Abstract
Purpose
To test the hypothesis that mucoadhesive microdiscs formulated in a rapidly dissolving tablet can increase preocular residence time.
Methods
Microparticles, smaller than 10 μm in diameter, were fabricated by emulsification with poly(lactic-co-glycolic acid) (PLG) as a core material and, in some cases, poly(ethylene glycol) (PEG) as a mucoadhesion promoter. To examine the effect of particle geometry, microparticles were also cut to possess flat surfaces (i.e., microdiscs) and compared with spherical particles (i.e., microspheres). In vitro mucoadhesion of microparticles was tested on a mucous layer under shear stress mimicking the human blink. The resulting microparticles were also formulated in two dosage forms – an aqueous suspension and dry tablet to – test the effect of formulation on the retention capacity of microparticles on the preocular space of rabbits in vivo.
Results
Mucoadhesive microdiscs adhered better to the simulated ocular surface than the other types of microparticles. When a dry tablet embedded with mucoadhesive microdiscs was administered in the cul-de-sac of the rabbit eye in vivo, these microdiscs exhibited longer retention than the other formulations tested in this work. More than 40% and 17% of mucoadhesive microdiscs remained on the preocular surface at 10 min and 30 min after administration, respectively. Fluorescence images from the eye surface showed that mucoadhesive microdiscs remain for at least 1 h in the lower fornix.
Conclusion
This study demonstrated that mucoadhesive microdiscs formulated in a dry tablet can achieve a prolonged residence time on the preocular surface and thus are a promising drug delivery system for ophthalmic applications.
INTRODUCTION
Topical drug administration is widely used to treat various eye disorders, but is nonetheless limited by low drug bioavailability due to rapid clearance by blinking, tear drainage and absorption into the conjunctival vasculature.1, 2 Topical administration using eye drops is known to lose approximately 75% of the applied dose via nasolacrimal drainage almost instantly and thus, less than 5% of the applied drug is ultimately bioavailable in the anterior segment of the eye. To maintain therapeutic drug levels, frequent administration or large doses is often required, which can reduce patient compliance, cause local side effects, and lead to undesirable systemic exposure. These problems could be addressed by a drug delivery system that remains on the preocular surface and slowly delivers drug for a prolonged period of time.
Various attempts have been made to increase drug residence time on the preocular surface. Drug solutions formulated with viscosity enhancers, such as carboxymethyl cellulose, hydroxypropyl cellulose, poly(vinylpyrrolidone), and polyvinyl alcohol, slowed drug clearance and increased drug bioavailability in rabbits.3–5 However, despite their prolonged residence time, the rate of drug release was not controlled, which often caused a rapid drug efflux out of the polymer network. Gels, ointments and ocular inserts can increase residence time and also provide sustained release from a drug depot typically in the conjunctival sac.6–8 However, discomfort, ocular irritation and blurred vision are often associated with such systems, which can adversely affect patient compliance.
Across medicine, biocompatible microparticles are widely used for controlled-release drug delivery due to their ease of fabrication, simplicity of administration and possible use in localized and targeted delivery.9 Drug molecules encapsulated in microparticles are released by drug diffusion and/or polymer degradation, which results in sustained release. In addition, microparticles can be easily formulated into a variety of dosage forms, such as suspensions, tablets and gels, which makes them applicable to many different localized or systemic treatments. Various therapeutic agents, such as acyclovir, dexamethasone, piroxicam and vancomycin, have been delivered via microparticles topically to the eye and exhibited enhanced targeting and drug bioavailability.10, 11
Microparticles designed to have increased residence time at the preocular surface would further improve drug retention on the eye and, hence, be more effective for the therapy of ocular diseases, such as glaucoma, keratoconjunctivitis sicca or dry eye disease. Microparticles have been made using mucoadhesive polymers to enhance their adhesion to the mucus layer on the preocular surface.12–15 Microparticles formulated using numerous mucoadhesive polymers, such as chitosan, pectin, hyaluronic acid, sodium carboxymethylcellulose and polyacrylic acid, have achieved slower preocular clearance and increased drug bioavailability.14–17
In this work, we propose ophthalmic drug delivery using microparticles engineered to optimize three design parameters: (i) mucoadhesion, (ii) geometry and (iii) dosage form. We hypothesize that mucoadhesive microdiscs formulated in a rapid-dissolving tablet can increase preocular residence time. Although previous studies have examined the use of mucoadhesive microparticles before, this is the first study to consider microparticle geometry (i.e., in particular a flat, microdisc geometry) and to embed microparticles in a rapidly dissolving tablet formulation for ophthalmic delivery. As discussed below, these attributes were designed to promote mucoadhesion by increasing contact area between the microparticles and the preocular surface and by reducing convective forces of tear fluid flow that carry particles away.
Microparticles were made of poly(lactic-co-glycolic acid) (PLG) and poly(ethylene glycol) (PEG), which were employed as the core material11 and mucoadhesion promoter,18 respectively. Mucoadhesiveness of PEG is known to be generated by hydrogen bonds with mucins that contain hydroxyl, carboxylic acid and sulfate groups.19 These materials are also widely established as safe for various medical applications.20, 21
Two different shapes of microparticles, i.e., spheres and discs, were compared to examine the effect of particle geometry. We were interested to study microdiscs because their flattened surface can increase frictional contact area with the preocular mucus surface and reduce hydrodynamic resistance when aligned parallel to the direction of tear flow.22 The diameter of microparticles was also controlled to be less than 10 μm to avoid possible eye irritation and for safe clearance through the lacrimal canals, which are 300 – 500 μm in diameter.2
Finally, microparticles were formulated in aqueous suspension and as a rapidly dissolving dry tablet. We were interested to study the dry tablet formulation because tablet dissolution would increase the local viscosity, which should facilitate initial contact between mucoadhesive microparticles and the preocular mucus surface, allowing particles to become anchored before exposure to the full hydrodynamic shear of tear fluid flow. Dry tablets were prepared by embedding microparticles in mannitol, which has been widely used in ophthalmic formulations.23
MATERIALS AND METHODS
Materials
Poly(lactic-co-glycolic acid) (PLG, 50:50; Lot number: LP-353; MW = 15 kDa; i.v. = 0.15 – 0.25 dl/g) and poly(ethylene glycol) (PEG; average MW = 6 kDa) were purchased from Lakeshore Biomaterias (Birmingham, AL, USA) and Acros Organics (Morris Plains, NJ, USA), respectively. Polyvinyl alcohol (PVA; 87 – 89% hydrolyzed, average MW = 31 – 50 kDa), mannitol, Nile Red and Type II mucin from porcine stomach were obtained from Sigma Chemicals (St. Louis, MO, USA). Methylene chloride and acetone in high purity were purchased from Fisher Scientific (Pittsburgh, PA, USA). Hank’s buffered saline solution (HBSS) was obtained from Mediatech (Manassas, VA, USA). Optimal cutting temperature (OCT) compounds, generally used as embedding media for frozen tissue specimens, were obtained from Sakura Finetek USA (Torrance, CA, USA) and utilized as the microparticle embedding media for cryo-sectioning. Proparacaine HCl (0.5% ophthalmic solution) was purchased from Bausch & Lomb (Tampa, FL, USA). The anesthetics for subcutaneous injection to the rabbits, i.e., ketamine, xylazine and acepromazine, were obtained from Fort Dodge Animal Health (Fort Dodge, IA, USA), Lloyd (Shenandoah, IA, USA) and Boehringer Ingelheim (St. Joseph, MO, USA), respectively.
