Abstract
The increased sensitivity of peripheral pain-sensing neurons, or nociceptors, is a major cause of the sensation of pain that follows injury. This plasticity is thought to contribute to the maintenance of chronic pain states. Although we have a broad knowledge of the factors that stimulate changes in nociceptor sensitivity, the cellular mechanisms that underlie this plasticity are still poorly understood; however, they are likely to involve changes in gene expression required for the phenotypic and functional changes seen in nociceptive neurons after injury. While the regulation of gene expression at the transcriptional level has been studied extensively, the regulation of protein synthesis, which is also a tightly controlled process, has only recently received more attention. Despite the established role of protein synthesis in the plasticity of neuronal cell bodies and dendrites, little attention has been paid to the role of translation control in mature undamaged axons. In this regard, several recent studies have demonstrated that the control of protein synthesis within the axonal compartment is crucial for the normal function and regulation of sensitivity of nociceptors. Pathways and proteins regulating this process, such as the mammalian target of rapamycin signaling cascade and the fragile X mental retardation protein, have recently been identified. We review here recent evidence for the regulation of protein synthesis within a nociceptor’s axonal compartment and its contribution to this neuron’s plasticity. We believe that an increased understanding of this process will lead to the identification of novel targets for the treatment of chronic pain.
Keywords: chronic pain, ERK, FMRP, hyperalgesia, mTOR, translation regulation
Introduction: nociceptor plasticity, a key factor in chronic pain
The transduction and transmission of information encoding noxious stimuli in peripheral tissues occurs through specialized sensory neurons called nociceptors that project to the central nervous system (CNS) where this peripheral input is processed and transmitted rostrally to higher brain structures leading to the perception of pain. Plasticity at multiple sites in pain pathways is now widely accepted as critical in the emergence and maintenance of pathological pain states (Hunt & Mantyh, 2001). Changes in synaptic strength and efficacy of pain modulatory circuits can occur throughout the pain axis. These include long-term potentiation of dorsal horn neurons (Sandkuhler, 2007) within spinal cord circuits receiving input from sensory neurons and enhancements of descending influences from brainstem circuits that exert a powerful control on spinal pain processing (Porreca et al., 2002). In addition to changes within the CNS, plasticity of peripheral nociceptors also contributes to the overall changes in sensitivity that support injury-induced pain (Woolf & Ma, 2007). This peripheral plasticity can manifest in a variety of ways such as changes in receptor and channel expression, distribution and activation threshold. For example, alterations in sodium channel expression or physiology can lead to hyperexcitability, a process thought to contribute to the ectopic discharge (the generation of action potentials in the absence of external stimulation) seen after peripheral nerve injury (Devor, 2006). Changes in transient potential channel vanilloid type 1 (TRPV1) receptor expression and activation threshold after inflammation are also well documented and have been linked to increased thermal sensitivity, referred to as thermal hyperalgesia (Caterina & Julius, 2001). Although more controversial, some mechanical sensitization of primary afferents has also been reported (at least to suprathreshold stimulation; Andrew & Greenspan, 1999a). Subsets of damaged nociceptors regenerating or sprouting after nerve injury do indeed show reduced mechanical thresholds (Jankowski et al., 2009) and enhanced responses to suprathreshold mechanical stimulation (Andrew & Greenspan, 1999b) and these result in mechanical hyperalgesia. While human and animal studies suggest that mechanical allodynia (a painful response to a previously non-noxious stimulus) is due to a change of central processing of incoming low-threshold inputs (Price et al., 2008), it is clear that plasticity in the physiology of peripheral nociceptors also plays a key role in the hyperalgesia and spontaneous nociceptor discharge seen after injury (Andrew & Greenspan, 1999b; a; Caterina & Julius, 2001; Devor, 2006; Woolf & Ma, 2007; Jankowski et al., 2009). In conclusion, the plasticity of peripheral nociceptors observed after injury is a key factor to the three primary characteristics of most preclinical pain models: thermal hyperalgesia, mechanical hyperalgesia/allodynia and sensory neuron ectopic activity. Therefore, understanding the molecular physiology of peripheral nociceptor plasticity has the potential to advance the generation of new therapeutic avenues for the treatment of pain states.
The crucial contribution of the peripheral nociceptor in the emergence and maintenance of pain states has led to an exponential increase in our understanding of the molecular phenotypes and functions of these neurons. Nociceptor-specific ion channels (Chen et al., 1995; Akopian et al., 1996) and G protein-coupled receptors (Dong et al., 2001; Lembo et al., 2002) have been identified, signaling pathways that enhance the sensitivity of ligand- and voltage-gated ion channels have been elucidated (Woolf & Salter, 2000; Woolf & Ma, 2007) and phenotypic changes in nociceptors have been described after inflammation and peripheral nerve injury (Noguchi et al., 1994; Woolf, 1996; Woolf & Ma, 2007). These studies have led to the view that changes in gene expression occur in nociceptors during chronic inflammation or after nerve injury and there is now ample evidence to support the conclusion that such changes contribute greatly to the transition from the initiation to the maintenance phase of a pain state (Woolf & Ma, 2007).
Gene expression is tightly regulated at both the transcriptional and translational levels and until recently the vast majority of studies have investigated changes in transcription (eg. mRNA content measured with microarrays or in situ hybridisation) or translation (protein expression measured by Western blot and immunohistochemistry) within the cell body of nociceptors in the dorsal root ganglion (DRG). However, the regulation of translation is highly dependent on subcellular compartmentalization, signaling pathways and signaling microdomains (Martin & Zukin, 2006; Bramham & Wells, 2007; Costa-Mattioli et al., 2009), suggesting that changes in protein expression within the neuronal soma may not reflect the full repertoire of the nociceptor’s capacity to regulate the expression of proteins.
Here, we review the current evidence supporting the view that local protein synthesis occurs in axons of subsets of DRG neurons, and more specifically nociceptors. Moreover we discuss data demonstrating that local axonal translation is a feature which is key to their normal function and critical for the regulation of their sensitivity (Weragoda et al., 2004; Price et al., 2006, 2007; Huang et al., 2008; Jimenez-Diaz et al., 2008). Collectively, these findings indicate that the regulation of nociceptor physiology at the translational level is more complex than has been previously recognized and that a greater knowledge of how gene expression is regulated within localized regions of these neurons, such as at the peripheral terminals, may represent a fruitful avenue for the exploration of novel therapeutic opportunities.
Regulation of translation: a critical factor for gene expression and synaptic plasticity
Control of gene expression is a crucial factor for the survival and function of all cells, and happens at both the transcriptional and translational level. The best-known aspect of this process in relation to neuronal plasticity is transcriptional control (Lee et al., 2008). Transcription activity depends on the conformation of DNA and the accessibility to transcription factors. It can be altered by the expression of other genes that feed back onto the transcriptional machinery through a multitude of different mechanisms. The resulting production of mRNAs is tightly controlled but, interestingly, does not necessarily have any direct bearing on changes in protein levels. This is because translation is closely regulated by a number of factors: (i) the binding of mRNAs by RNA binding proteins and other trafficking components and their trafficking to distinct subcellular locations (Bramham & Wells, 2007), (ii) the binding of miRNAs and the recruitment of mRNA-miRNA complexes into particular cellular RNA granules (Leung & Sharp, 2006; Kedersha & Anderson, 2007; Anderson & Kedersha, 2008) and (iii) the signaling pathways that converge on proteins that either bind the 5′CAP structure of mRNAs (a specially altered nucleotide on the 5′ end of mRNA that ensures the mRNA stability while it undergoes translation) or are involved in the recruitment of ribosomal subunits to modulate the initiation step of translation (Costa-Mattioli et al., 2009).
RNA binding proteins and miRNAs
Spatial and temporal control of translation are key features for the modulation of gene expression. A large number of RNA-binding proteins involved in this regulatory process have been identified and many of these are thought to be involved in the localization of RNAs to distal sites in neurons via transport granules and/or in the recruitment of RNAs into specialized RNA-containing cellular structures such as processing bodies (P-bodies) or stress granules (Bramham & Wells, 2007; Anderson & Kedersha, 2008). A key RNA binding protein is the fragile X mental retardation protein (FMRP). Silencing of the gene that codes for FMRP in humans (FMR1) causes fragile X mental retardation, the most common form of inherited mental retardation. FMRP contains several RNA-binding domains that associate with primary sequences or secondary structures which generally reside in untranslated regions (UTRs) of mRNAs (Bassell & Warren, 2008). FMRP appears to be involved in the localization of RNAs in cells (Estes et al., 2008), probably through association with transport granules, and is a critical translation repressor when bound to its target mRNAs (Bassell & Warren, 2008) or through protein–protein interactions by inhibiting translation initiation (Napoli et al., 2008). Current evidence suggests that cellular signals such as mGluR1/5 activation are capable of stimulating FMRP dissociation from target mRNAs after dephosphorylation by protein phosphatase 2A (PP2A), subsequently causing a derepression of translation of these targets (Narayanan et al., 2007). The phosphorylation state of FMRP is tightly regulated. Indeed, following FMRP dephosphorylation by PP2A, FMRP can be rephosphorylated by S6 kinase 1, downstream of extracellular signal regulated kinase (ERK) and the mammalian target of rapamycin (mTOR) (see below for discussion of ERK and mTOR) (Narayanan et al., 2008). These findings support a model wherein dephosphorylation and subsequent rephosphorylation of FMRP downstream of mGluR1/5 offers a high degree of temporal control of translation upon synaptic activation.
