Abstract
Purpose
To compare pregnancy and implantation rates when embryos are selected based on a single Day 3 (D 3) morphology score vs. a GES score plus sHLA-G expression.
Methods
A prospective randomized study (n = 214) undergoing fresh ICSI cycles. Embryos were selected for transfer based on either Day 3 morphology score (Group A) or GES-scoring plus sHLA-G expression (Group B).
Results
Clinical [35/107 (33%) vs. 52/107 (49%)] and ongoing pregnancy [20/107 (19%) vs. 52/107 (49%)] rates were significantly different between Group A and Group B (p < 0.05). Implantation rates were not significantly different between Group A [52/353 (15%)] and Group B [73/417 (18%)] (p < 0.05). The number of pregnancies lost during the first trimester was nearly 12 times higher in Group A [25/52 (48%)].
Conclusion
The miscarriage rate was significantly lower in Group B than Group A and the pregnancy results were superior when embryos were selected based on GES plus sHLA-G expression.
Keywords: Pregnancy, Implantation, Miscarriage, sHLA-G
Introduction
Generating criteria to select “competent embryos” for transfer has become of utmost importance. Historically, approaches to identifying the “competent” embryos prior to transfer focused mainly on morphological assessment of embryos. Evidently, morphological evaluations furnish clues that enhance ability in choosing the best pre-embryos for transfer. However, it is severely limited to provide reliable evidence for predicting subsequent normal embryo implantation [1–7]. Desai et al. [8] reported the first scoring system for day 3 embryos, whereas all previous scoring systems were based on the second day of culture (Day 2). Later on the graduated embryo scoring (GES) system was introduced by Fisch et al. [9] in which each embryo was individually cultured, allowing for sequential microscopic assessment of developmental stages starting on day 1 through day 3 of embryo culture.
Determining which morphologic evaluation system [10–13] to use for transferring embryos on Day 2/3, and to achieve pregnancy rates similar to embryos that are transferred at the blastocyst stage, is a controversial subject [14, 15]. Some researchers claimed that transferring selected embryos on day 3 yielded a pregnancy rate equivalent to embryos transferred on day 5 or 6 [16, 17].
Jurisicova et al. [18] reported that HLA-G expression was present during preimplantation human embryo development. The presence of soluble Human Leukocyte Antigen-G (sHLA-G) mRNA was detected in culture medium surrounding grouped embryos [19]. The detection of sHLA-G in the culture medium of individually cultured early cleaving embryos (as early as 44–46 h after insemination) provided evidence that embryos could be selected by their maternal genomic function. This is due to the correlation between sHLA-G expression by an embryo and its potential to reach a pregnancy [20].
In our study sHLA-G expression within a range of 0.184–0.196 was considered positive. This original optimal range was determined by Keskintepe (unpublished data) and was slightly different from other investigators [20, 24].
While several tools are available for the evaluation of embryos for transfer, other unknown factors such as oocyte quality and embryo dismorphism, still remain unanswered. The purpose of this study was to compare pregnancy and implantation rates when embryos are selected based on a single Day 3 (D 3) score vs. sHLA-G expression plus a graduated embryo scoring (GES).
Materials and methods
Patients
An initial pilot study was performed on 58 patients to compare pregnancy outcome when using a single D 3 morphology versus the GES-scoring system. We reported no statistical differences between these two treatment groups. Based on the findings of our pilot study, a subsequent study was designed. In Group A, the most common way (morphology and developmental rate) by which embryo selection is performed, was compared to Group B, a combination of non-invasive criteria ( the expression of soluble HLA-G plus the GES-score).
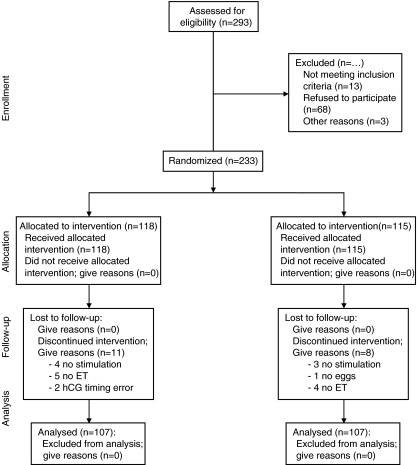
A Statistical program (Epi-info, UMassAmherst) was used to perform a power calculation for this study. Using the given parameters—confidence (95%) and the power (80%) and the ratio of cases to controls—it was evident that using one hundred and seven patients per treatment group would be sufficient. Two hundred and ninety seven (297) patients were assessed for eligibility to participate in this study (Fig. 1). Two hundred and thirty three (233) patients underwent randomization prior to egg retrieval. At this time, patients drew a single tab from a prepared envelope to determine their study group and whether their embryos will be transferred based on a single Day 3 score (Group A) or an sHLA-G expression plus a GES-score (Group B). (Furthermore, in Group A, the culture medium also underwent sHLA-G testing but the embryologist was blinded for the sHLA-G results of these embryos prior to transfer). Patient diagnosis included endometriosis, tubal factor, PCOS, unknown and male factor. All patients were <39 years of age and had a normal uterine cavity and normal endometrial thickness (≥ 9 mm) at the time of hCG administration).
