Abstract
The improved efficacy of cutaneous vaccination at lower doses can address the poor immunogenicity of intramuscular DNA vaccines. However, a simple and inexpensive cutaneous vaccination method is lacking. This study assessed use of micron-scale needles coated with DNA as a simple, inexpensive device for delivery targeted to skin. Delivery of 8 μg plasmid encoding hepatitis C virus NS3/4A protein using microneedles induced in vitro functional NS3/4A-specific cytotoxic T lymphocytes (CTLs) comparable to 4 μg DNA delivered using complex gene gun technology, and the in vivo CTL response from 3.2 μg DNA was comparable to a 100 μg intramuscular dose.
Keywords: cellular immune response, DNA-coated microneedle, DNA vaccine, hepatitis C virus, microfabricated needle
Introduction
DNA vaccines are simple to produce and can generate strong cellular and humoral immune responses, making them attractive vaccine candidates 1. However, a major shortcoming of DNA vaccines is their poor immunogenicity when administered intramuscularly, characteristically requiring at least 5-10 mg of DNA to generate a robust immune response in humans 2. Cutaneous immunization using gene gun or electroporation has improved potency by approximately three orders of magnitude in humans and non-human primates 2. However, gene gun and electroporation require elaborate vaccination protocols and equipment 3. Therefore, there is a need for a convenient and low-cost cutaneous DNA vaccine delivery method that can generate robust cellular responses.
Recently, microneedles have been developed by adapting technology of microelectronics industry to fabricate micron-scale needles that painlessly deliver compounds into skin using methods suitable for inexpensive mass production 4. In one approach, solid microneedles have been coated with drugs or vaccines as a solid film on the microneedle surface. After insertion into skin, cutaneous interstitial fluid rapidly dissolves the film and releases drug or vaccine into skin. Using this approach, humoral responses have been shown in mice using ovalbumin as a model antigen 5 and in nonhuman primates in combination with electroporation, using a smallpox DNA vaccine 6.
Using a related approach, humoral and cellular responses have been demonstrated by scraping blunt-tipped microneedles on the skin after topical application of a solution of plasmid DNA vaccine in mice 7. Using a different approach, hollow microneedles have been used to inject antigens into skin of animals and humans to generate humoral responses to a number of antigens 4,8.
Among the various approaches, coated microneedles are especially attractive as a method for rapid administration of vaccines with little or no training because microneedles are pre-coated with vaccines that can be dissolved in skin within seconds and can be prepared as adhesive patch-like devices for self application 9. Because coated microneedles have only been investigated previously for humoral responses, this study tested the hypothesis that DNA-coated microneedles can elicit a robust cellular immune response.
We tested this hypothesis using a well characterized DNA vaccine encoding hepatitis C virus non-structural (NS) 3/4A protein that has previously been shown to induce strong in vivo functional T cell responses in mice when delivered by gene gun or intramuscular injection followed by in vivo electroporation 10,11. In this study we compared cutaneous DNA delivery using microneedles to (i) intramuscular DNA delivery by hypodermic injection, which although is widely used in animal studies but is generally ineffective in humans, and to cutaneous delivery using gene gun, which can be effective in humans 12.
Results and discussion
Plasmid-coated microneedles
Rows of microneedles were designed to enable consistent penetration into the highly pliant skin of mice. Each microneedle row contained five microneedles measuring 700 μm in length and 160 μm in width at the base, which tapered to a sharp tip with less than 1 μm radius of curvature (Figure 1A). A previously developed dip-coating method was used to achieve uniform plasmid films on microneedles 9. The coating formulation and process are described in legend of Figure 1. Because of difficulty in visualizing plasmid coatings microneedles were independently coated with vitamin B2 as a model, colored compound to visually assess coating uniformity. After coating with vitamin B2, all five microneedles of the row were found to be uniformly coated (Figure 1B). Subsequently, microneedles were coated with DNA and each microneedle row was found to be coated with 1.6 ± 0.2 μg of DNA.