In addition to the functions highlighted above, FMRP has also been identified as a key molecular component of the miRNA translational arrest pathway and has been found in stress granules (Mazroui et al., 2002; Linder et al., 2008; Didiot et al., 2009) and P body-like structures (Barbee et al., 2006). The miRNA machinery plays an important role in regulating gene expression both at the transcriptional level, by modifying chromatin and DNA rearrangement (Lippman & Martienssen, 2004), and at the translational level, by repressing translation through binding to target mRNAs and by controlling mRNA degradation (for a complete review see Filipowicz et al., 2005). FMRP forms a complex with miRNAs and dicer and argonaute, two key proteins in the process of RNA silencing (Barbee et al., 2006). In drosophila, FMRP and argonaute are found together in neuronal P-bodies (Barbee et al., 2006). In a recent study, loss of FMRP was shown to decrease the formation of stress granules, suggesting that FMRP may be involved in shuttling mRNAs to cellular RNA granules where translational arrest occurs rather than being directly linked to mRNA degradation (Didiot et al., 2009), which occurs in P bodies (Parker & Sheth, 2007). Hence, FMRP is a multifunctional RNA-binding protein involved in many facets of the control of translation. Altogether these studies highlight the extent to which RNA binding proteins and miRNAs are crucial for the temporal and spatial control of translation in neurons.
Signaling pathways and the control of translation initiation
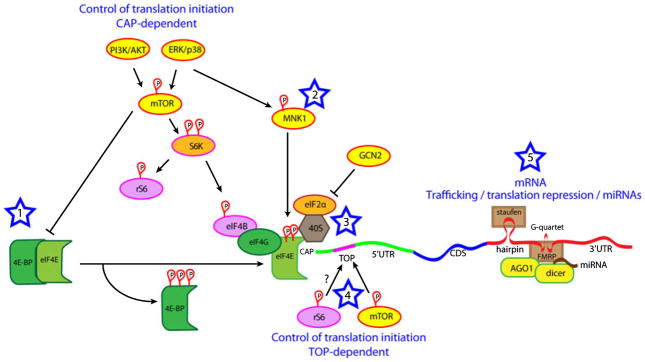
Translation proceeds in three major steps: initiation, elongation and termination. Initiation is the rate-limiting step in this process and is regulated by a group of initiation proteins that are targets for cellular protein kinases. The activity of these upstream kinases, which differs between subcellular compartments, controls the rate-limiting step of translation (Raught et al., 2001; Gingras et al., 2004). Moreover, as these kinases can be activated downstream of transmembrane receptor activation, translation initiation is controlled by hormones, cell-to-cell signaling and neurotransmission. Indeed, a key discovery in the control of gene expression in synaptic plasticity was the finding that neurotransmitter receptors are capable of signaling to translation initiation factors through protein kinases (Kelleher et al., 2004; Costa-Mattioli et al., 2009). This signaling process appears to occur primarily through two pathways, the ERK and mTOR signaling cascades (Kelleher et al., 2004; Costa-Mattioli et al., 2009). Both pathways regulate the efficiency of translation initiation via the phosphorylation of proteins that influence the formation of the translation initiation complex. Mechanisms of translation control of gene expression are shown in Fig. 1.
Fig. 1.
Mechanisms of translation control of gene expression. At least three mechanisms have been identified via which extracellular signals can regulate the rate-limiting step of translation, initiation (Star 1 to 3). (Star 1) The kinase mTOR can be activated downstream of PI3Kinase/AKT signaling and under certain circumstances by ERK. The primary target for mTOR (when it is associated with raptor, forming the mTORC1 complex) is 4E-BP. When 4E-BP is not phosphorylated, it binds to eIF4E and suppresses translation. When 4E-BP is phosphorylated it dissociates from eIF4E, allowing the binding of eIF4G and the modulation of eIF4E activity by ERK/MNK1 signaling. mTOR also phosphorylates S6 kinase (S6K), leading to phosphorylation of eIF4B and ribosomal S6 protein (rS6). (Star 2) ERK and p38 signal via MNK1 to increase eIF4E phosphorylation. This phosphorylation of eIF4E is linked to an increased affinity for eIF4G and the formation of this complex is involved in the recruitment of the 40S ribosomal subunit. (Star 3) The initiation factor eIF2α, when unphosphorylated, is also involved in the recruitment of the 40S ribosome subunit. eIF2α is phosphorylated by GCN2 in conditions of cellular stress, and genetic modulation of eIF2α phosphorylation has shown that eIF2α plays an important role in synaptic plasticity. (Star 4) A subset of mRNAs contain a 5′ terminal oligopyrimidine tract (TOP) and largely encode ribosomal proteins and elongation initiation factors. Increased mTOR activity is positively linked to the translation of these mRNAs as well as rS6 phosphorylation; however, genetic studies clearly show that rS6 is dispensable for this effect. (Star 5) Translation is also regulated by mRNA localization, miRNAs and the miRNA pathway. Key RNA binding proteins involved in RNA localization in neurons are staufen (which binds to hairpins) and FMRP (which binds to G-quartets, among other structures and sequences). FMRP has also been shown to bind to miRNAs and proteins involved in the miRNA pathway such as dicer and argonaute (AGO1).
ERK, mTOR and eIF4E
ERK can be activated downstream of a number of transmembrane receptors (e.g. mGluR1/5) via its upstream kinase the mitogen-activated protein kinase (MEK). ERK is capable of phosphorylating a large number of ion channel targets, such as A-type potassium channels (Adams et al., 2000; Hu et al., 2006); moreover, it also phosphorylates a downstream effector called the mitogen-activated kinase (MAPK)-interacting kinase 1 (MNK1), a kinase for the initiation factor and 5′CAP-binding protein: the eukaryotic initiation factor (eIF) 4E (Banko et al., 2006; Gelinas et al., 2007). MNK1 can also be phosphorylated by another member of the MAPK family, p38, which enhances eIF4E phosphorylation (Waskiewicz et al., 1997), an event in general closely correlated with the rate of translation (Costa-Mattioli et al., 2009). Hence, both ERK and p38 signal to the translation machinery via MNK1-induced phosphorylation of eIF4E.
Although mTOR can also be a substrate for phosphorylation via the ERK pathway (Shaw & Cantley, 2006), it is primarily activated downstream of a number of transmembrane receptors and phosphorylated following upstream activation of the phosphoinositol 3 kinase (PI3K)–AKT pathway (Gingras et al., 2004; Jaworski & Sheng, 2006). mTOR phosphorylates a protein that tonically inhibits the 5′CAP-binding protein eIF4E, called 4E-binding protein (4E-BP) (Gingras et al., 1999). Under normal conditions, 4E-BP binds to eIF4E and prevents its association with eIF4G, a required step for the initiation of translation. When 4E-BP is phosphorylated by mTOR it becomes uncoupled from eIF4E, allowing eIF4E to bind eIF4G (Gingras et al., 1999). 4E-BP is phosphorylated hierarchically and, while several sites contributing to 4E-BP dissociation from eIF4E are targets for mTOR, the complete repertoire of kinases responsible for hyperphosphorylation of 4E-BP have not yet been identified (Gingras et al., 2001). When eIF4E binds eIF4G, eIF4E can be phosphorylated by MNK1 which uses eIF4G as a docking site (Gingras et al., 1999; Pyronnet et al., 1999). This phosphorylation, which occurs downstream of ERK and p38, as discussed above, enhances eIF4E–eIF4G association, allowing the stabilization of this complex and the recruitment of the 40S ribosomal subunits to initiate elongation of mRNAs that have a 5′CAP (Morley et al., 1997; Dever, 1999; Scheper & Proud, 2002). CGP57380, a specific inhibitor of MNK1, has been shown to inhibit eIF4E phosphorylation and therefore reduce the interaction between eIF4E and eIF4G (Zhang et al., 2008). While ERK and mTOR signaling to 5′CAP-binding elongation initiation factors (eIF4E and 4E-BP, respectively) is a critical step for the stimulation of CAP-dependent translation, mTOR and ERK signaling have also specifically been linked to increases in the translation of 5′ terminal oligopyrimidine tract (TOP) mRNAs, i.e. mRNAs that contain an oligopyrimidine sequence in their 5′ regions (Ruvinsky & Meyuhas, 2006; Gobert et al., 2008; Patursky-Polischuk et al., 2009). As most TOP-containing mRNAs encode proteins involved in protein biosynthesis (such as ribosomal proteins and eIFs; Meyuhas, 2000; Hamilton et al., 2006) it is clear that mTOR or ERK activation can fundamentally alter gene expression via an increase in protein biosynthesis machinery. This process also suggests autoregulation of translation as some stimuli can trigger the translation of factors involved in translation (Lin & Holt, 2008) leading not only to an enhancement of translation efficiency but also to an increase in the absolute quantity of proteins required for translation.