Fig. 1.
CONSORT statement flow diagram
Individually cultured embryos whose surrounding media expressed sHLA-G with an OD = 0.190 ± 0.006 (the geometric mean) were defined as positive for sHLA-G expression, whereas those outside the range (0.184–0.196) were designated as negative (Table 2).
Table 2.
Comparative analysis data analysis between day 3 score and GES-Score Plus sHLA-Ga
| Parameter | D.3 Score (n = 107) | GES score plus sHLA-G expression (n = 107) | P-value |
|---|---|---|---|
| Age (years) | 35.2 ± 4.0a | 35.1 ± 4.0a | 0.58 |
| Total no. oocytes | 9.7 ± 6.2a | 11.4 ± 6.2a | 0.20 |
| Mature oocytes | 7.3 ± 5.0a | 9.2 ± 4.2a | 0.30 |
| No. Ooacytes fertilized | 7.1 ± 4.4a | 8.5 ± 4.7a | 0.36 |
| Total no. embryos | 6.8 ± 3.3a | 8.0 ± 3.9a | 0.32 |
| No. embryo transferred | 3.3 ± 0.8a | 3.9 ± 0.8a | 0.15 |
| Biochemical pregnancies (%) | 42 (39)b | 60 (56)b | 0.05* |
| Clinical pregnancies (%) | 35 (33)b | 52 (49)b | 0.05* |
| Ongoing pregnancies (%) | 20 (19)b | 52 (49)b | 0.05* |
| Implantation rates (%) | (15)b | (18)b | 0.15 |
aData expressed as means±SD
bData expressed as (%)
*Significance p < 0.05
Stimulation
Patients were pre-treated with oral contraceptives received Lupron (TAP Pharmaceuticals, Lake Forest, IL) in a long protocol after pre-treatment with oral contraceptive (OC) birth control pills for 1–3 weeks and were treated by a human derived gonadotropin (Bravelle; Ferring Pharmaceuticals Inc, Suffern, NY) to activate ovarian follicular stimulation. Ovulation was triggered when at least two follicles were 18 mm and half the remaining were ≥15 mm. Oocytes were harvested transvaginally using ultrasound guidance 35 h post hCG. All patients underwent controlled ovarian hyper-stimulation (COH) by the same physician.
Embryo culture
All metaphase II (MII) embryos were injected by ICSI 3–4 hours post retrieval. All zygotes were cultured individually in 50 µl droplets of P-1 medium, supplemented with 10% Synthetic Serum Substitute (SSS) (Irvine Scientific, Santa Ana, CA). After 44–46 h embryos were moved to Complete Blastocyst Medium (Irvine Scientific, Santa Ana, CA). At this point, ±35 µl from the remaining P-1 culture media drops were collected in 200 µl micro-centrifuge tubes and immediately frozen. All samples were shipped to a central location and tested for sHLA-G, using an enzyme-linked immunosorbent sandwich (ELISA) assay. All embryos were transferred on Day 3.
Embryos scoring
The GES-score
To apply the GES-score, all mature oocytes underwent ICSI with a single sperm and were individually cultured. In order to apply the GES-score, oocytes were evaluated at 16–18 h post ICSI when the presence of pronuclear as well as nucleoli alignment along the pronuclear axis was evident, a score of 20 was allotted where nucleoli alignment was prevalent. The second observation took place at 25–27 h, at which time early cleavage was noted. A score of 30 was allotted when cleavage was observed. Furthermore, at this time a score was given based on the presence/absence of fragmentation. When fragmentation was absent a score of 30 was given, <20% fragmentation received a score of 25 and >20% received a score of zero (0). The third and final score was given 64–67 h after ICSI and involved the number of blastomeres and embryo grade. Example: six cell grade one—6(I),7(I), 8(I), 8(II), 9(I) scored 20 points, seven cell, grade II [7(II)], 9(II), 10(I), 11(I) and compacting(I) scored ten points. The maximum GES-score totaled 100 points (Table 1).
Table 1.
Graduated embryo scoring (GES) of cleavage stage embryos
| Evaluation | Hours after insemination | Developmental milestone | Score |
|---|---|---|---|
| 1 | 16–18 | Nucleoli aligned along pronuclear axis | 20 |
| 2 | 25–27 | Cleavage regular and symmetrical Fragmentationa | 30 |
| Absent | 30 | ||
| <20% | 25 | ||
| >20% | 0 | ||
| 3 | 64–67 | Cell number and gradeb 7CI, 8CI, 8CII, 9CI | 20 |
| 7CII, 9CII, 10CI, 11CI, Compacting I | 10 | ||
| Total score | 100 |
Fisch. Graduated embryo score (GES). Fertil Steril 2003
aIf the embryo was not cleaved at 25–27 h, grading of fragmentation should occur at the 64–67 h evaluation if the embryo reached the seven-cell stage and had <20% fragmentation
bGrade I = symmetrical blastomeres and absent fragmentation. Grade II = slightly uneven blastomeres and <20% fragmentation. Grade III = uneven blastomeres and >20% fragmentation. Grade A embryos are seven or more cells with <20% fragmentation
-The day 3 score
The Day 3 score that was applied in this study was a modification of Veeck’s [21] criteria for Day 3 embryos combined with our laboratory’s criteria (unpublished data DK). Embryos were scored based on their blastomere number, size and symmetry as well as the percentage of fragmentation that was present. Example: a grade one embryo: 8–11 cell with even sized blastomeres (+8 points) and without fragmentation (+2 points) scored a max of ten points, a grade two embryo: 8–11 cell with even sized blastomeres (+8 points) and with <10% fragmentation (0 points) scored a max of eight points, a grade three embryo 8–11 cell (+8 points) with uneven blastomere (−1 points) and >10% fragmentation (−3 points) scored a max of four points.
