Hamelink C, Tjurmina O, Damadzic R, Young WS, Weihe E, Lee HW, Eiden LE. Pituitary adenylate cyclase-activating polypeptide is a sympathoadrenal neurotransmitter involved in catecholamine regulation and glucohomeostasis. Proc Natl Acad Sci U S A. 2002;99:461–466. doi: 10.1073/pnas.012608999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J. Roles of PACAP-containing retinal ganglion cells in circadian timing. Int Rev Cytol. 2006;251:1–39. doi: 10.1016/S0074-7696(06)51001-0. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Brabet P, Fahrenkrug J. Mice lacking the PACAP type I receptor have impaired photic entrainment and negative masking. Am J Physiol Regul Integr Comp Physiol. 2008;295:R2050–R2058. doi: 10.1152/ajpregu.90563.2008. [DOI] [PubMed] [Google Scholar]
- Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD. Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Jamen F, Nielsen HS, Journot L, Brabet P, Fahrenkrug J. Dissociation between light-induced phase shift of the circadian rhythm and clock gene expression in mice lacking the pituitary adenylate cyclase activating polypeptide type 1 receptor. J Neurosci. 2001;21:4883–4890. doi: 10.1523/JNEUROSCI.21-13-04883.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Moller M, Ottersen OP, Fahrenkrug J. PACAP and glutamate are co-stored in the retinohypothalamic tract. J Comp Neurol. 2000;418:147–155. [PubMed] [Google Scholar]
- Kawaguchi C, Tanaka K, Isojima Y, Shintani N, Hashimoto H, Baba A, Nagai K. Changes in light-induced phase shift of circadian rhythm in mice lacking PACAP. Biochem Biophys Res Commun. 2003;310:169–175. doi: 10.1016/j.bbrc.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus. New York: Oxford University Press; 1991. [Google Scholar]
- Kopp MD, Schomerus C, Dehghani F, Korf HW, Meissl H. Pituitary adenylate cyclase-activating polypeptide and melatonin in the suprachiasmatic nucleus: effects on the calcium signal transduction cascade. J Neurosci. 1999;19:206–219. doi: 10.1523/JNEUROSCI.19-01-00206.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornhauser JM, Mayo KE, Takahashi JS. Light, immediate-early genes, and circadian rhythms. Behav Genet. 1996;26:221–240. doi: 10.1007/BF02359382. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- Lindberg PT, Hamelink C, Damadzic R, Eiden LE, Gillette MU. Pituitary Adenylate Cyclase-Activating Peptide Plays a Time-dependent Role in Light-induced Phase Shifts of Circadian Rhythms. In: Sf Neuroscience, editor. Annual Meeting; San Diego, CA. 2004. [Google Scholar]
- Matsuyama S, Matsumoto A, Hashimoto H, Shintani N, Baba A. Impaired long-term potentiation in vivo in the dentate gyrus of pituitary adenylate cyclase-activating polypeptide (PACAP) or PACAP type 1 receptor-mutant mice. Neuroreport. 2003;14:2095–2098. doi: 10.1097/00001756-200311140-00017. [DOI] [PubMed] [Google Scholar]
- Mice BCoGBi. Mutant mice and neuroscience: recommendations concerning genetic background. Banbury Conference on genetic background in mice. Neuron. 1997;19:755–759. doi: 10.1016/s0896-6273(00)80958-7. [DOI] [PubMed] [Google Scholar]
- Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain Res Brain Res Rev. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Otto C, Kovalchuk Y, Wolfer DP, Gass P, Martin M, Zuschratter W, Grone HJ, Kellendonk C, Tronche F, Maldonado R, Lipp HP, Konnerth A, Schutz G. Impairment of mossy fiber long-term potentiation and associative learning in pituitary adenylate cyclase activating polypeptide type I receptor-deficient mice. J Neurosci. 2001;21:5520–5527. doi: 10.1523/JNEUROSCI.21-15-05520.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A Functional Analysis of Circadian Pacemakers in Noctural Rodents. V. Pacemaker Structure: A Clock for All Seasons. J Comp Physiol. 1976;106:333–355. [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Sharma VK, Chandrashekaran MK, Singaravel M, Subbaraj R. Relationship between light intensity and phase resetting in a mammalian circadian system. J Exp Zool. 1999;283:181–185. doi: 10.1002/(sici)1097-010x(19990201)283:2<181::aid-jez8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Stanewsky R, Kaneko M, Emery P, Beretta B, Wager-Smith K, Kay SA, Rosbash M, Hall JC. The cryb mutation identifies cryptochrome as a circadian photoreceptor in Drosophila. Cell. 1998;95:681–692. doi: 10.1016/s0092-8674(00)81638-4. [DOI] [PubMed] [Google Scholar]
- Tischkau SA, Mitchell JW, Tyan SH, Buchanan GF, Gillette MU. Ca2+/cAMP response element-binding protein (CREB)-dependent activation of Per1 is required for light-induced signaling in the suprachiasmatic nucleus circadian clock. J Biol Chem. 2003;278:718–723. doi: 10.1074/jbc.M209241200. [DOI] [PubMed] [Google Scholar]
- von Gall C, Duffield GE, Hastings MH, Kopp MD, Dehghani F, Korf HW, Stehle JH. CREB in the mouse SCN: a molecular interface coding the phase-adjusting stimuli light, glutamate, PACAP, and melatonin for clockwork access. J Neurosci. 1998;18:10389–10397. doi: 10.1523/JNEUROSCI.18-24-10389.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]