Fabrication of Microparticles
Four different kinds of microparticles were prepared: microspheres of PLG (PLG MS), microspheres of a blend of PLG and PEG (PLG/PEG MS), microdiscs (i.e., cut microspheres) of PLG (PLG MD) and microdiscs of a blend of PLG and PEG (PLG/PEG MD). To prepare PLG and PLG/PEG microparticles, a polymer solution was prepared by dissolving either 200 mg PLG or a mixture of 200 mg PLG and 20 mg PEG in 2 ml methylene chloride. Two milligrams of the fluorescent tracer, Nile Red was also dissolved in these solutions to label the microparticles.
The resulting solutions were then each dispersed in 15 ml water containing PVA (2% w/v) and sonicated at 100 W for 10 s to obtain oil-in-water emulsion droplets of appropriate size (ultrasonic converter: CV33, power supply: VC505, Sonics & Materials, Newtown, CT, USA). The emulsion was stirred overnight to evaporate the methylene chloride and obtain solid microparticles. The solid microparticles were filtered (nylon net filter, 11-μm pore, Millipore, Billerica, MA, USA) to remove particles larger than 10 μm, suspended in OCT compound and frozen at − 75 °C for 2 h. The distribution of PEG inside of the microparticles is expected to be fairly homogeneous due to fast polymer-phase inversion of small microparticles (< 10 μm) during solvent evaporation.24
To prepare microspheres, the frozen OCT compound was thawed at room temperature without further treatment, washed thoroughly with DI water, and freeze dried (VirTis Advantage, Gardiner, NY, USA) for 2 days. To fabricate microdiscs, a piece of the frozen OCT compound embedded with microparticles was sectioned into 1 μm thick films on a cryostat (HM 560 Cryo-Star, Microm International, Waldorf, Germany), which were then thawed, washed with DI water and freeze dried.
Preparation of Microparticle Formulations
Microparticles were formulated in two different dosage forms, i.e., an aqueous suspension and a dry tablet. Preparing each of the four different microparticle types in each of these two dosage forms yielded eight different microparticle formulations: PLG MS suspension, PLG MS tablet, PLG MD suspension, PLG MD tablet, PLG/PEG MS suspension, PLG/PEG MS tablet, PLG/PEG MD suspension and PLG/PEG MD tablet. Suspensions were prepared in 100 μl of HBSS with a concentration of 10 mg/ml and 5 mg/ml of microparticles for the in vitro and in vivo mucoadhesion tests, respectively. To fabricate a dry tablet used for in vivo tests, a homogeneous mixture of 20 mg of mannitol and 0.5 mg of microparticles was hand-pressed in a 3 mm diameter bore formed in a 1 cm thick acrylic sheet (Goodfellow, Oakdale, PA, USA). In this way, all eight formulations used for the in vivo study were each prepared to contain 0.5 mg microparticles for single use.
Characterization of Microparticles
The size and shape of microparticles were examined using a LEO 1530 scanning electron microscope (Carl Zeiss SMT, Peabody, MA, USA). A droplet of an aqueous suspension of microparticles was placed on a small piece of silicon wafer attached to the top of a scanning electron microscope sample holder. The samples were dried with desiccant overnight and sputter-coated with gold. The microparticles were imaged at 2–10 kV. A Coulter Multisizer 3 (Beckman Coulter, Fullerton, CA, USA) equipped with a 50-μm aperture was used to determine the size distribution of microparticles. The microparticles were suspended in Isoton electrolyte (Beckman Coulter) with a dispersant (Dispersant IA, Beckman Coulter) to prevent aggregation. At least 5000 particles were counted for each sample.
Nile Red was used as a fluorescent tracer to facilitate measuring the amount of microparticles remaining on the preocular surface over time. Although the initial loading of Nile Red (1% w/w) was known, the amount of Nile Red actually entrapped in microparticles was also measured by dissolving 4 – 5 mg of Nile Red-loaded microparticles in 10 ml of acetone with strong agitation and then measuring the Nile Red concentration in the solution using calibrated fluorescence spectroscopy (Photon Technology International, Birmingham, NJ, USA).
A differential scanning calorimeter (DSC, STA409, Netzsch, Exton, PA, USA) was used for thermal analysis of microparticles. Briefly, 5 – 15 mg of pure PLG, pure PEG, or a microparticle formulation was placed in an aluminum pan with hermetic sealing and heated from 25 to 150 °C at a rate of 10 °C/min. The locations of endothermal peaks were determined to identify the peak shift associated with the presence of PEG in microparticles made of a blend of PLG and PEG.
In Vitro Mucoadhesion Study
Microparticle adhesion to the mucous layer was examined under a condition mimicking the shear force applied to the preocular surface during the blink of a human (150 dyne/cm2) 25. A mucous layer was prepared on a hydrophilic membrane of 1 cm × 1 cm (cellulose nitrate membrane, 0.45 μm pore size, Whatman, Florham Park, NJ, USA), which was first soaked for 2 h with an aqueous mucin solution prepared in HBSS (0.1% w/v).15 Then, 20 μl of a 10 mg/ml suspension of microparticles was applied as a single drop at the center of the membrane. After 30 s, the membrane was placed on a stress/strain controlled rheometer (MCR 300, Anton Paar, Ashland, VA, USA) to apply a continuous shear stress in the range of 140 – 160 dyne/cm2 for 60 s, which was performed using parallel surfaces separated by 50 mm and filled with HBSS. Peltier temperature control was used to maintain the temperature of HBSS at 33 – 34 °C during the experiment since the temperature of tear fluid is known to be about 2 – 3 °C lower than the body core.25 After applying the shear stress, the microparticles remaining on the membrane were dissolved in acetone under strong agitation and quantified using calibrated fluorescence spectroscopy (Photon Technology International, Birmingham, NJ, USA). The experiments were performed in triplicate for each type of microparticle.