ERK/MNK1 signaling has been shown to be an important step in synaptic plasticity including mGluR-dependent long-term depression (LTD) and β-adrenergic-dependent LTP (Banko et al., 2006; Gelinas et al., 2007). mTOR-mediated translation has also been implicated in many forms of synaptic plasticity including NMDA receptor-mediated LTP and mGluR-mediated LTD (Kelleher et al., 2004; Jaworski & Sheng, 2006). Importantly, both ERK and mTOR converge on the eIF4E complex (Banko et al., 2006; Gelinas et al., 2007; Tsokas et al., 2007) which is a significant regulator of the rate-limiting step of translation, initiation.
eIF2α
Another critical mode of control of initiation of translation for synaptic plasticity is via the eIF2α protein. In the nonphosphorylated state, eIF2α is involved in the recruitment of the 40S ribosomal subunit. However, phosphorylation of eIF2α, a substrate for a number of kinases including general control nonderepressible 2 (GCN2), inhibits translation (Dever et al., 1992). While cellular stress is known to stimulate GCN2-mediated eIF2α phosphorylation (Dever et al., 1992), neuro-transmitter receptor-linked pathways that engage GCN2 have yet to be elucidated. Finally, genetic manipulation of a key eIF2α phosphorylation site (S51) or knockout of GCN2 have indicated that eIF2α plays a major role in synaptic plasticity, presumably via its influence on translation initiation (Costa-Mattioli et al., 2005, 2007, 2009).
Dendritic protein synthesis
The emergence of the role of dendritic translation in synaptic plasticity over the past decade has largely reversed the commonly held misconception that protein synthesis only occurs in the neuronal soma. The discovery that ribosomes are found at the base of dendritic spines immediately suggested that protein synthesis at these sites could contribute to postsynaptic plasticity (Steward & Levy, 1982). A large number of studies have borne this hypothesis out and it is now widely accepted that dendritic translation is an important component of at least two major forms of synaptic plasticity, LTP and LTD (Kelleher et al., 2004; Schuman et al., 2006). The critical nature of localized, dendritic translation for neuronal morphology and function is supported by specific mRNA localization to defined neuronal compartments. For example, the short 3′UTR form of brain-derived neurotrophic factor (BDNF) mRNA is restricted to the soma while the long 3′UTR form is enriched in dendrites (An et al., 2008). In a mutant mouse in which the long 3′UTR BDNF mRNA is truncated, BDNF protein fails to localize to dendrites, dendritic spine morphology is aberrant and LTP is selectively abrogated in dendrites but not in the soma (An et al., 2008).
The role of the miRNA machinery in mediating local translation-induced synaptic plasticity in dendrites has recently been demonstrated. Indeed, the miRNA miR-134 has been shown to regulate spine size in hippocampal dendrites by repressing Lim kinase 1 translation and this repression was relieved by BDNF stimulation (Schratt et al., 2006). In Drosophila, synaptic activity relieves miRNA repression of calcium–calmodulin-dependent kinase II (CaMKII) translation, a process required for long-term memory (Ashraf et al., 2006). These results suggest that the miRNA machinery is a key factor in the regulation of dendritic translation and could play an important role in the regulation of axonal mRNA translation.
Finally, the discovery of so-called Golgi outposts in dendrites have changed our views on how transmembrane proteins can be made and processed in the dendritic region (Horton & Ehlers, 2003, 2004; Kennedy & Ehlers, 2006; Hanus & Ehlers, 2008), and their recent demonstration in axons of peripheral neurons (Merianda et al., 2009) is an important step forward in our understanding of sensory neuron biology.
Peripheral axons contain the machinery for protein synthesis
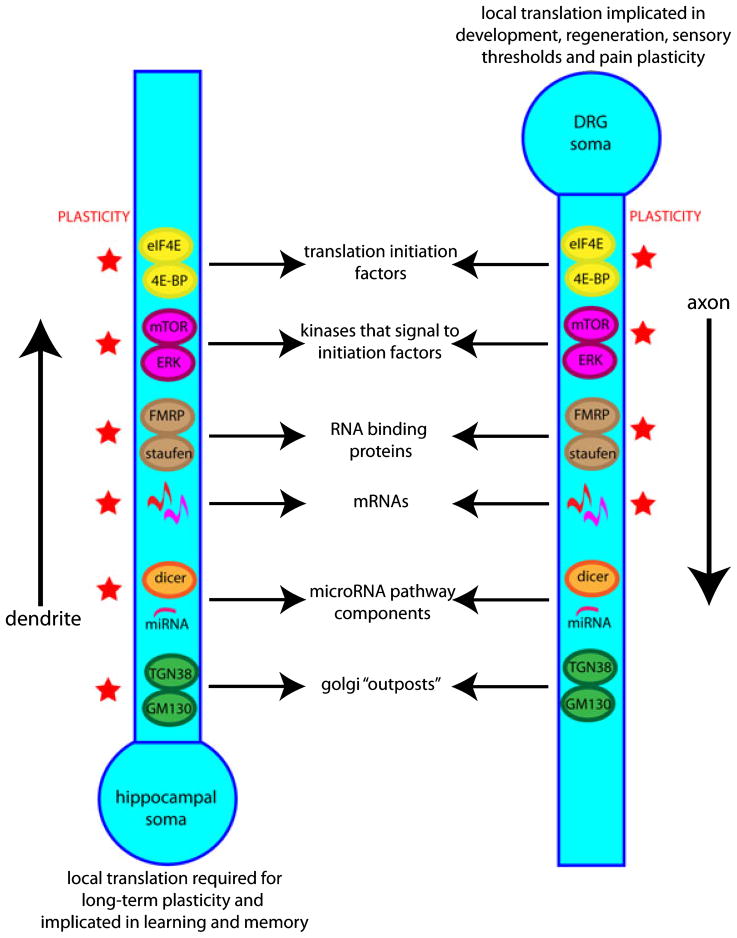
Although evidence that proteins can be synthesized in the axons of invertebrate neurons, away from the cell body, has actually existed for more than 40 years (Giuditta et al., 1968), translation in axons has remained a more controversial issue, largely because of the difficulty in identifying ribosomes and Golgi components required for the processing of many vesicular and transmembrane proteins within mammalian axons (Alvarez et al., 2000). However, it has been argued that slow axonal transport rates are wholly incompatible with protein half-lives for neurons with long axonal processes such as mammalian DRG neurons (Alvarez et al., 2000), and the existence of axonal translation seems a necessary means of maintaining axonal integrity. A number of recent findings form a strong case for protein synthesis within the axon. Although methods used in the past to identify ribosomes in axons have not always had sufficient sensitivity (see Alvarez et al., 2000; for a comprehensive examination of these experimental limitations), more detailed examinations have now demonstrated that ribosomes do indeed localize to axons (Koenig, 1979, 1991; Koenig & Giuditta, 1999; Koenig et al., 2000; Alvarez, 2001; Martin, 2004). Axons also contain mRNAs (Zheng et al., 2001; Willis et al., 2005) and molecules involved in the regulation of translation. These include Staufen 2 and FMRP, two proteins that bind mRNAs and traffic them to distal sites in neurons (Price et al., 2006) and the functionally active miRNA machinery involved in the regulation of translation (Hengst et al., 2006; Murashov et al., 2007). Finally, peripheral axons contain mTOR (Jimenez-Diaz et al., 2008), ERK (Agthong et al., 2006) and the initiation factors they signal to (e.g. 4E-BP), and are endowed with ribosomal kinases (P70-S6kinase and ribosomal S6kinase; Jimenez-Diaz et al., 2008). These pathways are expected to regulate CAP-dependent translation as well as to play an important role in stimulating TOP-containing mRNA protein biosynthesis and therefore increase the axonal protein synthesis capacity. Similarities between translation control in CNS dendrites and PNS axons are shown in Fig. 2.
Fig. 2.
Similarities between translation control in CNS dendrites and PNS axons. Advances in our understanding of how translation can be controlled at distal sites in neurons has shown a number of parallels between translation control in CNS dendrites (which is linked to synaptic plasticity) and in PNS axons (which is linked to regeneration, repair and nociceptive plasticity). Both of these structures contain initiation factors (4E-BP and eIF4E), kinases that signal to initiation factors (mTOR and ERK), RNA binding proteins (FMRP and staufen), a diverse subset of mRNAs, miRNAs and miRNA pathway components (dicer and argonaute) and Golgi outposts for the proper processing of transmembrane and other proteins (TGN38 and GM130). Many of these components and related mechanisms have been experimentally linked to synaptic plasticity in CNS dendrites (red stars). Many of them have also been shown to be involved in PNS axon regeneration, repair and/or nociceptive plasticity (red stars).
In conclusion, it is clear that peripheral axons contain the machinery for protein synthesis. They contain ribosomes, mRNAs, miRNAs, Golgi components, RNA biding proteins and, finally, kinases and initiation factors that regulate translation.