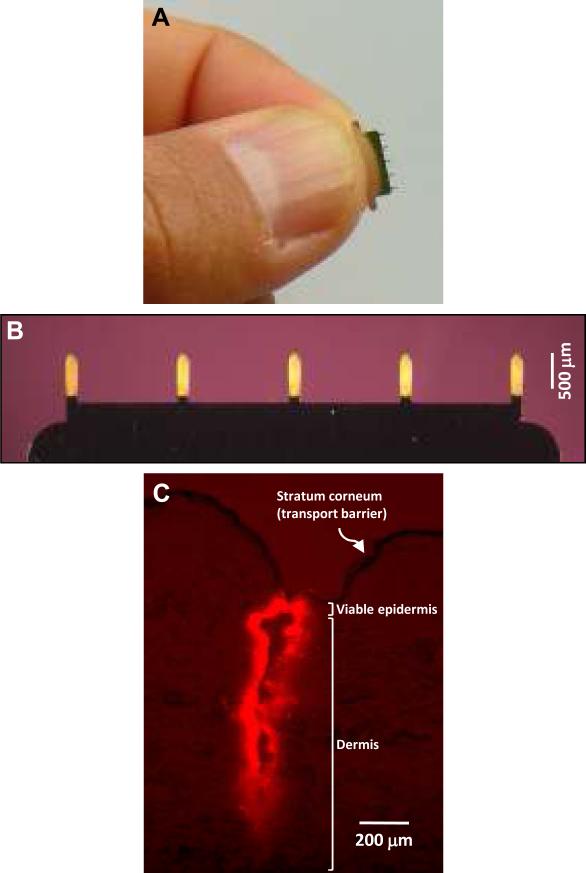
Figure 1. Coated microneedles.
Microneedles were cut from stainless steel sheets using an infrared laser and electropolished as described before 9. (A) Photograph of a representative microneedle row held in a human hand. (B) Microneedle row with five microneedles uniformly coated with vitamin B2 as a model compound. Because plasmid coatings were difficult to visualize, uniformity of coatings was assessed by coating microneedles with a model colored compound, vitamin B2 (riboflavin-5'-phosphate sodium salt dihydrate) (Fisher Scientific, Fair Lawn, NJ, USA). The aqueous dip-coating solution contained 1% (w/v) carboxymethylcellulose sodium salt (CMC, low viscosity, USP grade, CarboMer, San Diego, CA, USA), 0.5% (w/v) Lutrol F-68 NF (BASF, Mt. Olive, NJ, USA) and 20 mg/ml vitamin B2 18. Microneedles were coated using a custom dip-coating device and air-dried for >24h 9. For immunization, microneedles were coated using the same formulation, but by replacing vitamin B2 with 5 mg/ml codon optimized NS3/4A plasmid DNA. To determine the amount of DNA coated on microneedles, DNA concentration was measured by a validated technique using UV absorbance at 260 nm in a solution prepared by vortexing coated microneedles for 1 min in 1ml deionized water. (C) Histological section of porcine cadaver skin after inserting sulforhodamine-coated microneedle. To histologically characterize insertion of microneedles into skin, microneedles were coated with 0.1% (w/v) sulforhodamine (Molecular Probes, Eugene, OR, USA) and inserted into porcine cadaver skin for 1 min.
Microneedles were designed with a length of 700 μm for intracutaneous delivery to human skin, which has a representative thickness of 2-3 mm. Histological evaluation of skin after piercing with sulforhodamine-coated microneedles demonstrates that the microneedles deposited the coating in pig skin, which is a good model of human skin anatomy (Figure 1C). Although mouse skin is approximately three times thinner, microneedles are expected to remain equally within the skin of mice.
This result is consistent with previous work in which microneedles were uniformly coated with luciferase plasmid DNA that was fluorescently labeled with YOYO-1. Furthermore, coated microneedles have upto 90% delivery efficiency9, Accordingly, 1.4 μg of the total 1.6±0.2 μg DNA coated on microneedles is expected to be delivered into the skin.
