Abstract
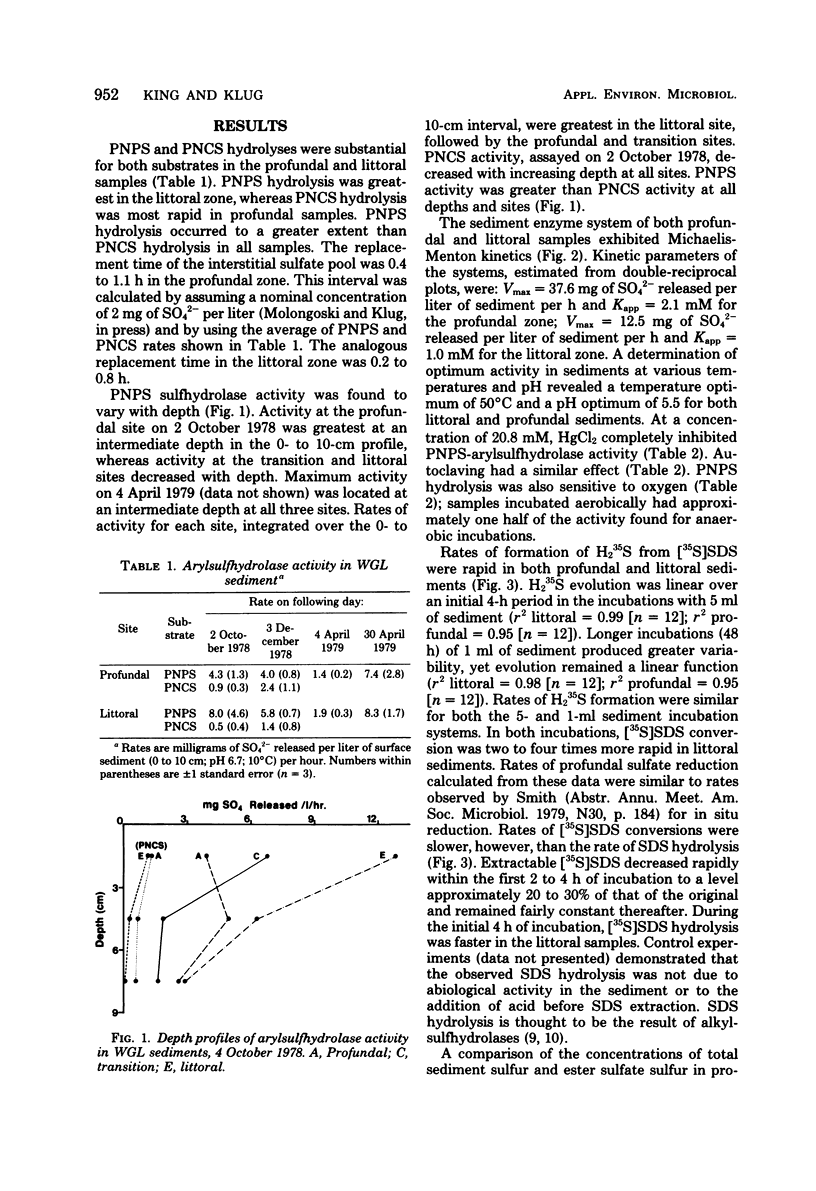
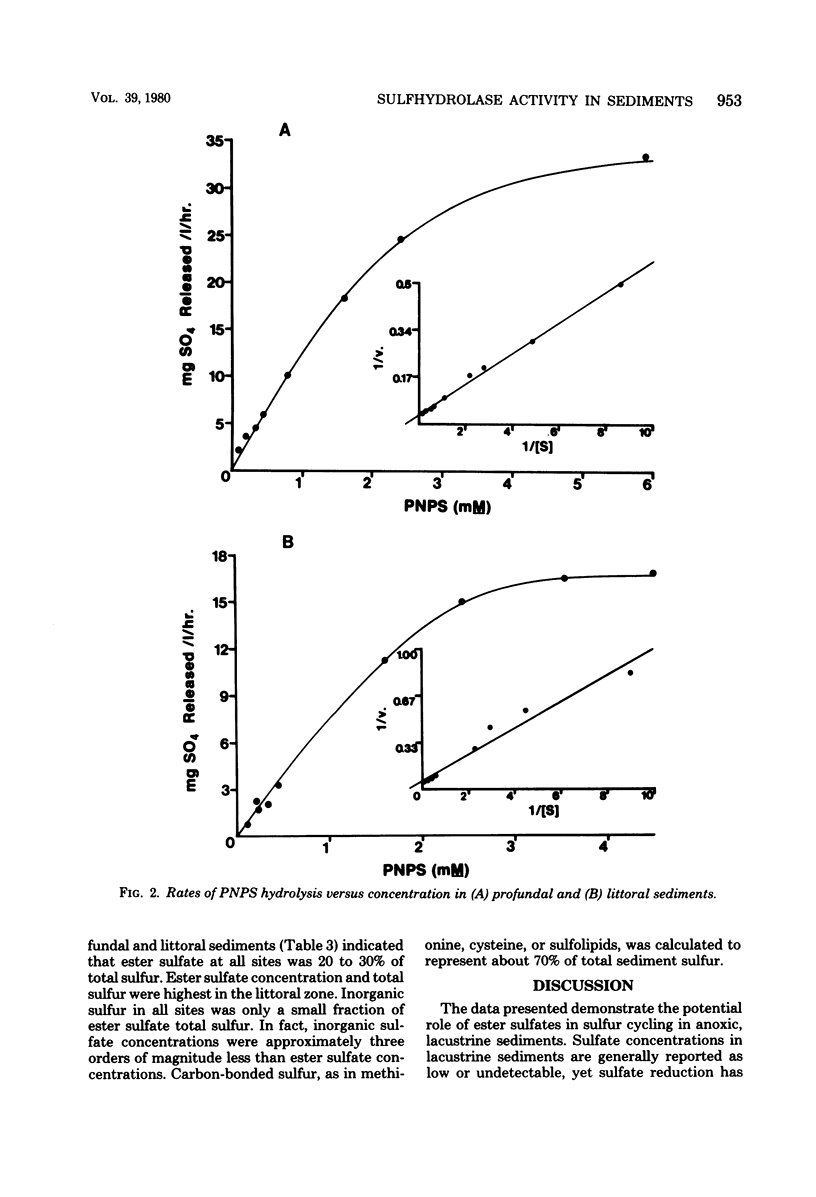
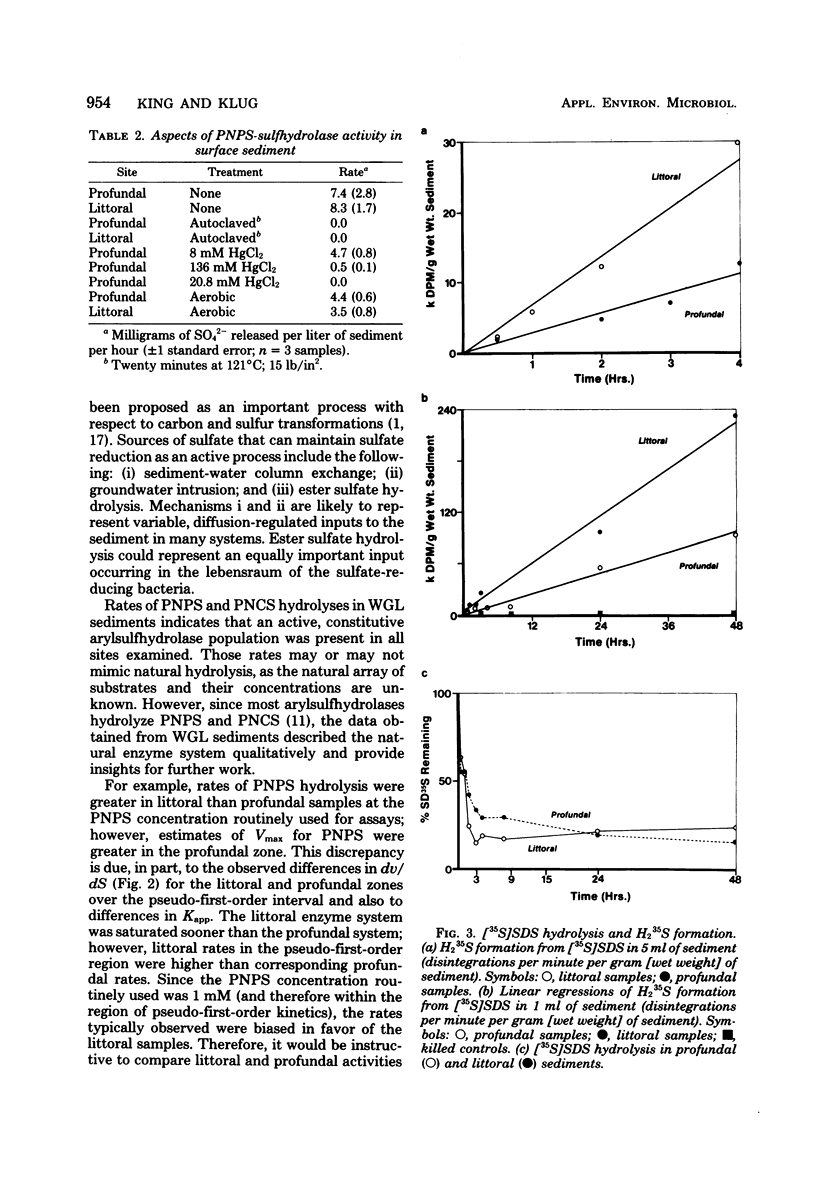
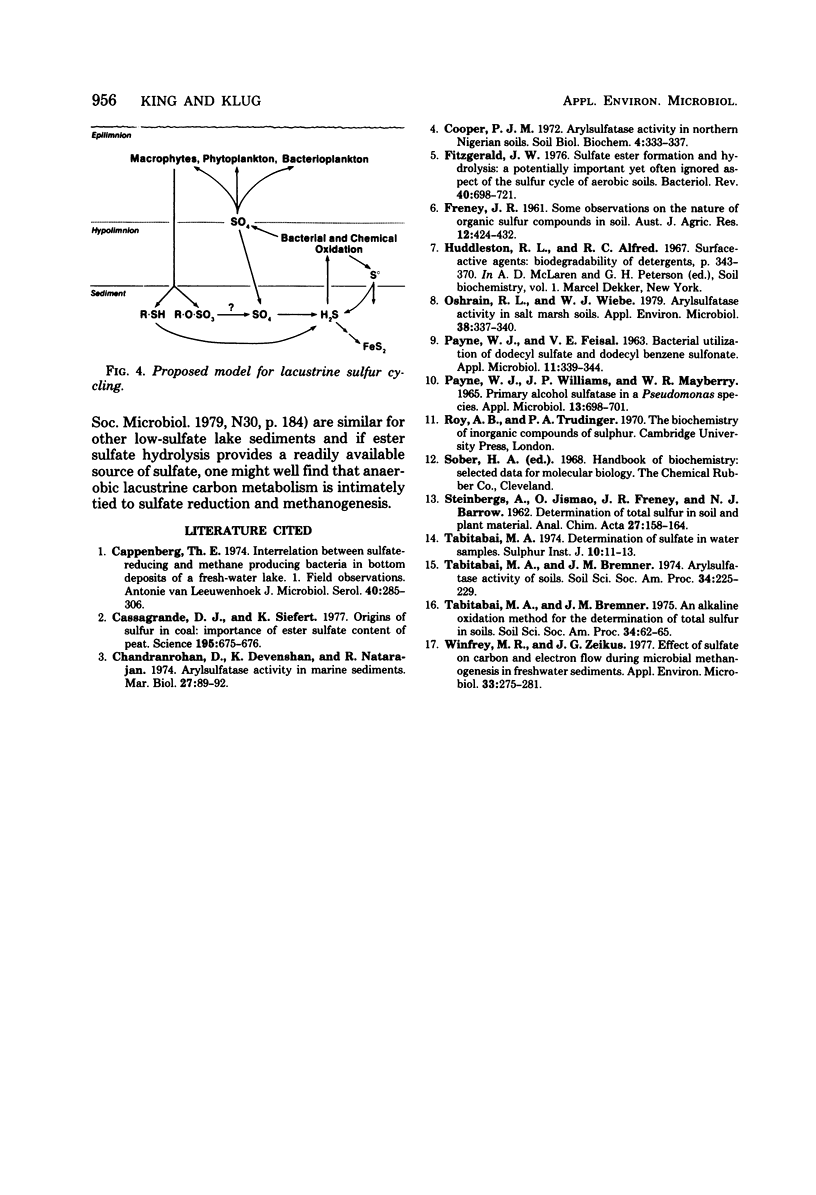
The hydrolysis of p-nitrophenyl sulfate, p-nitrocatechol sulfate, and [35S]sodium dodecyl sulfate was examined in anoxic sediments of Wintergreen Lake, Michigan. Significant levels of sulfhydrolase activity were observed in littoral, transition, and profundal sediment samples. Rates of sulfate formation suggest that the sulfhydrolase system would represent a major source of sulfate within these sediments. Sulfate formed by ester sulfate hydrolysis can support dissimilatory sulfate reduction as shown by the incorporation of 35S from labeled