Compartmentalized control of translation in developing and regenerating axons
Blockade of protein synthesis in growing axons causes a rapid retraction of the growth cone, suggesting that local translation is involved in axonal growth (Verma et al., 2005; Wu et al., 2005). This indicates a possible functional role for axonal translation in both development and regeneration of axons (Piper & Holt, 2004; Willis & Twiss, 2006). This notion is now supported by detailed studies indicating that the mRNA for the small GTPase, rhoA, which is involved in growth cone guidance, localizes to the growth cone and is translated downstream of external cues which direct growth cone guidance and collapse (Wu et al., 2005). Similar results have been obtained with the guidance cue receptor EphA2, confirming that axonal translation is an important aspect of axonal development, guidance and possibly repair and regeneration (Brittis et al., 2002). In the mammalian system, axonal translation of RanBP1 in injured adult sciatic nerve axons has been shown to be an important component of peripheral injury-induced signaling for nerve regeneration (Yudin et al., 2008).
Hence, peripheral axons are functionally capable of regulating gene expression via translation. Below we review the evidence that the control of translation in the axonal compartment is a novel mechanism for nociceptor plasticity and a critical factor for the normal function of a subset of sensory neurons that may present unique therapeutic opportunities.
Axonal protein synthesis: a mechanism for the plasticity of peripheral nociceptors
The first evidence for translational control in axonal plasticity came from studies in Aplysia. When Aplysia axons are crushed, they show a type of plasticity called long-term facilitation wherein the axons have a lower activation threshold accompanied by a greater discharge. Crush-induced long-term facilitation of Aplysia axons is completely blocked by the general protein synthesis inhibitor anisomycin and by the specific mTOR inhibitor rapamycin (Weragoda et al., 2004). These results suggest that axonal hyperexcitability in response to injury is mediated by nascent protein synthesis downstream of mTOR activation.
FMRP and nociceptor sensitivity
Following these initial studies in Aplysia, several lines of evidence have been presented to support the concept that peripheral translation in axons contributes to nociceptive plasticity in mammals. FMRP controls translation following metabotropic glutamate receptor 1/5 (mGluR1/5) activation and, in FMRP-knockout (KO) mice, mGluR1/5-mediated plasticity is dramatically altered (Todd et al., 2003; Hou et al., 2006; Muddashetty et al., 2007). In a study from Price et al. (2007), a peripheral injection of the mGluR1/5 agonist dihydroxyphenylglycine (DHPG), in wild-type mice, caused thermal hyperalgesia. However, DHPG failed to stimulate thermal hyperalgesia in FMRP-KO mice, suggesting that peripheral translation was necessary for DHPG-induced thermal hyperalgesia. Moreover, in the same study a peripheral injection of the mTOR inhibitor rapamycin attenuated the second phase of formalin-induced pain-related behaviors in wild-type mice. The formalin test is a model of pain with a biphasic response. While the first phase is thought to result from direct activation of primary afferent sensory neurons, the second phase is believed to reflect the combined effects of afferent input and central sensitization (Coderre et al., 1990; Puig & Sorkin, 1996; McNamara et al., 2007). The attenuation of the behavior seen after peripheral rapamycin administration suggests that mTOR activity is involved in the primary afferent response to formalin (Price et al., 2007). Because subsequent examinations have shown that mTOR is present in a small subset of C-fibers and more widely expressed in the axonal compartment of A-fibers (Jimenez-Diaz et al., 2008) this may predominately reflect rapamycin-dependent changes in the plasticity of Aδ-fibers which are known to be active during the second phase of the formalin test (Puig & Sorkin, 1996). Formalin-induced responses were decreased in FMRP-KO mice and rapamycin failed to further reduce the pain behavior in these animals, again suggesting that mechanisms of peripheral translation are abrogated in the absence of FMRP. In addition to chemical-induced injury, the emergence of neuropathic pain was delayed in FMRP-KO mice. While this effect may be attributable to either peripheral or central deficits in plasticity, evidence indicating that the translational capacity of the axonal compartment is increased following a distal injury to DRG axons (Twiss et al., 2000; Willis et al., 2005) highlights the potential importance of FMRP in the response to peripheral nerve injury. Although further experimentation will be required to fully understand the role that FMRP may play in nerve injury-induced plasticity and repair, these findings represent early evidence that translational regulation via FMRP and the mTOR pathway are involved in the regulation of the sensitivity of peripheral nociceptors.
mTOR-mediated protein synthesis in cutaneous terminals of nociceptors
A second line of investigation on mTOR-controlled axonal translation from Jimenez-Diaz et al. (2008) suggests that axonal protein synthesis is key to the normal function of a subset of peripheral nociceptors. Primary afferents fall into two broad categories: myelinated A-fibers that signal noxious or innocuous stimuli and mediate ‘first’ pain perceived as rapid and sharp, and unmyelinated C-fibers that are largely nociceptors and signal ‘second’ pain, delayed, diffuse and dull. First, the authors showed that phospho-mTOR and its phosphorylated downstream targets, phospho-4E-BP, phospho-S6K and phospho-S6 protein, were found extensively in naïve, untreated, myelinated fibers in rat cutaneous tissue. In further electromyographic studies, in unstimulated rat tissue, rapamycin reduced the sensitivity of a population of A-nociceptors known to be important for the increased mechanical sensitivity that follows injury. Moreover, local administration of rapamycin in the hind paw significantly blunted the A-nociceptor-mediated mechanical hypersensitivity that develops around a site of injury and reduced the long-term mechanical hypersensitivity that follows partial peripheral nerve damage, a widely used model of chronic pain. Taken together these results suggested that constitutively active mTOR-dependent translation participates in maintaining the sensitivity of a subpopulation of fast-conducting nociceptors and therefore is likely to be important for the changes in nociceptor sensitivity after nerve injury. In other words, local protein synthesis seems to be responsible for preserving a specific pool of proteins within sensory fiber terminations in cutaneous tissue, essential for the full response of the fiber to noxious stimulation.
These findings shed new light on the control of A-nociceptor sensitivity. Previously, most A-fibers had not been thought to possess the inherent plasticity of C-fibers. Indeed, modulation of A-fiber sensitivity was assumed to be wholly contingent upon central sensitization established by activation of C-fibers. The data presented by Jimenez-Diaz et al. (2008) implies that, even in the absence of C-fiber activation, mTOR-dependent axonal translation regulates the sensitivity of some A-fibers. These data also raise the possibility that a similar process is operating at the sites of termination of sensory afferents within the dorsal horn. In most of the studied cases, the transport of molecules from the DRG is in both directions: to the periphery and to the superficial dorsal horn, including the RNA binding proteins FMRP and staufen 2 (Price et al., 2006). It therefore seems highly likely that local translation of mRNA occurs in central terminals of A-fiber nociceptors but this remains to be fully explored. In support of this hypothesis, intrathecal injections of anisomycin (Kim et al., 1998) or rapamycin (Price et al., 2007) reduced pain behavior in the second phase of the formalin test. As anisomycin and rapamycin have been shown to inhibit LTP (Tang et al., 2002; Hu et al., 2003), which is induced during the second phase of the formalin test (Ikeda et al., 2006), these effects have previously been largely attributed to a decrease in the sensitization of second-order spinal neurons. However, these effects could also be attributed, at least partly, to blunted protein synthesis in the central terminals of nociceptive axons.
Axonal translation and retrograde signaling
Axonal translation has been linked to retrograde axonal signaling. An elegant study from Hanz et al. (2003) showed how injury-induced importin β was locally synthesized in DRG axons and retrogradely transported to the nucleus, along with nuclear localization signal-bearing proteins. A recent study potentially linked retrograde signaling and sensory neuron plasticity. Therein, nerve growth factor (NGF), a well-known sensitizer of mammalian nociceptors (Bennett, 2001), was shown to stimulate axonal translation of the transcription factor cAMP response element-binding protein (CREB) in embryonic DRG neurons (Cox et al., 2008). After local axonal translation, CREB was retrogradely transported to the neuronal soma where it altered CREB-mediated transcription. Although the authors did not link their findings to nociceptor sensitization by NGF, their results provide the exciting possibility that injured axons may locally synthesized CREB and induce changes in gene expression within the soma. Moreover, CREB has previously been linked to changes in gene expression in the neuronal nucleus correlated with the long-term sensitization and regeneration of nociceptors (Herdegen et al., 1992; Fields et al., 1997; Teng & Tang, 2006). Hence, these findings may represent an important mechanism through which local translation, possibly provoked by noxious stimulation, can lead to the generation of positive retrograde signals in the periphery that induce enduring, transcription-based, changes in gene expression in the neuronal nucleus.