Priming of NS3-specific CTLs by microneedle delivery
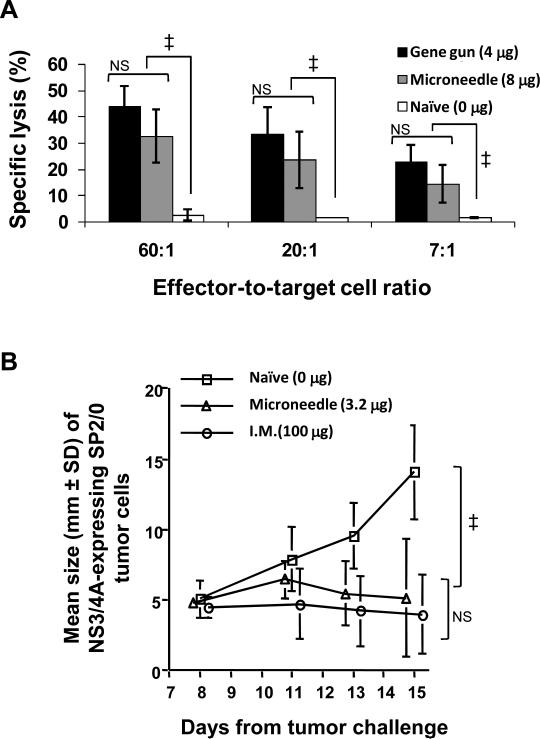
To determine if cutaneous immunization of mice with microneedles coated with plasmid encoding hepatitis C virus NS3/4A protein could elicit a lytic cellular immune response,mice were vaccinated with 8 μg DNA using microneedles and 4 μg DNA using gene gun. Figure 2A shows that DNA-coated microneedle-based immunization induced lytic CTLs. At each effector-to-target ratio, cell lysis was significantly higher after microneedle treatment compared to naïve mice (p < 0.05). This indicates that microneedles released coated DNA within skin and thereby induced a cellular immune response.
Figure 2. Microneedle-based immunization induces antigen-specific CTLs.
Groups of 4-8 week old female C57BL/6 or Balb/c mice (Charles River, Uppsala, Sweden) were immunized cutaneously with microneedles or gene gun, or intramusculary using hypodermic needles.For microneedle-based immunization two or five rows per mouse were used to control the dose. Each microneedle row was coated with 1.6 μg DNA. DNA-coated microneedle rows were manually inserted into trimmed abdominal or back skin and held for 1 min to allow dissolution of coated DNA into skin. For gene gun-based immunization mice were immunized by gene gun (Bio-Rad Laboratories, Hercules, CA, USA) at a dose of 4 μg/mouse as previously described 11. Plasmid DNA was linked to 1-μm diameter gold particles for gene gun-based immunizations according to protocols supplied by the manufacturer. For intramuscular immunization mice were injected with 100 μg DNA in the tibialis anterior using a hypodermic needle. Un-treated (naive) mice were used as a negative control. All experimental protocols were approved by the ethical committee for animal research at Karolinska Institutet. (A) C57B1/6 mice immunized once with codon-optimized NS3/4A DNA using either microneedles (1.6 μg per row × 5 microneedle rows per mouse = 8 μg dose; n=4 mice; gray bars), gene gun (4 μg dose; n=4 mice; black bars) or no immunization (n=2 mice; white bars) and euthanized after two weeks. As described previously 11,13, cells harvested from the spleen (effector cells, 2.5×107 cells per mouse) were restimulated with a NS3 H-2Db-specific peptide and 2.5×107 irradiated naïve C57B1/6 spleenocytes cells. After five days, 5×103 RMA-S cells pulsed with NS3 H-2Db-specific peptide and labeled with 51Cr were used as target cells. The specific cell lysis of target cells was then measured at different effector-to-target cell ratios by measuring 51Cr released from lysed target cells. (B) Balb/C mice were either immunized intramuscularly (100 μg; n=5 mice),cutaneously with microneedles (1.6 μg per row × 2 microneedle rows per mouse = 3.2 μg dose; n=5 mice) or were not immunized (n=2 mice). Presence of in vivo functional CTLs was determined using a tumor challenge model 13. Two weeks after immunization, mice were subcutaneously injected with 1×106 SP2/0 myeloma cells stably transfected with NS3/4A. Tumor growth was then monitored daily through skin by recording mean tumor size (thickness of skin flap at tumor injection site) for 14 days and compared to growth of the same tumor cell line in non-vaccinated mice. Mean tumor sizes were compared by analysis of variance (ANOVA, α=0.05). Error bars represent SD; Symbol ‡ represents p<0.05; NS means not significant.