In Vivo Mucoadhesion Study
The in vivo studies were performed using male New Zealand White rabbits (Myrtle’s Rabbitry, Thompsons Station, TN, USA) weighing 3.4 – 3.6 kg and without any ocular defects. All experiments were conducted in compliance with the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Georgia Institute of Technology. The rabbits were housed singly in a standard cage without any restriction of food and water.
To administer the microparticle formulation, the rabbit was removed from the cage, placed in a soft cloth restraint bag (Four Flags Over Aspen, Janesville, MN, USA), which was opened just enough to expose its head. The rabbits lay on the stomach during this whole procedure. A total of 100 μl of microparticle suspension (5 mg/ml) was added topically to the rabbit eye by four consecutive administrations of 25 μl each with 1 min intervals in between, which collected to a large extent almost instantly in the lower cul-de-sac due to gravity. Alternatively, a tablet was placed in the lower cul-de-sac and the eyelid was closed manually for 5 min to have the tablet completely dissolve in the tear fluid. The same dose of microparticles (0.5 mg) was administered using suspensions and tablets. No anesthesia was required for these procedures, which were well tolerated by the rabbits. After administration, the rabbit was placed back into the cage and allowed to move freely until samples were collected.
The microparticles remaining on the preocular surface were collected 10 min, 30 min or 60 min after administration of the suspension or complete dissolution of the tablet. Only one sampling time was used per animal. The preocular surface was wiped thoroughly using a cellulose surgical sponge (Ultracell Medical Technologies, North Stonington, CT, USA) while the eye was locally anesthetized with topical administration of 25 μl of 0.5% proparacaine HCl ophthalmic solution. The surgical sponge containing microparticles was then submerged in acetone for 1 h. The acetone was then assayed with calibrated fluorescence spectroscopy (Photon Technology International, Birmingham, NJ, USA) to determine the amount of microparticles collected. For each sample and each time after administration, at least three eyes were selected randomly among eight different eyes (i.e., four rabbits were used in this study). The interval between two consecutive sample collections for each eye was a minimum of 48 h to permit the animals to recover from any experimental stress.
To obtain images of microparticles on the eye, the rabbit was anesthetized with a single subcutaneous injection of a cocktail of 17.5 mg/kg ketamine, 8.5 mg/kg xylazine and 0.5 mg/kg acepromazine. One or two additional boosters (5.6 mg/kg ketamine, 2.8 mg/kg xylazine and 0.16 mg/kg acepromazine) were used if needed. Each of the eight microparticle formulations was administered under the same condition as the quantitative analysis described above. The images of microparticles on the preocular surface were obtained using a fluorescence stereomicroscope (SZX 12, Olympus America, Center Valley, PA, USA) 10 min, 30 min and 60 min after administration while the rabbit lay on its side. The locations imaged were cornea, lacrimal caruncle, upper fornix and lower fornix. Between the intervals of imaging, the rabbit lay on its stomach and the eye was manually blinked once per 5 min, as if the rabbit were awake.
Statistical Analysis
The percentage of remaining microparticles was assessed based on the amount applied initially for both in vitro and in vivo experiments. The mean percentages of remaining microparticles among the different microparticle formulations were statistically analyzed using a generalized linear model ANOVA with α = 0.05, followed by pairwise comparisons using a Tukey’s post hoc test.
RESULTS
Characterization of Microparticles
Four different kinds of microparticles were prepared as shown in Figure 1. PLG MS were prepared as a control formulation of spherical geometry and no mucoadhesion promoter. PLG MD were prepared to assess the effect of a microdisc geometry in the absence of a mucoadhesion promoter. PLG/PEG MS were prepared to assess the effect of a mucoadhesion promoter in spherical microparticles. PLG/PEG MD were prepared to assess the combined effect of a mucoadhesion promoter and microdisc geometry.
Figure 1.
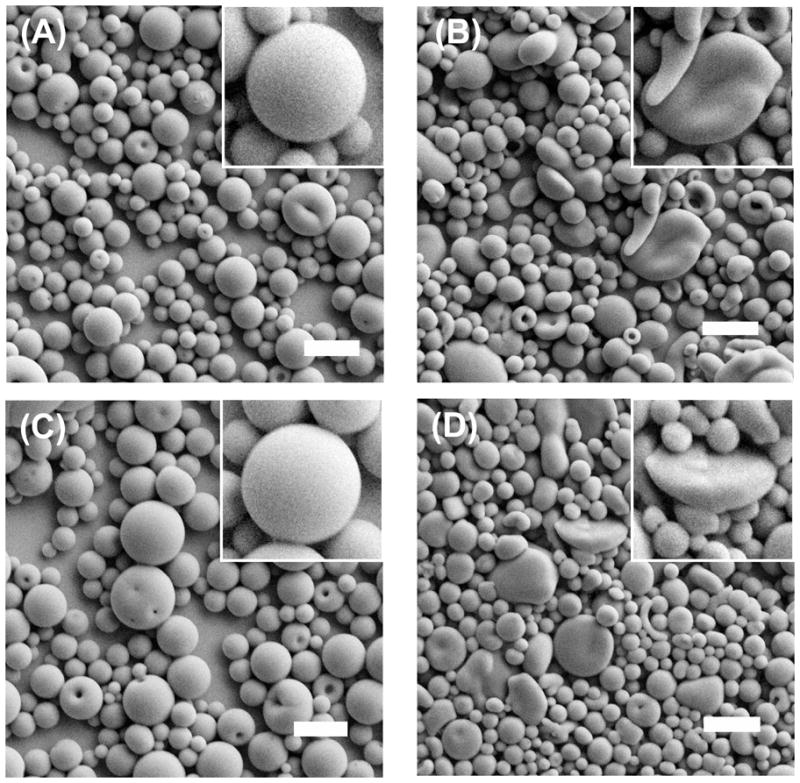
Scanning electron micrographs of microparticles prepared for ocular adhesion studies. (A) PLG MS, (B) PLG MD, (C) PLG/PEG MS and (D) PLG/PEG MD. The scale bars = 10 μm.
The average diameter of all microparticles was less than 10 μm (Table 1), which was achieved by removing larger particles by filtration. Microparticles in this size range should be appropriate for topical ophthalmic delivery.2 After cutting, the microdiscs exhibited flat surfaces (Figures 1(B) and 1(D)) while the uncut particles were spherical (Figures 1(A) and 1(C)). The amount of Nile Red entrapped in microparticles was determined to be 7 – 8 μg per mg microparticles (Table 1).
Table 1.