Translation control in injured nerves
A recent study of proteomic profiling of peripheral neuromas (which are associated with neuropathic pain) suggests that local changes in protein expression do not parallel transcriptomic analysis of injured DRG neurons (Huang et al., 2008). In this study, the authors reported localized translation of a subset of neuronal proteins in the neuroma which may contribute to neuropathic pain. In addition to changes in translation in response to injury it is also clear that mRNA transport can be controlled by environmental cues or by injury to the axon. For example, DRG neurons can regulate the axonal transport of distinct subsets of mRNAs in response to neurotrophins, including the pain-promoting neurotrophic factor NGF (Willis et al., 2007). Peripheral nerve injury also increases the axonal localization and/or translation of mRNAs for transcripts that are critical for pain plasticity. Sural nerve injury increases local axonal calcitonin gene-related peptide (CGRP) synthesis within the injured nerve suggesting that this local generation of CGRP may be important for nerve regeneration and may also play a role in promoting pain signaling (Toth et al., 2009). Finally, peripheral nerve injury causes an increase in the axonal localization of Nav1.8 mRNA, suggesting that increases in Nav1.8 expression in injured nerves are due, at least in part, to local synthesis of the sodium channel (Thakor et al., 2009). As neuropathic pain can be alleviated by knockdown (Lai et al., 2002) or pharmacological inhibition (Jarvis et al., 2007) of Nav1.8, a channel required for ectopic activity in injured nociceptors (Roza et al., 2003), this finding is particularly significant because it raises the possibility that local synthesis of Nav1.8 after nerve injury contributes strongly to ectopic activity and neuropathic pain. These reports highlight the importance of examining changes in gene expression at the site of injury to unveil new mechanisms and targets for the control of injury-induced pain at its source. These findings, along with previous studies linking FMRP to neuropathic pain (Price et al., 2007) and mTOR to nerve injury-induced hypersensitivity (Jimenez-Diaz et al., 2008), make a strong case for a role of axonal translation in nerve injury-induced pain.
Role of glia in translation in the axonal compartment
As discussed above, there is now ample evidence supporting the conclusion that adult sensory axons are endowed with functional machinery for protein biosynthesis and DRG culture studies indicate that this machinery is of neuronal origin (Zheng et al., 2001; Willis et al., 2005). However, others have hypothesized that glial cells may also contribute to the protein synthesis capacity of distal axons (Alvarez et al., 2000). Indeed, some studies in invertebrates have indicated that transfer of mRNA, and possibly ribosomes, can occur from glial cells to mature axons (Cutillo et al., 1983; Eyman et al., 2007). A similar process has recently been described for mammalian DRG axons wherein Schwann cells are capable of transferring polyribosomes to desomatized axons by invaginations (Court et al., 2008). Because these ribosomes have been identified as polyribosomes consisting of several ribosomes translating a strand of mRNA, it is likely that this mechanism also involves the transfer of mRNAs (Court et al., 2008). It is therefore possible for the control of gene expression in sensory axons to be under the influence of Schwann cells (Twiss & Fainzilber, 2009). Although the contribution of this process to injury-induced changes in nociceptor plasticity remains to be investigated, it does provide a potential explanation for the relative paucity of ribosomes in adult DRG axons despite the clear presence of RNAs, RNA binding proteins and kinases that signal to initiation factors constitutively present in the axoplasm of these neurons (Agthong et al., 2006; Price et al., 2006; Jimenez-Diaz et al., 2008).
Conclusion and future directions
The studies described above form the basis for a novel theory: local axonal protein synthesis as a modulator of peripheral nociceptor sensitivity. Such mechanisms are likely to contribute to the establishment and maintenance of chronic pain conditions. While the exploration of this novel mechanism is just beginning, the available evidence indicates that translation in the axonal compartment is critical for peripheral nociceptors’ normal sensitivity and their plasticity after injury (Price et al., 2007; Huang et al., 2008; Jimenez-Diaz et al., 2008). The mTOR pathway appears to play a key role in both these processes (Price et al., 2007; Jimenez-Diaz et al., 2008). Inhibition of mTOR activity therefore represents a novel mechanism for the modulation of nociceptor sensitivity. Sirolimus®, also known as rapamycin, is already available for use in cancer patients to reduce tumor proliferation and in transplant patients to reduce rejection, and is used as an elutant in coronary stents (Easton & Houghton, 2004). However, its immunosuppressant properties might make it unsuitable for long-term treatment of chronic pain states. A recent approach taken by pharmaceutical companies is to develop non-immunosuppressive rapamycin analogs (Cruzado, 2008). Whether these new drugs will possess the same properties as rapamycin remain to be explored.
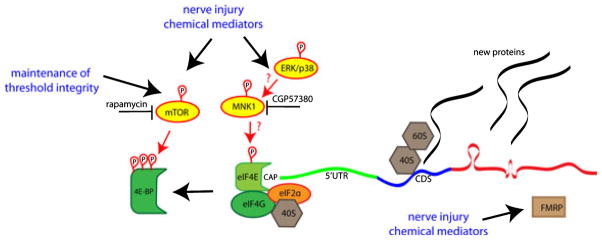
Identified and potential key players involved in the regulation of translation in the axonal compartment and nociceptor plasticity are shown in Fig. 3.
Fig. 3.
Identified and potential key players involved in the regulation of translation in the axonal compartment and nociceptor plasticity. The available evidence suggests that mTOR activity is critical for maintaining the sensitivity of a subset of nociceptive neurons and that mTOR is also an important mediator of chemically-mediated nociceptor plasticity. Rapamycin, a specific inhibitor of mTOR activity, is a powerful tool for regulating protein synthesis of TOP mRNAs. Furthermore, findings from studies in FMRP-KO mice suggest that FMRP is crucial for mediating activity-dependent synthesis of new proteins that promote the hypersensitivity of nociceptors in response to mGluR1/5 agonists, chemically mediated pain (formalin) and perhaps after nerve injury. Although ERK and/or p38 activity have been positively linked to plasticity after inflammation or nerve injury, its role in regulating translation in the axonal compartment has not yet been explored. However, the availability of a specific MNK1 inhibitor, CGP57380, has been shown to reduce the interaction between eIF4E and eIF4G, making the ERK–p38–MNK1 pathway an attractive target to explore. We propose that gaining a better understanding of how algogens and/or nerve injury modulate the expression of new proteins via these signaling pathways will lead to the discovery of new targets for the control of chronic pain directly at its source either via inhibition of these pathways (e.g. mTOR or ERK) or identification of proteins locally synthesized which may be targets for therapeutic intervention.
Several studies have linked increased ERK and/or p38 activity in sensory neurons to pain plasticity (Ji et al., 2002; Obata et al., 2004; Agthong et al., 2006; Hensellek et al., 2007). However, these studies have not assessed the possible involvement of ERK signaling through mTOR or ERK and/or p38 signaling to MNK1/eIF4E to this effect. As ERK plays a critical role in controlling translation in dendrites during the induction of neuronal plasticity in the CNS (Kelleher et al., 2004; Gelinas et al., 2007; Tsokas et al., 2007; Gobert et al., 2008) it is also possible that similar effects would occur in the axonal compartment, contributing to the enhanced sensitivity of sensory neurons. As our knowledge of the mechanisms of activation and the role of mTOR, ERK and p38 in the control of nociceptor sensitivity continues to progress, it is highly likely that therapeutic opportunities for the treatment of pain will arise.
Regulation of gene expression in the axonal compartment implies that mRNAs are trafficked to distal sites and proteins are synthesized there, offering technical opportunities to determine their identity. Identifying them and assessing their potential role in nociceptor function will be key to the discovery of new therapeutic treatments. Directly and locally modulating their expression pharmacologically (e.g. with mTOR inhibitors) or with RNAi (Murashov et al., 2007) offers a new approach for the control of increased sensitivity found in many chronic pain states. mRNAs that are associated with RNA binding proteins in the axons of sensory neurons are the first obvious candidates. As FMRP has been linked with mechanisms of nociceptor plasticity (Price et al., 2006, 2007), identification of such mRNAs may be facilitated by determining which transcripts are substrates for FMRP in sensory neurons.
Understanding the molecular events that lead to changes in nociceptor sensitivity holds great promise for the advancement of understanding and treatment of chronic pain conditions in humans. We propose that the control of translation in the axonal compartment of nociceptors represents a novel mechanism for modulating gene expression in these neurons. Moreover, we suggest that gaining a better understanding of how this process occurs and identifying proteins that are translated, on demand, in the axonal compartment will lead to the elucidation of new therapeutic targets. Hence, the control of gene expression in the axonal compartment represents a powerful new paradigm not only for understanding nociceptor function and plasticity but also for the discovery of novel therapeutics.
Acknowledgments
T.J.P. is supported by grants from The American Pain Society, The University of Arizona Foundation and the Rita Allen Foundation and by startup funds from The University of Arizona School of Medicine. S.G. is supported by the Wellcome Trust (grant numbers 065374 and 080506). The authors wish to thank Steve Hunt, Frank Porreca and Todd Vanderah for helpful comments and discussion during the preparation of this manuscript.