Potency of coated microneedle-based immunization measured through in vitro cytolytic activity was found to be comparable to that of gene gun-based immunization (p > 0.05, Figure 2A). A significant target-cell lysis was observed even at low effector-to-target ratios, indicating a robust immune response from both the DNA-coated microneedle and gene gun-based immunizations. These results are consistent with previous cutaneous immunization studies using a gene gun, where it was found that gene gun-based cutaneous delivery of low (≤ 10 μg) doses of coNS3/4A DNA plasmid effectively primed NS3-specific CTLs in Balb/c and C57BL/6 mice 13. Although the IM immunization group was not included in this experiment, our previous data shows that a single intramuscular injection of the same dose does not induce significant CTL activity 11. These results indicate that, similar to the gene gun, microneedle-based cutaneous immunization in mice induces a robust CTL response at low microgram doses without the use of an adjuvant. However, in contrast to gene gun, microneedle delivery does not require sophisticated instrumentation or leave behind gold particle debris in skin.
In vivo functionality of primed NS3-specific CTLs
In vivo functionality of primed CTLs was determined as the ability to inhibit growth of NS3/4A-expressing tumor cells in vivo after challenge. This assay determines whether CTLs are functional in vivo such that they recognize and lyse a syngeneic cell line stably transfected to express NS3/4A. This assay showed that tumors in naïve mice grew until the experiment had to be terminated to avoid undue animal suffering (Figure 2B). In contrast, tumor growth was significantly inhibited in mice treated with microneedles coated with 3.2 μg plasmid (p < 0.05). Tumor growth after intramuscular delivery of 100 μg plasmid was also inhibited relative to naïve mice (p < 0.05) and to a similar extent as microneedles (p > 0.05). Altogether, this result shows that microneedle immunization activates NS3-specific CTLs that are functional in vivo, and that primed CTLs can recognize, enter, and eliminate an NS3/4A-expressing tumor. Importantly, microneedle immunized mice received much less DNA compared to intramuscularl injected mice, but were equally effective. This supports our previous observations that NS3/4A-DNA effectively primes specific in vivo functional CTLs by low-dose cutaneous administration 11. Although we did not include a gene gun-immunized group, our previous data has shown that cutaneous delivery of 4μg DNA in mice using gene gun also leads to regression of tumor13.
Overall, this study assessed the potential of DNA-coated microneedles to induce a cellular immune response and compared it to gene gun and intramuscular routes. Microneedles are an attractive delivery system because they have previously been shown to cause little or no pain in human subjects 14, can be produced at disposable cost on an industrial scale 15, and are expected to be easily administered by minimally trained personnel, with possibility of self administration. Although rows of microneedles were used in this study to facilitate penetration into highly deformable mouse skin, two-dimensional arrays of microneedles can easily be fabricated as patches containing up to a few hundred microneedles each, allowing delivery of up to hundreds of micrograms of DNA. Non-coated microneedle patches have been applied to the skin of human subjects hundreds of times in our laboratory using simple manual insertion and have generated no adverse effects 14,16.
Gene gun-mediated delivery has consistently shown up to 1000-fold higher potency than non-adjuvanted intramuscular injection in various animal models, including larger animals and humans 12. However, clinical and logistical applicability of gene gun remain uncertain. Based on this study, microneedles can generate immune responses similar to gene gun. Thus, it is our hypothesis that microneedle patches may provide a DNA vaccine delivery method with adequate efficacy that is also simple, inexpensive and apparently safe.
This study showed, for the first time, that coated microneedle- and gene gun-based cutaneous immunizations using low doses of coNS3/4A DNA (without adjuvants) resulted in comparable levels of in vitro and in vivo priming of antigen specific CTLs. The same level of protection through intramuscular administration used a >30-fold higher dose. Previous work showed that intramuscular dose can be reduced if injection is followed by in vivo electroporation 10. The gene gun and intramuscular injection control experiments in this study are consistent with previous observations 13. Altogether, this suggests that microneedles may provide a delivery method with efficacy suitable for vaccine applications. Additional research in larger animals and humans is needed to more fully validate this hypothesis.
Mechanistically, we expect that several different cell populations may have helped generate a potent immune response from DNA-coated microneedle-based immunization. Both Langerhans and epithelial cells of the skin may be transfected by the NS3/4A DNA, in addition to dendritic and other cells of the dermis. Thus, both direct antigen presentation and cross-presentation, as reported when using the gene gun 17, could be responsible for priming the immune response.