Properties of microparticles tested for ocular adhesion.
| Microparticles | Mean diameter (μm) | Nile Red (μg/mg) |
|---|---|---|
| PLG MS1 | 4.13 ± 1.76 | 8.07 ± 0.12 |
| PLG MD1 | 3.59 ± 1.19 | 7.51 ± 0.17 |
| PLG/PEG MS1 | 4.21 ± 1.93 | 7.53 ± 0.07 |
| PLG/PEG MD1 | 3.09 ± 1.24 | 7.01 ± 0.31 |
PLG = poly(lactic-co-glycolic acid), PEG = poly(ethylene glycol), MS = microspheres and MD = microdiscs
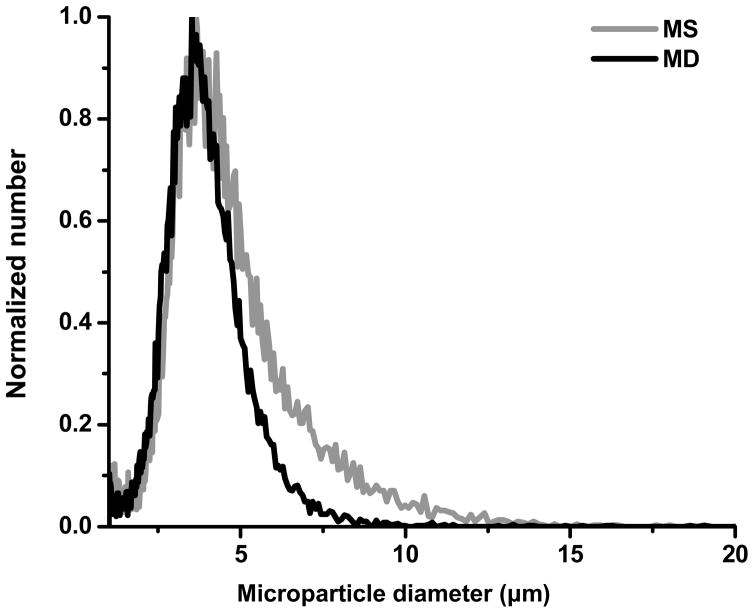
To further characterize the effect of cutting, the particle size distribution was measured using a multisizer. As shown in Figure 2 and Table 1, the cut microdiscs were smaller than the uncut microspheres. Although the 4 μm diameter microspheres were cut to 1 μm thickness to make microdiscs, the multisizer shows a relatively small change in size. This is because the multisizer measurement depends strongly on particle orientation in the electric field formed in the measuring orifice.26 If the long axis of a microdisc is oriented perpendicular to the electric field, that particle appears to the instrument to be similar in size to the original microspheres. If a microdisc is oriented parallel to the electric field, then it will appear close to 1 μm in size. In addition, due to limitations inherently present in the cryo-sectioning method (e.g., bluntness of the blade, particle deformability in cryostat, etc.), very small microspheres might not have been cut satisfactorily. In this way, the data make better sense, when considering the biased averaging of microdisc size performed by the Coulter method.
Figure 2.
Size distribution of PLG MS (gray curve) and PLG MD (black curve) measured by a Beckman Coulter Multisizer.
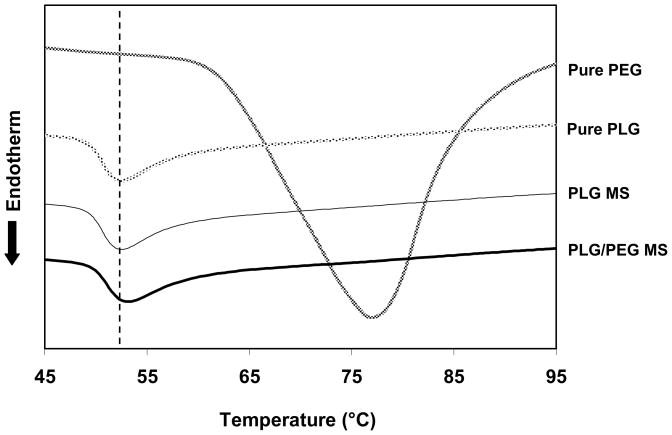
DSC analysis was also performed on microparticles to validate the presence of PEG in microparticles made of a blend of PLG and PEG. As control experiments, Figure 3 shows that the Tg (glass transition temperature) of pure PLG appeared at 52 °C and Tm (melting temperature) of pure PEG appeared at 77 °C. Microparticles of PLG alone exhibited the same Tg as pure PLG. However, microparticles composed of a blend of PLG and PEG exhibited a higher Tg of 54 °C, which is attributed to the presence of PEG in the microparticles. This is consistent with a previous report, where a similar transition of Tg was observed with a film made by solvent casting of PLG (75:25) and PEG (10 kDa).27 The lack of PEG melting endotherm for the PLG/PEG microparticles could be explained by the high miscibility and small mass fraction of PEG in microparticles, probably smaller than the initial loading of 10 % w/w, due to partial PEG diffusion out of the particles during the solvent evaporation step of fabrication.
Figure 3.
Differential scanning calorimetry thermograms of PLG MS and PLG/PEG MS. Thermograms from pure PLG and PEG are also plotted for comparison.
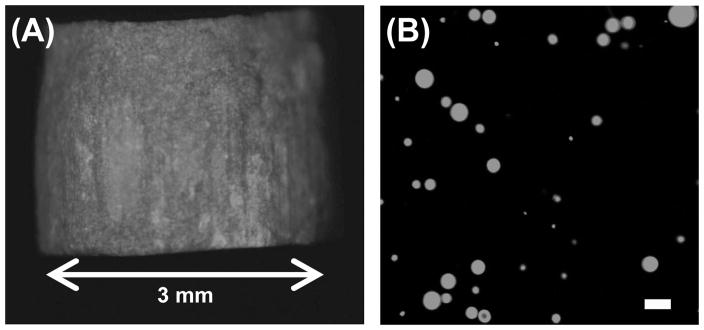
Microparticles were formulated in two different dosage forms – an aqueous suspension and dry tablet to – examine the effect of formulation on microparticle adhesion to the preocular surface. For ophthalmic application, a tablet should be small and dissolve rapidly in tear fluid to minimize possible discomfort on the sensitive eye surface. Figure 4 shows the fluorescence images of a dry tablet containing microparticles and the resulting microparticle suspension after tablet dissolution in HBSS. The fluorescence signal observed from the tablet indicates the presence of embedded microparticles labeled with Nile Red (Figure 4(A)). The cylindrical tablet measured 3 mm in diameter and 3 mm in height, which corresponded to a volume of 21 μl and was small enough to be applied in the lower cul-de-sac of the eye. Mannitol, a highly water soluble and biologically inert material, was used as the embedding medium of microparticles, which enabled the tablet to fully dissolve in HBSS within 5 min and release the individual microparticles (Figure 4(B)).