Abbreviations
- 4E-BP
eIF4E binding protein
- BDNF
brain-derived neurotrophic factor
- CGRP
calcitonin gene-related peptide
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- DRG
dorsal root ganglion
- eIF
eukaryotic initiation factor
- ERK
extracellular regulated kinase
- FMRP
fragile X mental retardation protein
- GCN2
general control nonderepressible 2
- KO
knockout
- LTD
long-term depression
- LTP
long-term potentiation
- MEK
mitogen-activated protein kinase kinase
- mGluR
metabotropic glutamate receptor
- MNK1
mitogen-activated kinase-interacting kinase 1
- mTOR
mamallian target of rapamycin
- NGF
nerve growth factor
- PI3K
phosphoinositol 3 kinase
- TOP
terminal oligopyrimidine tract
- UTR
untranslated region
References
- Adams JP, Anderson AE, Varga AW, Dineley KT, Cook RG, Pfaffinger PJ, Sweatt JD. The A-type potassium channel Kv4.2 is a substrate for the mitogen-activated protein kinase ERK. J Neurochem. 2000;75:2277–2287. doi: 10.1046/j.1471-4159.2000.0752277.x. [DOI] [PubMed] [Google Scholar]
- Agthong S, Kaewsema A, Tanomsridejchai N, Chentanez V. Activation of MAPKERK in peripheral nerve after injury. BMC Neurosci. 2006;7:45. doi: 10.1186/1471-2202-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- Alvarez J. The autonomous axon: a model based on local synthesis of proteins. Biol Res. 2001;34:103–109. doi: 10.4067/s0716-97602001000200014. [DOI] [PubMed] [Google Scholar]
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. doi: 10.1016/s0301-0082(99)00062-3. [DOI] [PubMed] [Google Scholar]
- An JJ, Gharami K, Liao GY, Woo NH, Lau AG, Vanevski F, Torre ER, Jones KR, Feng Y, Lu B, Xu B. Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell. 2008;134:175–187. doi: 10.1016/j.cell.2008.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Mechanical and heat sensitization of cutaneous nociceptors after peripheral inflammation in the rat. J Neurophysiol. 1999a;82:2649–2656. doi: 10.1152/jn.1999.82.5.2649. [DOI] [PubMed] [Google Scholar]
- Andrew D, Greenspan JD. Modality-specific hyper-responsivity of regenerated cat cutaneous nociceptors. J Physiol. 1999b;516 (Pt 3):897–906. doi: 10.1111/j.1469-7793.1999.0897u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf SI, McLoon AL, Sclarsic SM, Kunes S. Synaptic protein synthesis associated with memory is regulated by the RISC pathway in Drosophila. Cell. 2006;124:191–205. doi: 10.1016/j.cell.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbee SA, Estes PS, Cziko AM, Hillebrand J, Luedeman RA, Coller JM, Johnson N, Howlett IC, Geng C, Ueda R, Brand AH, Newbury SF, Wilhelm JE, Levine RB, Nakamura A, Parker R, Ramaswami M. Staufen- and FMRP-containing neuronal RNPs are structurally and functionally related to somatic P bodies. Neuron. 2006;52:997–1009. doi: 10.1016/j.neuron.2006.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST. Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron. 2008;60:201–214. doi: 10.1016/j.neuron.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DL. Neurotrophic factors: important regulators of nociceptive function. Neuroscientist. 2001;7:13–17. doi: 10.1177/107385840100700105. [DOI] [PubMed] [Google Scholar]
- Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–789. doi: 10.1038/nrn2150. [DOI] [PubMed] [Google Scholar]
- Brittis PA, Lu Q, Flanagan JG. Axonal protein synthesis provides a mechanism for localized regulation at an intermediate target. Cell. 2002;110:223–235. doi: 10.1016/s0092-8674(02)00813-9. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Vaccarino AL, Melzack R. Central nervous system plasticity in the tonic pain response to subcutaneous formalin injection. Brain Res. 1990;535:155–158. doi: 10.1016/0006-8993(90)91835-5. [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Harding H, Herdy B, Azzi M, Bruno M, Bidinosti M, Ben Mamou C, Marcinkiewicz E, Yoshida M, Imataka H, Cuello AC, Seidah N, Sossin W, Lacaille JC, Ron D, Nader K, Sonenberg N. Translational control of hippocampal synaptic plasticity and memory by the eIF2alpha kinase GCN2. Nature. 2005;436:1166–1173. doi: 10.1038/nature03897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjevic K, Lacaille JC, Nader K, Sonenberg N. eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court FA, Hendriks WT, Macgillavry HD, Alvarez J, van Minnen J. Schwann cell to axon transfer of ribosomes: toward a novel understanding of the role of glia in the nervous system. J Neurosci. 2008;28:11024–11029. doi: 10.1523/JNEUROSCI.2429-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LJ, Hengst U, Gurskaya NG, Lukyanov KA, Jaffrey SR. Intra-axonal translation and retrograde trafficking of CREB promotes neuronal survival. Nat Cell Biol. 2008;10:149–159. doi: 10.1038/ncb1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruzado JM. Nonimmunosuppressive effects of mammalian target of rapamycin inhibitors. Transplant Rev (Orlando) 2008;22:73–81. doi: 10.1016/j.trre.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Cutillo V, Montagnese P, Gremo F, Casola L, Giuditta A. Origin of axoplasmic RNA in the squid giant fiber. Neurochem Res. 1983;8:1621–1634. doi: 10.1007/BF00964163. [DOI] [PubMed] [Google Scholar]
- Dever TE. Translation initiation: adept at adapting. Trends Biochem Sci. 1999;24:398–403. doi: 10.1016/s0968-0004(99)01457-7. [DOI] [PubMed] [Google Scholar]
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006;7:S3–S12. doi: 10.1016/j.jpain.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Didiot MC, Subramanian M, Flatter E, Mandel JL, Moine H. Cells lacking the fragile X mental retardation protein (FMRP) have normal RISC activity but exhibit altered stress granule assembly. Mol Biol Cell. 2009;20:428–437. doi: 10.1091/mbc.E08-07-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Han S, Zylka MJ, Simon MI, Anderson DJ. A diverse family of GPCRs expressed in specific subsets of nociceptive sensory neurons. Cell. 2001;106:619–632. doi: 10.1016/s0092-8674(01)00483-4. [DOI] [PubMed] [Google Scholar]
- Easton JB, Houghton PJ. Therapeutic potential of target of rapamycin inhibitors. Expert Opin Ther Targets. 2004;8:551–564. doi: 10.1517/14728222.8.6.551. [DOI] [PubMed] [Google Scholar]
- Estes PS, O’Shea M, Clasen S, Zarnescu DC. Fragile X protein controls the efficacy of mRNA transport in Drosophila neurons. Mol Cell Neurosci. 2008;39:170–179. doi: 10.1016/j.mcn.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Eyman M, Cefaliello C, Ferrara E, De Stefano R, Lavina ZS, Crispino M, Squillace A, van Minnen J, Kaplan BB, Giuditta A. Local synthesis of axonal and presynaptic RNA in squid model systems. Eur J Neurosci. 2007;25:341–350. doi: 10.1111/j.1460-9568.2007.05304.x. [DOI] [PubMed] [Google Scholar]
- Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipowicz W, Jaskiewicz L, Kolb FA, Pillai RS. Post-transcriptional gene silencing by siRNAs and miRNAs. Curr Opin Struct Biol. 2005;15:331–341. doi: 10.1016/j.sbi.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta -adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent LTP. J Biol Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Gingras AC, Gygi SP, Raught B, Polakiewicz RD, Abraham RT, Hoekstra MF, Aebersold R, Sonenberg N. Regulation of 4E-BP1 phosphorylation: a novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Gygi SP, Niedzwiecka A, Miron M, Burley SK, Polakiewicz RD, Wyslouch-Cieszynska A, Aebersold R, Sonenberg N. Hierarchical phosphorylation of the translation inhibitor 4E-BP1. Genes Dev. 2001;15:2852–2864. doi: 10.1101/gad.912401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N. mTOR signaling to translation. Curr Top Microbiol Immunol. 2004;279:169–197. doi: 10.1007/978-3-642-18930-2_11. [DOI] [PubMed] [Google Scholar]
- Giuditta A, Dettbarn WD, Brzin M. Protein synthesis in the isolated giant axon of the squid. Proc Natl Acad Sci U S A. 1968;59:1284–1287. doi: 10.1073/pnas.59.4.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobert D, Topolnik L, Azzi M, Huang L, Badeaux F, Desgroseillers L, Sossin WS, Lacaille JC. Forskolin induction of late-LTP and up-regulation of 5′ TOP mRNAs translation via mTOR, ERK, and PI3K in hippocampal pyramidal cells. J Neurochem. 2008;106:1160–1174. doi: 10.1111/j.1471-4159.2008.05470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton TL, Stoneley M, Spriggs KA, Bushell M. TOPs and their regulation. Biochem Soc Trans. 2006;34:12–16. doi: 10.1042/BST20060012. [DOI] [PubMed] [Google Scholar]
- Hanus C, Ehlers MD. Secretory Outposts for the Local Processing of Membrane Cargo in Neuronal Dendrites. Traffic. 2008;9:1437–1445. doi: 10.1111/j.1600-0854.2008.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanz S, Perlson E, Willis D, Zheng JQ, Massarwa R, Huerta JJ, Koltzenburg M, Kohler M, van-Minnen J, Twiss JL, Fainzilber M. Axoplasmic importins enable retrograde injury signaling in lesioned nerve. Neuron. 2003;40:1095–1104. doi: 10.1016/s0896-6273(03)00770-0. [DOI] [PubMed] [Google Scholar]