In conclusion, this study shows that a NS3/4A-expressing DNA plasmid can be delivered into skin using coated microneedles to elicit CTLs specific for hepatitis C virus. Importantly, CTL priming using microneedles was similar to gene gun at similar doses, which suggests that immune responses generated using microneedles may be sufficient for DNA vaccine applications.
Acknowledgements
This study was supported by the Swedish Science Council, Cancer Foundation, Stockholm Count Council, U.S. National Institutes of Health, and Robert M. Nerem Travel Award to HSG.
References
- 1.Garmory HS, Perkins SD, Phillpotts RJ, Titball RW. DNA vaccines for biodefence. Adv Drug Deliver Rev. 2005;57:1343–1361. doi: 10.1016/j.addr.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 2.Donnelly J, Berry K, Ulmer JB. Technical and regulatory hurdles for DNA vaccines. Int J Parasitol. 2003;33:457–467. doi: 10.1016/s0020-7519(03)00056-0. [DOI] [PubMed] [Google Scholar]
- 3.Nicolas JF, Guy B. Intradermal, epidermal and transcutaneous vaccination: from immunology to clinical practice. Expert Rev Vaccines. 2008;7:1201–1214. doi: 10.1586/14760584.7.8.1201. [DOI] [PubMed] [Google Scholar]
- 4.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. In: Compans RW, Orenstein WA, editors. Current Topics in Microbiology & Immunology: Vaccines for Pandemic Influenza. Springer-Verlag; Berlin/Heidelberg, Germany: 2009. [Google Scholar]
- 5.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 6.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 8.Van Damme P, Oosterhuis-Kafeja F, Van der Wielen M, Almagor Y, Sharon O, Levin Y. Safety and efficacy of a novel microneedle device for dose sparing intradermal influenza vaccination in healthy adults. Vaccine. 2009;27:454–459. doi: 10.1016/j.vaccine.2008.10.077. [DOI] [PubMed] [Google Scholar]
- 9.Gill HS, Prausnitz MR. Coated microneedles for transdermal delivery. J Controlled Release. 2007;117:227–237. doi: 10.1016/j.jconrel.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahlen G, Soderholm J, Tjelle T, Kjeken R, Frelin L, Hoglund U, et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J Immunol. 2007;179:4741–4753. doi: 10.4049/jimmunol.179.7.4741. [DOI] [PubMed] [Google Scholar]
- 11.Frelin L, Alheim M, Chen A, Soderholm J, Rozell B, Barnfield C, et al. Low dose and gene gun immunization with a hepatitis C virus nonstructural (NS) 3 DNA-based vaccine containing NS4A inhibit NS3/4A-expressing tumors in vivo. Gene Ther. 2003;10:686–699. doi: 10.1038/sj.gt.3301933. [DOI] [PubMed] [Google Scholar]
- 12.Fuller DH, Loudon P, Schmaljohn C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods. 2006;40:86–97. doi: 10.1016/j.ymeth.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 13.Frelin L, Ahlen G, Alheim M, Weiland O, Barnfield C, Liljestrom P, et al. Codon optimization and mRNA amplification effectively enhances the immunogenicity of the hepatitis C virus nonstructural 3/4A gene. Gene Ther. 2004;11:522–533. doi: 10.1038/sj.gt.3302184. [DOI] [PubMed] [Google Scholar]
- 14.Gill HS, Denson DD, Burris BA, Prausnitz MR. Effect of microneedle design on pain in human volunteers. Clin J Pain. doi: 10.1097/AJP.0b013e31816778f9. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prausnitz MR, Mikszta JA, Cormier M, Andrianov AK. Microneedle-based vaccines. Curr Top Microbiol Immunol. doi: 10.1007/978-3-540-92165-3_18. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci U S A. 2008;105:2058–2063. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho JH, Youn JW, Sung YC. Cross-priming as a predominant mechanism for inducing CD8(+) T cell responses in gene gun DNA immunization. J Immunol. 2001;167:5549–5557. doi: 10.4049/jimmunol.167.10.5549. [DOI] [PubMed] [Google Scholar]
- 18.Gill HS, Prausnitz MR. Coating formulations for microneedles. Pharm Res. 2007;24:1369–1380. doi: 10.1007/s11095-007-9286-4. [DOI] [PubMed] [Google Scholar]