Figure 4.
Fluorescence micrographs of (A) red-fluorescent PLG MS embedded in a dry mannitol tablet and (B) a PLG MS suspension after dissolution of a tablet for 5 min in HBSS. The scale bar = 10 μm.
In Vitro Mucoadhesion of Microparticles
Using this collection of microparticle formulations, we determined the effect of mucoadhesion and microparticle geometry on preocular residence time using an in vitro model of the preocular surface. This test was carried out for 60 s under a shear stress (140 – 160 dyne/cm2) that mimicked the blink of a human eye because most particle clearance is expected during blinking.28
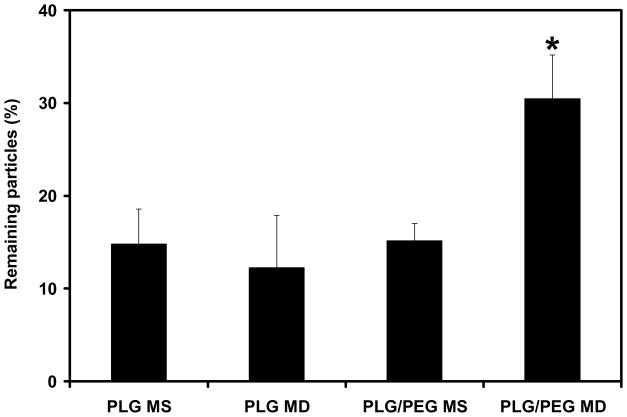
Under these conditions, 15% of PLG MS, having spherical geometry and no mucoadhesion promoter, remained on the model preocular surface, as shown in Figure 5. Neither cutting the particles into microdiscs nor adding a mucoadhesion promoter significantly changed the extent of particle adhesion (p > 0.10). However, the combination of the microdisc geometry and the mucoadhesion promoter doubled the percentage of microparticles remaining to 30% (p < 0.05). This can be explained by the increased force of adhesion of microparticles to the model preocular surface enabled by the mucoadhesion promoter and the reduced fluid mechanical force acting to remove microparticles enabled by the microdisc geometry.
Figure 5.
In vitro mucoadhesion of microparticles. The percentage of microparticles remaining on a model mucous membrane was measured after applying a shear stress similar to that of a human blink (140 – 160 dyne/cm2) for 60 s. *PLG/PEG MD were significantly different from the other types of microparticles (p < 0.05). The data points represent the average of n = 3 measurements ± standard deviation.
In Vivo Mucoadhesion of Microparticles
We next sought to validate these in vitro findings using the rabbit as an in vivo model. The same four types of microparticles were formulated either as an aqueous suspension and a dry tablet and administered topically to the rabbit eye. The percentage of microparticles remaining on the preocular surface was determined after 10, 30 and 60 min, as shown in Figure 6. Retention of any of the four microparticle types, when administered as a suspension, was relatively poor, with just 1 – 10 % remaining after 10 min and even less at later times. We believe that this poor retention can be partially explained by the co-administration of eye drop carrier fluid to the preocular space, which is known to expedite tear drainage and thus remove microparticles more rapidly.29, 30
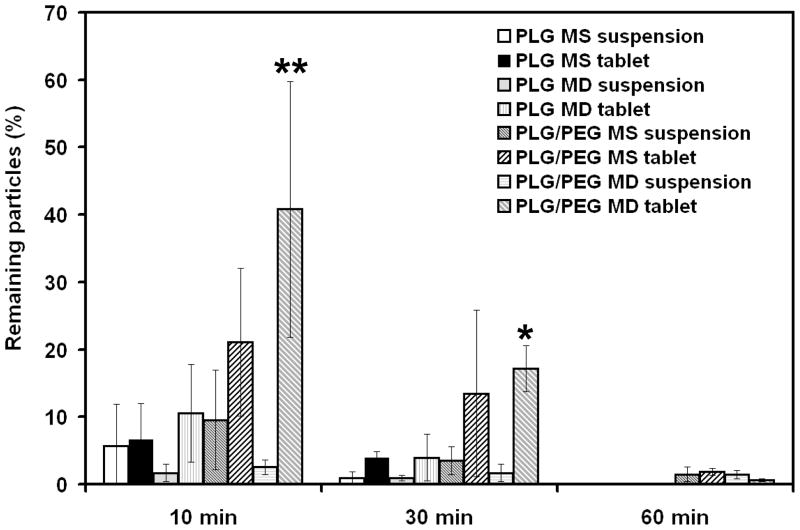
Figure 6.
In vivo residence time of microparticles on the rabbit eye. The percentage of microparticles remaining on the preocular surface of rabbits was measured for eight different microparticle formulations. **At 10 min, PLG/PEG MD tablet was significantly different from all other formulations (p < 0.05) except PLG/PEG MS tablet. *At 30 min, PLG/PEG MD tablet was significantly different from all suspensions (p < 0.05). The data points represent the average of n = 3 – 5 measurements ± standard deviation.
To reduce the effect of rapid removal of microparticles by excess tear drainage, we formulated the same dose of microparticles into rapidly dissolving mannitol-based tablets. These small cylindrical tablets dissolved in tear fluid on the preocular surface within 5 min (data not shown). This tablet formulation had no significant effect on PLG MS relative to the suspension formulations (p > 0.1). PLG MD tablets increased microdisc retention from 1.7 % to 10.5 % at 10 min and from 0.9 % to 3.9 % at 30 min compared to suspension, but this was not statistically significant (p > 0.1). The tablet formulation of PLG/PEG MS also made non-significant increases in mucoadhesive microsphere retention relative to PLG/PEG MS suspension (p > 0.1).
Finally, PLG/PEG MD tablets gave the best result with 41 % remaining at 10 min and 17 % at 30 min. The use of PLG/PEG MD tablets increased microparticle retention by 8 fold at 10 min and 17 fold at 30 min compared to a PLG MS suspension. Retention of PLG/PEG MD tablets was significantly greater at 10 min compared to all other formulations except PLG/PEG MS tablets, and at 30 min compared to all kinds of suspensions (p < 0.05). This shows that the combination of a mucoadhesion promoter, microdisc geometry and tablet formulation provided the greatest increase in preocular residence time.