- Hengst U, Cox LJ, Macosko EZ, Jaffrey SR. Functional and selective RNA interference in developing axons and growth cones. J Neurosci. 2006;26:5727–5732. doi: 10.1523/JNEUROSCI.5229-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNFalpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36:381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Fiallos-Estrada CE, Schmid W, Bravo R, Zimmermann M. The transcription factors c-JUN, JUN D and CREB, but not FOS and KROX-24, are differentially regulated in axotomized neurons following transection of rat sciatic nerve. Brain Res Mol Brain Res. 1992;14:155–165. doi: 10.1016/0169-328x(92)90170-g. [DOI] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Dual modes of endoplasmic reticulum-to-Golgi transport in dendrites revealed by live-cell imaging. J Neurosci. 2003;23:6188–6199. doi: 10.1523/JNEUROSCI.23-15-06188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AC, Ehlers MD. Secretory trafficking in neuronal dendrites. Nat Cell Biol. 2004;6:585–591. doi: 10.1038/ncb0704-585. [DOI] [PubMed] [Google Scholar]
- Hou L, Antion MD, Hu D, Spencer CM, Paylor R, Klann E. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51:441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Hu NW, Zhang HM, Hu XD, Li MT, Zhang T, Zhou LJ, Liu XG. Protein synthesis inhibition blocks the late-phase LTP of C-fiber evoked field potentials in rat spinal dorsal horn. J Neurophysiol. 2003;89:2354–2359. doi: 10.1152/jn.01027.2002. [DOI] [PubMed] [Google Scholar]
- Hu HJ, Carrasquillo Y, Karim F, Jung WE, Nerbonne JM, Schwarz TL, Gereau RWT. The kv4.2 potassium channel subunit is required for pain plasticity. Neuron. 2006;50:89–100. doi: 10.1016/j.neuron.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Huang HL, Cendan CM, Roza C, Okuse K, Cramer R, Timms JF, Wood JN. Proteomic profiling of neuromas reveals alterations in protein composition and local protein synthesis in hyper-excitable nerves. Mol Pain. 2008;4:33. doi: 10.1186/1744-8069-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Mantyh PW. The molecular dynamics of pain control. Nat Rev Neurosci. 2001;2:83–91. doi: 10.1038/35053509. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Stark J, Fischer H, Wagner M, Drdla R, Jager T, Sandkuhler J. Synaptic amplifier of inflammatory pain in the spinal dorsal horn. Science. 2006;312:1659–1662. doi: 10.1126/science.1127233. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Lawson JJ, McIlwrath SL, Rau KK, Anderson CE, Albers KM, Koerber HR. Sensitization of cutaneous nociceptors after nerve transection and regeneration: possible role of target-derived neurotrophic factor signaling. J Neurosci. 2009;29:1636–1647. doi: 10.1523/JNEUROSCI.3474-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, Chapman M, Joshi S, Zhang XF, Kort M, Carroll W, Marron B, Atkinson R, Thomas J, Liu D, Krambis M, Liu Y, McGaraughty S, Chu K, Roeloffs R, Zhong C, Mikusa JP, Hernandez G, Gauvin D, Wade C, Zhu C, Pai M, Scanio M, Shi L, Drizin I, Gregg R, Matulenko M, Hakeem A, Gross M, Johnson M, Marsh K, Wagoner PK, Sullivan JP, Faltynek CR, Krafte DS. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci U S A. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaworski J, Sheng M. The growing role of mTOR in neuronal development and plasticity. Mol Neurobiol. 2006;34:205–219. doi: 10.1385/MN:34:3:205. [DOI] [PubMed] [Google Scholar]
- Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- Jimenez-Diaz L, Geranton SM, Passmore GM, Leith JL, Fisher AS, Berliocchi L, Sivasubramaniam AK, Sheasby A, Lumb BM, Hunt SP. Local translation in primary afferent fibers regulates nociception. PLoS ONE. 2008;3:e1961. doi: 10.1371/journal.pone.0001961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha N, Anderson P. Mammalian stress granules and processing bodies. Methods Enzymol. 2007;431:61–81. doi: 10.1016/S0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, III, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Kennedy MJ, Ehlers MD. Organelles and trafficking machinery for postsynaptic plasticity. Annu Rev Neurosci. 2006;29:325–362. doi: 10.1146/annurev.neuro.29.051605.112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Thomas KS, Calejesan AA, Zhuo M. Macromolecular synthesis contributes to nociceptive response to subcutaneous formalin injection in mice. Neuropharmacology. 1998;37:1091–1093. doi: 10.1016/s0028-3908(98)00099-9. [DOI] [PubMed] [Google Scholar]
- Koenig E. Ribosomal RNA in Mauthner axon: implications for a protein synthesizing machinery in the myelinated axon. Brain Res. 1979;174:95–107. doi: 10.1016/0006-8993(79)90806-0. [DOI] [PubMed] [Google Scholar]
- Koenig E. Evaluation of local synthesis of axonal proteins in the goldfish Mauthner cell axon and axons of dorsal and ventral roots of the rat in vitro. Mol Cell Neurosci. 1991;2:384. doi: 10.1016/1044-7431(91)90025-j. [DOI] [PubMed] [Google Scholar]
- Koenig E, Giuditta A. Protein-synthesizing machinery in the axon compartment. Neuroscience. 1999;89:5–15. doi: 10.1016/s0306-4522(98)00282-6. [DOI] [PubMed] [Google Scholar]
- Koenig E, Martin R, Titmus M, Sotelo-Silveira JR. Cryptic peripheral ribosomal domains distributed intermittently along mammalian myelinated axons. J Neurosci. 2000;20:8390–8400. doi: 10.1523/JNEUROSCI.20-22-08390.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Gold MS, Kim CS, Bian D, Ossipov MH, Hunter JC, Porreca F. Inhibition of neuropathic pain by decreased expression of the tetrodotoxin-resistant sodium channel, NaV1.8. Pain. 2002;95:143–152. doi: 10.1016/s0304-3959(01)00391-8. [DOI] [PubMed] [Google Scholar]
- Lee YS, Bailey CH, Kandel ER, Kaang BK. Transcriptional regulation of long-term memory in the marine snail Aplysia. Mol Brain. 2008;1:3. doi: 10.1186/1756-6606-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo PM, Grazzini E, Groblewski T, O’Donnell D, Roy MO, Zhang J, Hoffert C, Cao J, Schmidt R, Pelletier M, Labarre M, Gosselin M, Fortin Y, Banville D, Shen SH, Strom P, Payza K, Dray A, Walker P, Ahmad S. Proenkephalin A gene products activate a new family of sensory neuron–specific GPCRs. Nat Neurosci. 2002;5:201–209. doi: 10.1038/nn815. [DOI] [PubMed] [Google Scholar]
- Leung AK, Sharp PA. Function and localization of microRNAs in mammalian cells. Cold Spring Harb Symp Quant Biol. 2006;71:29–38. doi: 10.1101/sqb.2006.71.049. [DOI] [PubMed] [Google Scholar]
- Lin AC, Holt CE. Function and regulation of local axonal translation. Curr Opin Neurobiol. 2008;18:60–68. doi: 10.1016/j.conb.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder B, Plottner O, Kroiss M, Hartmann E, Laggerbauer B, Meister G, Keidel E, Fischer U. Tdrd3 is a novel stress granule-associated protein interacting with the Fragile-X syndrome protein FMRP. Hum Mol Genet. 2008;17:3236–3246. doi: 10.1093/hmg/ddn219. [DOI] [PubMed] [Google Scholar]
- Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature. 2004;431:364–370. doi: 10.1038/nature02875. [DOI] [PubMed] [Google Scholar]
- Martin KC. Local protein synthesis during axon guidance and synaptic plasticity. Curr Opin Neurobiol. 2004;14:305–310. doi: 10.1016/j.conb.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Martin KC, Zukin RS. RNA trafficking and local protein synthesis in dendrites: an overview. J Neurosci. 2006;26:7131–7134. doi: 10.1523/JNEUROSCI.1801-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazroui R, Huot ME, Tremblay S, Filion C, Labelle Y, Khandjian EW. Trapping of messenger RNA by Fragile X Mental Retardation protein into cytoplasmic granules induces translation repression. Hum Mol Genet. 2002;11:3007–3017. doi: 10.1093/hmg/11.24.3007. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merianda TT, Lin AC, Lam JS, Vuppalanchi D, Willis DE, Karin N, Holt CE, Twiss JL. A functional equivalent of endoplasmic reticulum and Golgi in axons for secretion of locally synthesized proteins. Mol Cell Neurosci. 2009;40:128–142. doi: 10.1016/j.mcn.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O. Synthesis of the translational apparatus is regulated at the translational level. Eur J Biochem. 2000;267:6321–6330. doi: 10.1046/j.1432-1327.2000.01719.x. [DOI] [PubMed] [Google Scholar]
- Morley SJ, Curtis PS, Pain VM. eIF4G: translation’s mystery factor begins to yield its secrets. RNA. 1997;3:1085–1104. [PMC free article] [PubMed] [Google Scholar]