sodium dodecyl sulfate into H235S. Sulfhydrolase activity varied with sediment depth, was greatest in the littoral zone, and was sensitive to the presence of oxygen. Estimations of ester sulfate concentrations in sediments revealed large quantities of ester sulfate (∼30% of total sulfur). Both total sulfur and ester sulfate concentrations varied with the sediment type and were two to three orders of magnitude greater than the inorganic sulfur concentration.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cappenberg T. E. Interrelations between sulfate-reducing and methane-producing bacteria in bottom deposits of a fresh-water lake. II. Inhibition experiments. Antonie Van Leeuwenhoek. 1974;40(2):297–306. doi: 10.1007/BF00394388. [DOI] [PubMed] [Google Scholar]
- Casagrande D., Siefert K. Origins of sulfur in coal: importance of the ester sulfate content of peat. Science. 1977 Feb 18;195(4279):675–676. doi: 10.1126/science.195.4279.675. [DOI] [PubMed] [Google Scholar]
- Fitzgerald J. W. Sulfate ester formation and hydrolysis: a potentially important yet often ignored aspect of the sulfur cycle of aerobic soils. Bacteriol Rev. 1976 Sep;40(3):698–721. doi: 10.1128/br.40.3.698-721.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshrain R. L., Wiebe W. J. Arylsulfatase activity in salt marsh soils. Appl Environ Microbiol. 1979 Aug;38(2):337–340. doi: 10.1128/aem.38.2.337-340.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAYNE W. J., FEISAL V. E. Bacterial utilization of dodecyl sulfate and dodecyl benzene sulfonate. Appl Microbiol. 1963 Jul;11:339–344. doi: 10.1128/am.11.4.339-344.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne W. J., Williams J. P., Mayberry W. R. Primary alcohol sulfatase in a Pseudomonas species. Appl Microbiol. 1965 Sep;13(5):698–701. doi: 10.1128/am.13.5.698-701.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfrey M. R., Zeikus J. G. Effect of sulfate on carbon and electron flow during microbial methanogenesis in freshwater sediments. Appl Environ Microbiol. 1977 Feb;33(2):275–281. doi: 10.1128/aem.33.2.275-281.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