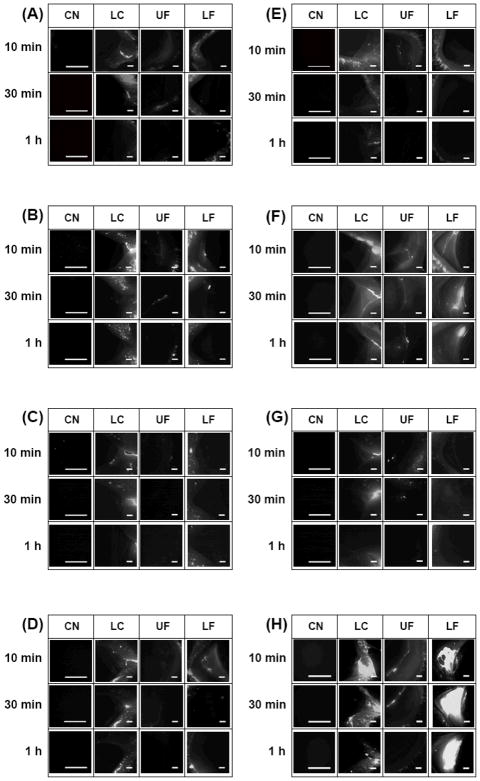
As a companion to these quantitative results, we next imaged the distribution of microparticles on the preocular surface using the same eight microparticle formulations at the same three time points, as shown in Figure 7. Four different regions of the eye were imaged: cornea (CN), lacrimal caruncle (LC), upper fornix (UF) and lower fornix (LF). Microparticles applied as a suspension were not seen much in any region of the eye. Some microparticles observed at the LC could be attributed to accumulated microparticles draining toward the lacrimal duct. Similarly, tablets embedded with PLG MS and PLG MD also disappeared rapidly, probably due to the absence of a mucoadhesion promoter (Figures 7(B) and (D)).
Figure 7.
Fluorescence micrographs of red-fluorescent microparticles remaining on the preocular surface of rabbits as a function of time and location on the eye. The formulations used were (A) PLG MS suspension, (B) PLG MS tablet, (C) PLG MD suspension, (D) PLG MD tablet, (E) PLG/PEG MS suspension, (F) PLG/PEG MS tablet, (G) PLG/PEG MD suspension and (H) PLG/PEG MD tablet. Each column presents images from a specific ocular region: cornea (CN), lacrimal caruncle (LC), upper fornix (UF) and lower fornix (LF). Each row presents images at different times after microparticle administration. The scale bars = 500 μm.
Much more extensive microparticle retention was observed with PLG/PEG MS and PLG/PEG MD tablets (Figures 7(F) and (H)) at all regions except the CN. The UF showed moderate accumulation of microparticles that mostly disappeared at 1 h after administration. The LF showed extensive microparticle retention, especially for the PLG/PEG MD, which persisted for at least 1 h. The extensive accumulation in the LF can be explained because the goblet cells in the lower cul-de-sac are known to be mainly responsible for production of mucin on the preocular surface.13, 25 Therefore, the local mucin concentration should be higher in the LF than other locations on the ocular surface, which would promote mucoadhesion of PLG/PEG microparticles. The LF was also the site of tablet administration, which would further help localize mucoadhesion at that location.
The absence of microparticles on the CN could be explained by the locally vigorous hydrodynamics and lack of mucin. During blinking, tear fluid on the CN experiences the strongest shear and highly dynamic mixing.25 This, combined with the thin film thickness of the tear fluid (i.e. < 10 μm),31 makes microparticle adhesion to the CN surface difficult.
DISCUSSION
Due to rapid clearance of drug from the preocular surface, topical delivery to the eye would benefit from a delivery system with longer preocular residence time to improve drug bioavailability and efficacy of therapy. To achieve this goal, we designed microparticles considering three design parameters: mucoadhesion, disc geometry and tablet formulation. Using this approach, we achieved microparticle residence times on the preocular surface of up to 1 h, which is an order-of-magnitude increase over conventional eye drops, which have a typical residence time of just 3 – 7 min.32
The turnover rate of mucin on the preocular surface is known to be much slower than that of the tear fluid.13 Thus, microparticles formulated with a mucoadhesion promoter, such as PEG,18 are expected to remain on the eye for a longer time. Considering tear flow and blinking, a disc shape should also help retain microparticles in the preocular space due to the low hydrodynamic resistance and high static frictional adhesion to the eye surface enabled by this geometry. The in vitro test in this study supports these expectations by showing synergetic enhancement of preocular residence time due to mucoadhesion and disc geometry. The use of mucoadhesive microdiscs (i.e., PLG/PEG MD) increased adhesion to the simulated ocular surface by a factor of two (Figure 5).
Use of a tablet formulation also played a key role to increase residence time of microparticles in vivo. Mucoadhesive microdiscs, when administered in a dry tablet to rabbits eyes in vivo, exhibited the best retention of all, which provided an increase in residence time of up to 17 fold compared to a PLG MS suspension (Figures 6 and 7). In contrast to aqueous suspensions, dissolution of the mannitol-based tablet probably increased the viscosity of tear fluid at the site of administration, which gave time for particles to react with the mucin better and thereby enhance microparticle attachment to the preocular surface.
Based on these observations, we propose mucoadhesive microdiscs formulated in a rapidly dissolving tablet as a novel topical drug delivery technology for the eye. In addition to prolonged residence time on the preocular surface, we further propose that controlled drug release from microdiscs can be achieved by encapsulating drug within the core material, i.e., PLG.33 Depending on the ratio of lactic to glycolic acids, polymer molecular weight, degree of drug loading, addition of other excipients, and other factors, the drug release rate can be tailored to achieve sustained or otherwise controlled release. Previously, suspensions of PLG MS (i.e., non-mucoadhesive microspheres), when administered topically to the rabbit eye, already showed significant improvement in drug bioavailability due to controlled release.10, 11, 16
In addition to mucoadhesivenss, PEG may also play a role in modulating the drug release profile. For example, release of transforming growth factor β1 was reported to be more sustained from microparticles blended with PEG.34 In contrast, different microparticles with water-soluble PEG became more porous due to rapid PEG dissolution in the release media, which accelerated the release of a number of compounds, such as ovalbumin, immunoglobin and dextran.35, 36
Optimal bioavailability might be achieved by matching the duration of drug release from the microparticles to the residence time of microparticles on the preocular surface. Note that PLG microparticles can be formulated for drug release over times much shorter than the time it takes to fully degrade the microparticles, especially for small drug molecules and at high drug loading in the microparticles.9 In this way, controlled release from PLG microparticles can be achieved over time scales most useful to topical delivery to the eye (e.g., hours) using microparticles that may biodegrade over much longer times (e.g., days or weeks). The biodegradation time of the microparticles may not be a critical parameter, because after they are cleared from the preocular surface through the lacrimal ducts, they will probably be eliminated through the gastrointestinal tract on a time scale of hours.37
In some previous studies, microparticles made solely out of mucoadhesive materials, such as pectin and poly(acrylic acid), were shown to achieve residence times of up to 2 h on the preocular surface.15, 16 Although it is difficult to compare quantitatively between studies due to different experimental methods, analytical detection limits, etc., this study showed that mucoadhesive microdiscs in a dry tablet had a similar residence time of at least 1 h (Figure 7(H)), but have the added advantage of using a PLG formulation that lends itself to controlled release of encapsulated drugs. It should also be noted that this increased residence time was achieved with a small mass fraction of mucoadhesive PEG (i.e., 10% w/w at most). Thus, addition of more mucoadhesion promoter, along with improved optimization of microdisc and tablet formulation, could increase residence time still further.