- Muddashetty RS, Kelic S, Gross C, Xu M, Bassell GJ. Dysregulated metabotropic glutamate receptor-dependent translation of AMPA receptor and postsynaptic density-95 mRNAs at synapses in a mouse model of fragile X syndrome. J Neurosci. 2007;27:5338–5348. doi: 10.1523/JNEUROSCI.0937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashov AK, Chintalgattu V, Islamov RR, Lever TE, Pak ES, Sierpinski PL, Katwa LC, Van Scott MR. RNAi pathway is functional in peripheral nerve axons. FASEB J. 2007;21:656–670. doi: 10.1096/fj.06-6155com. [DOI] [PubMed] [Google Scholar]
- Napoli I, Mercaldo V, Boyl PP, Eleuteri B, Zalfa F, De Rubeis S, Di Marino D, Mohr E, Massimi M, Falconi M, Witke W, Costa-Mattioli M, Sonenberg N, Achsel T, Bagni C. The fragile X syndrome protein represses activity-dependent translation through CYFIP1, a new 4E-BP. Cell. 2008;134:1042–1054. doi: 10.1016/j.cell.2008.07.031. [DOI] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Pallas DC, Ceman S, Bassell GJ, Warren ST. FMRP phosphorylation reveals an immediate-early signaling pathway triggered by group I mGluR and mediated by PP2A. J Neurosci. 2007;27:14349–14357. doi: 10.1523/JNEUROSCI.2969-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem. 2008;283:18478–18482. doi: 10.1074/jbc.C800055200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA. Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J Neurosci Res. 1994;37:596–603. doi: 10.1002/jnr.490370506. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Dai Y, Mizushima T, Fukuoka T, Tokunaga A, Noguchi K. Differential activation of MAPK in injured and uninjured DRG neurons following chronic constriction injury of the sciatic nerve in rats. Eur J Neurosci. 2004;20:2881–2895. doi: 10.1111/j.1460-9568.2004.03754.x. [DOI] [PubMed] [Google Scholar]
- Parker R, Sheth U. P bodies and the control of mRNA translation and degradation. Mol Cell. 2007;25:635–646. doi: 10.1016/j.molcel.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Patursky-Polischuk I, Stolovich-Rain M, Hausner-Hanochi M, Kasir J, Cybulski N, Avruch J, Ruegg MA, Hall MN, Meyuhas O. The TSC-mTOR pathway mediates translational activation of TOP mRNAs by insulin largely in a raptor- or rictor-independent manner. Mol Cell Biol. 2009;29:640–649. doi: 10.1128/MCB.00980-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper M, Holt C. RNA translation in axons. Annu Rev Cell Dev Biol. 2004;20:505–523. doi: 10.1146/annurev.cellbio.20.010403.111746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porreca F, Ossipov MH, Gebhart GF. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Price TJ, Flores CM, Cervero F, Hargreaves KM. The RNA binding and transport proteins staufen and fragile X mental retardation protein are expressed by rat primary afferent neurons and localize to peripheral and central axons. Neuroscience. 2006;141:2107–2116. doi: 10.1016/j.neuroscience.2006.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Rashid MH, Millecamps M, Sanoja R, Entrena JM, Cervero F. Decreased nociceptive sensitization in mice lacking the fragile X mental retardation protein: role of mGluR1/5 and mTOR. J Neurosci. 2007;27:13958–13967. doi: 10.1523/JNEUROSCI.4383-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2008;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Pyronnet S, Imataka H, Gingras AC, Fukunaga R, Hunter T, Sonenberg N. Human eukaryotic translation initiation factor 4G (eIF4G) recruits mnk1 to phosphorylate eIF4E. EMBO J. 1999;18:270–279. doi: 10.1093/emboj/18.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Gingras AC, Sonenberg N. The target of rapamycin (TOR) proteins. Proc Natl Acad Sci U S A. 2001;98:7037–7044. doi: 10.1073/pnas.121145898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roza C, Laird JM, Souslova V, Wood JN, Cervero F. The tetrodotoxin-resistant Na+ channel Nav1.8 is essential for the expression of spontaneous activity in damaged sensory axons of mice. J Physiol. 2003;550:921–926. doi: 10.1113/jphysiol.2003.046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruvinsky I, Meyuhas O. Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci. 2006;31:342–348. doi: 10.1016/j.tibs.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Sandkuhler J. Understanding LTP in pain pathways. Mol Pain. 2007;3:9. doi: 10.1186/1744-8069-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269:5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Schuman EM, Dynes JL, Steward O. Synaptic regulation of translation of dendritic mRNAs. J Neurosci. 2006;26:7143–7146. doi: 10.1523/JNEUROSCI.1796-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SJ, Reis G, Kang H, Gingras AC, Sonenberg N, Schuman EM. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc Natl Acad Sci U S A. 2002;99:467–472. doi: 10.1073/pnas.012605299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng FY, Tang BL. Axonal regeneration in adult CNS neurons–signaling molecules and pathways. J Neurochem. 2006;96:1501–1508. doi: 10.1111/j.1471-4159.2006.03663.x. [DOI] [PubMed] [Google Scholar]
- Thakor DK, Lin A, Matsuka Y, Meyer EM, Ruangsri S, Nishimura I, Spigelman I. Increased peripheral nerve excitability and local NaV1.8 mRNA up-regulation in painful neuropathy. Mol Pain. 2009;5:14. doi: 10.1186/1744-8069-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd PK, Mack KJ, Malter JS. The fragile X mental retardation protein is required for type-I metabotropic glutamate receptor-dependent translation of PSD-95. Proc Natl Acad Sci U S A. 2003;100:14374–14378. doi: 10.1073/pnas.2336265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth CC, Willis D, Twiss JL, Walsh S, Martinez JA, Liu WQ, Midha R, Zochodne DW. Locally synthesized calcitonin gene-related Peptide has a critical role in peripheral nerve regeneration. J Neuropathol Exp Neurol. 2009;68:326–337. doi: 10.1097/NEN.0b013e31819ac71b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twiss JL, Fainzilber M. Ribosomes in axons - scrounging from the neighbors? Trends Cell Biol. 2009;19:236–243. doi: 10.1016/j.tcb.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Twiss JL, Smith DS, Chang B, Shooter EM. Translational control of ribosomal protein L4 mRNA is required for rapid neurite regeneration. Neurobiol Dis. 2000;7:416–428. doi: 10.1006/nbdi.2000.0293. [DOI] [PubMed] [Google Scholar]
- Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weragoda RM, Ferrer E, Walters ET. Memory-like alterations in Aplysia axons after nerve injury or localized depolarization. J Neurosci. 2004;24:10393–10401. doi: 10.1523/JNEUROSCI.2329-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, Twiss JL. The evolving roles of axonally synthesized proteins in regeneration. Curr Opin Neurobiol. 2006;16:111–118. doi: 10.1016/j.conb.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Willis D, Li KW, Zheng JQ, Chang JH, Smit A, Kelly T, Merianda TT, Sylvester J, van Minnen J, Twiss JL. Differential transport and local translation of cytoskeletal, injury-response, and neurodegeneration protein mRNAs in axons. J Neurosci. 2005;25:778–791. doi: 10.1523/JNEUROSCI.4235-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis DE, van Niekerk EA, Sasaki Y, Mesngon M, Merianda TT, Williams GG, Kendall M, Smith DS, Bassell GJ, Twiss JL. Extracellular stimuli specifically regulate localized levels of individual neuronal mRNAs. J Cell Biol. 2007;178:965–980. doi: 10.1083/jcb.200703209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ. Phenotypic modification of primary sensory neurons: the role of nerve growth factor in the production of persistent pain. Philos Trans R Soc Lond B Biol Sci. 1996;351:441–448. doi: 10.1098/rstb.1996.0040. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Ma Q. Nociceptors – noxious stimulus detectors. Neuron. 2007;55:353–364. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–1769. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Wu KY, Hengst U, Cox LJ, Macosko EZ, Jeromin A, Urquhart ER, Jaffrey SR. Local translation of RhoA regulates growth cone collapse. Nature. 2005;436:1020. doi: 10.1038/nature03885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudin D, Hanz S, Yoo S, Iavnilovitch E, Willis D, Gradus T, Vuppalanchi D, Segal-Ruder Y, Ben-Yaakov K, Hieda M, Yoneda Y, Twiss JL, Fainzilber M. Localized regulation of axonal RanGTPase controls retrograde injury signaling in peripheral nerve. Neuron. 2008;59:241–252. doi: 10.1016/j.neuron.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li Y, Yang DQ. Phosphorylation of eIF-4E positively regulates formation of the eIF-4F translation initiation complex following DNA damage. Biochem Biophys Res Commun. 2008;367:54–59. doi: 10.1016/j.bbrc.2007.12.118. [DOI] [PubMed] [Google Scholar]
- Zheng JQ, Kelly TK, Chang B, Ryazantsev S, Rajasekaran AK, Martin KC, Twiss JL. A functional role for intra-axonal protein synthesis during axonal regeneration from adult sensory neurons. J Neurosci. 2001;21:9291–9303. doi: 10.1523/JNEUROSCI.21-23-09291.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]