Microdiscs developed in this study should also be distinguished from nanoparticles widely investigated for topical drug delivery to the eye. Such nanoparticles are often used based on their ability to penetrate across the cornea through intracellular or paracellular pathways.38–40 For example, mucoadhesive nanoparticles (i.e., PEG-coated nanoparticles) were previously suggested as drug carriers with accelerated transport across the epithelium on the eye.38 In contrast, microdiscs in this work are envisioned primarily as controlled release vehicles for release into the tear fluid. Indeed, drug released from microdiscs is also intended to transport into the eye, but the microdiscs themselves remained outside.
Microdiscs also differ from ocular inserts. Although both systems serve as a preocular reservoir to deliver drug into the tear fluid,8 ocular inserts remain on the preocular surface as macroscopic devices without dissolution or degradation, which is bothersome to many patients.41 In contrast, a mannitol-based tablet can rapidly dissolve in the tear fluid, leaving behind only tiny microdiscs smaller than 10 μm, which are expected to be well tolerated by patients.2
CONCLUSION
This study supported the hypothesis that mucoadhesive microdiscs formulated in a rapidly dissolving tablet can increase preocular residence time. Mucoadhesion can improve microparticle attachment to the mucous layer of the preocular surface. The microdisc geometry can further promote mucoadhesion by increasing contact area between microparticles and the preocular surface and by reducing convective forces of tear flow that carry particles away. In vitro tests showed that microparticles prepared with a mucoadhesion promoter (PEG) and a microdisc geometry can adhere to a model mucous membrane twice as well as microparticles without these design features.
Formulating in a rapidly dissolving tablet can also increase preocular residence time because dissolution of the mannitol-based tablet can increase the viscosity of tear fluid at the site of administration, which gives time for microparticles to react with the mucin better and thereby enhance microparticle attachment to the preocular surface. In vivo tests in rabbits showed that formulation of mucoadhesive microdiscs in a rapidly dissolving tablet can increase preocular residence up to one hour, which represents an order of magnitude increase over conventional microparticles and eye drop formulations. Overall, we conclude that mucoadhesive microdiscs formulated in a rapidly dissolving tablet, coupled with encapsulation of drug within the microparticles for controlled release into the tear fluid, is a novel method of topical drug administration for sustained delivery to the eye.
Acknowledgments
We thank Jae Kyu Cho for help with in vitro mucoadhesion studies and Tae-Hyun Bae for help with DSC analysis. This research was partially funded by the National Eye Institute of NIH.
References
- 1.Ghate D, Edelhauser HF. Ocular drug delivery. Expert Opin Drug Deliv. 2006;3:275–287. doi: 10.1517/17425247.3.2.275. [DOI] [PubMed] [Google Scholar]
- 2.Sultana Y, Jain R, Aqil M, Ali A. Review of ocular drug delivery. Curr Drug Del. 2006;3:207–217. doi: 10.2174/156720106776359186. [DOI] [PubMed] [Google Scholar]
- 3.Saettone MF, Giannaccini B, Ravecca S, Marca FL, Tota G. Polymer effects on ocular bioavailability - the influence of different liquid vehicles on the mydriatic response of tropicamide in humans and rabbits. Int J Pharm. 1984;20:187–202. [Google Scholar]
- 4.Saettone MF, Giannaccini B, Guiducci A, Marca F, Tota G. Polymer effects on ocular bioavailability: II. The influence of benzalkonium chloride on the mydriatic response of tropicamide in different polymeric vehicles. Int J Pharm. 1985;25:73–83. [Google Scholar]
- 5.Chang SC, Chien DS, Budgaard H, Lee VHL. Relative effectiveness of prodrug and viscous approaches in maximizing the ratio of ocular to systemic absorption of topically applied tomolol. Exp Eye Res. 1988;46:56–69. doi: 10.1016/s0014-4835(88)80093-9. [DOI] [PubMed] [Google Scholar]
- 6.Roziere A, Mazuel C, Grove J, Plazonnet B. Gelrite: a novel ion activated, in situ gelling polymer for ophthalmic vehicles: effect on bioavailability of timolol. Int J Pharm. 1989;57:163–168. [Google Scholar]
- 7.Miller SC, Donovan MD. Effect of poloxamer 407 gel on the miotic activity of pilocarpine nitrate in rabbits. Int J Pharm. 1982;12:147–152. [Google Scholar]
- 8.Macoul KL, Pavan-Langston D. Pilocarpine ocusert system for sustained control of ocular hypertension. Arch Ophthalmol. 1975;93:587–590. doi: 10.1001/archopht.1975.01010020571003. [DOI] [PubMed] [Google Scholar]
- 9.Varde NK, Pack DW. Microspheres for controlled release drug delivery. Expert Opin Biol Ther. 2004;4:35–51. doi: 10.1517/14712598.4.1.35. [DOI] [PubMed] [Google Scholar]
- 10.Loftsson T, Hreinsdottir D, Stefansson E. Cyclodextrin microparticles for drug delivery to the posterior segment of the eye: aqueous dexamethasone eye drops. J Pharm Pharmacol. 2007;59:629–635. doi: 10.1211/jpp.59.5.0002. [DOI] [PubMed] [Google Scholar]
- 11.Gavini E, Chetoni P, Cossu M, Alvarez MG, Saettone MF, Giunchedi P. PLGA microspheres for the ocular delivery of a peptide drug, vancomycin using emulsification/spray-drying as the preparation method: in vitro/in vivo studies. Eur J Pharm Biopharm. 2004;57:207–212. doi: 10.1016/j.ejpb.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Vasir JK, Tambwekar K, Garg S. Bioadhesive microspheres as a controlled drug delivery system. Int J Pharm. 2003;255:13–32. doi: 10.1016/s0378-5173(03)00087-5. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig A. The use of mucoadhesive polymers in ocular drug delivery. Adv Drug Del Rev. 2005;57:1595–1639. doi: 10.1016/j.addr.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Genta I, Conti B, Perugini P, Pavanetto F, Spadaro A, Puglisi G. Bioadhesive microspheres for ophthalmic administration of acyclovir. J Pharm Pharmacol. 1997;49:737–742. doi: 10.1111/j.2042-7158.1997.tb06103.x. [DOI] [PubMed] [Google Scholar]
- 15.Durrani AM, Farr SJ, Kellaway IW. Precorneal clearance of mucoadhesive microspheres from the rabbit eye. J Pharm Pharmacol. 1995;47:581–584. doi: 10.1111/j.2042-7158.1995.tb06718.x. [DOI] [PubMed] [Google Scholar]
- 16.Giunchedi P, Conte U, Chetoni P, Saettone MF. Pectin microspheres as ophthalmic carriers for piroxicam: evaluation in vitro and in vivo in albino rabbits. Eur J Pharm Sci. 1999;9:1–7. doi: 10.1016/s0928-0987(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 17.Zimmer AK, Chetoni P, Saettone MF, Zerbe H, Kreuter J. Evaluation of pilocarpine-loaded albumin particles as controlled drug delivery systems for the eye. II. Co-administration with bioadhesive and viscous polymers. J Control Rel. 1995;33:31–46. [Google Scholar]
- 18.Iwanaga K, Ono S, Narioka K, et al. Application of surface-coated liposomes for oral delivery of peptide: effects of coating the liposome’s surface on the GI transit of insulin. J Pharm Sci. 1999;88:248–252. doi: 10.1021/js980235x. [DOI] [PubMed] [Google Scholar]
- 19.Efremova NV, Huang Y, Peppas NA, Leckband DE. Direct measurement of interactions between tethered poly(ethylene glycol) chains and adsorbed mucin layers. Langmuir. 2002;18:836–845. [Google Scholar]
- 20.Anderson JM, Shive MS. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Del Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 21.Zalipsky S. Chemistry of polyethylene glycol conjugates with biologically active molecules. Adv Drug Del Rev. 1995;16:157–182. [Google Scholar]
- 22.Champion JA, Katare YK, Mitragotri S. Particle shape: a new design parameter for micro- and nanoscale drug delivery carriers. J Control Release. 2007;121:3–9. doi: 10.1016/j.jconrel.2007.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reader MJ. Influence of isotonic agents on the stability of thimerosal in ophthalmic formulations. J Pharm Sci. 1984;73:840–841. doi: 10.1002/jps.2600730637. [DOI] [PubMed] [Google Scholar]
- 24.Berkland C, Kim K, Pack DW. PLG Microsphere Size Controls Drug Release Rate Through Several Competing Factors. Pharm Res. 2003;20:1055–1062. doi: 10.1023/a:1024466407849. [DOI] [PubMed] [Google Scholar]
- 25.Dilly PN. Structure and Function of the Tear Film. In: Sullivan DA, editor. Lacrimal Gland, Tear Film, and Dry Eye Syndromes. New York: Plenum Press; 1994. [Google Scholar]
- 26.Eckhoff RK. A static investigation of the Coulter principle of particle sizing. J Sci Instrum. 1969;2:973–977. [Google Scholar]
- 27.Forcino RG, Jonnalagadda S. The effect of fabrication methods on the mechanical and thermal properties of poly(lactide-co-glycolide) scaffolds. J Appl Polym Sic. 2007;104:944–949. [Google Scholar]
- 28.Owens H, Philips J. Spreading of the tears after a blink. Cornea. 2001;20:484–487. doi: 10.1097/00003226-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Sieg JW, Triplett JW. Precorneal retention of topical instilled micronized particles. J Pharm Sci. 1980;69:863–864. doi: 10.1002/jps.2600690735. [DOI] [PubMed] [Google Scholar]
- 30.Zhu H, Chauhan A. A mathematical model for ocular tear and solute balance. Curr Eye Res. 2005;30:841–854. doi: 10.1080/02713680591004077. [DOI] [PubMed] [Google Scholar]
- 31.Watsky MA, Jablonski MM, Edelhauser HF. Comparison of conjuctival and corneal surface areas in rabbit and human. Curr Eye Res. 1988;7:483–486. doi: 10.3109/02713688809031801. [DOI] [PubMed] [Google Scholar]
- 32.Schoenwald RW. Ocular pharmacokinetics. In: Zimmerman TJ, editor. Textbook of Ocular Pharmacology. Lippincott-Raven Publishers; 1997. pp. 119–138. [Google Scholar]
- 33.Shive MS, Anderson JM. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv Drug Deliv Rev. 1997;28:5–24. doi: 10.1016/s0169-409x(97)00048-3. [DOI] [PubMed] [Google Scholar]
- 34.Lu L, Stamatas GN, Mikos AG. Controlled release of transforming growth factor beta-1 from biodegradable polymer microparticles. J Biomed Mater Res Part A. 2000;50:440–451. doi: 10.1002/(sici)1097-4636(20000605)50:3<440::aid-jbm19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 35.Cleek RL, Ting KC, Eskin SG, Mikos AG. Microparticles of poly(DL-lactic-co-glycolic acid)/poly(ethylene glycol) blends for controlled drug delivery. J Control Release. 1997;48:259–268. [Google Scholar]
- 36.Yeh M-K, Jenkins PG, Davis SS, Coombes AGA. Improving the delivery capacity of microparticle systems using blends of poly(DL-lactide co-glycolide) and poly(ethylene glycol) J Control Release. 1995;37:1–9. [Google Scholar]
- 37.Khan MZ, Prebeg Z, Kurjakovic N. A pH-dependent colon targeted oral drug delivery system using methacrylic acid copolymers. I. Manipulation Of drug release using Eudragit L100-55 and Eudragit S100 combinations. J Control Release. 1999;58:215–222. doi: 10.1016/s0168-3659(98)00151-5. [DOI] [PubMed] [Google Scholar]
- 38.De Campos AM, Sanchez A, Gref R, Calvo P, Alonso MJ. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci. 2003;20:73–81. doi: 10.1016/s0928-0987(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 39.Giannavola C, Bucolo C, Maltese A, et al. Influence of preparation conditions on acyclovir-loaded poly-d,l-lactic acid nanospheres and effect of PEG coating on ocular drug bioavailability. Pharm Res. 2003;20:584–590. doi: 10.1023/a:1023290514575. [DOI] [PubMed] [Google Scholar]
- 40.Kompella UB, Sundaram S, Raghava S, Escobar ER. Luteinizing hormone-releasing hormone agonist and transferrin functionalizations enhance nanoparticle delivery in a novel bovine ex vivo eye model. Mol Vis. 2006;12:1185–1198. [PubMed] [Google Scholar]
- 41.Saettone MF, Salminen L. Ocular inserts for topical delivery. Adv Drug Deliv Rev. 1995;16:95–106. [Google Scholar]