The same embryologist preformed all embryology and embryo scoring in this study.
Soluble HLA-G assay
A monoclonal antibody (mAb; MEM-G9 MCA 2044; Serotec, Raleigh, NC) against sHLA-G was used to coat a 96-well Nunc-Immunoplate (Fisher Scientific, Chino CA) using a concentration of 2 µg/ml in 0.1 mol/l carbonate buffer at pH 9.5 for 1 h at 37°C. The plate was then refrigerated overnight at 4°C. On the following day, the plate was thoroughly washed using 100 µl of phosphate-buffered saline (PBS) and 0.05% Tween-20. The wash was repeated twice using 100 µl PBS and 5% bovine serum albumin (BSA) for 15 min each. A 50 µl aliquot of PBS and 5% BSA was added to each well before adding the sample of 50 µl of embryo supernatant. Amniotic fluid (AF) was used as a positive control. AF (50 µL) and 50 µl of pure Complete Blastocyst culture media (Irvine Scientific) in which no embryos had been cultured (the negative control) Samples were incubated for a period of 1 h at 37°C. After incubation, the plates were washed three times with PBS, followed by incubation with a specific biotin conjugated mAB (w6/32 MCA81B; Serotec) at a 1:1000 dilution in PBS and 1% BSA for 45 min at 37°C and then washed five times with PBS. Streptavidin alkaline phosphatase conjugated (BD Bioscience Pharmigen, San Diego, CA) at a concentration of 1:1000 in carbonate buffer was incubated for 30 min at 37°C and washed five times with PBS, after which phosphatase substrate was added at a concentration of 1 mg/ml in 10% diethanolamine at pH 9.8 for 30 min. The colorimetric reaction was then stopped by the addition of 50 µl of 3 mol/l NaOH. The sHLA-G concentration was determined by absorbance at 405 nm on the EL800 Universal Microplate Reader (Bio-Tek Instruments Inc., Winooski, VT).
Embryo selection and transfer
Individual embryos were defined as having positive sHLA-G expression if their surrounding media expressed sHLA-G with an optical density inside the range of 0.184–0.196. Those outside the above mentioned range were designated as sHLA-G negative. In Group A, one hundred and seven (107) patients received embryos for transfer and all of these embryos were selected by using a Day 3 score only. In Group B, one hundred and seven (107) patients received embryos for transfer by first selecting any embryos that had a positive sHLA-G expression, and correlating such with the highest available GES score. Furthermore, in Group B the patients received embryos containing at least one sHLA-G positive in the cohort for transfer. In this study no more than four (4) embryos were recommended for transfer. However, the final decision regarding the number of embryos for transfer was left to the patient after an informed consent that was based on American Society for Reproductive Medicine guidelines. All embryos were transferred on day 3 of culture, using ultra sound guidance and an echogenic catheter (Wallace, Smith Medical, UK). Serum β-hCG levels were measured 11 days and 13 days after the transfer. The patient was considered positive for biochemical pregnancy when the first value was >5.0 IU and the next value 2 days later was double the first. Clinical pregnancy rates were based on a six (6) week ultrasound with a gestational sac containing a fetal heartbeat. Ongoing pregnancy rates were based on a ten to twelve (10–12) week ultrasound with gestational sac containing one or more growing fetuses with appropriate heart rates.
Statistical analysis
Statistical analysis was carried out using SPSS version 15 (Statistical Package for the Social Science). The number and percentage (%) of categorical data as well as the mean and standard deviation (SD) of continuous data were calculated. Comparison between mean values of continuous variables was calculated using the Students t-test for parametric and the Mann–Whitney-U test for non-parametric data, while the Chi-square was used for categorical data and the Odds ratio and 95% confidence calculated. Significance value was set at p < 0.05.
Miscarriage rates between treatment groups were calculated as a percentage of pregnancy loss between biochemical, clinical and ongoing rates. Fetal loss between treatment groups were calculated between clinical and ongoing pregnancies. Retrospective data were calculated as a percentage.
Institutional review
Since January 2005, all embryos at SIRM underwent routine sHLA-G assay to determine the expression of this potential biochemical marker in the culture medium surrounding embryos. All patients were counseled regarding the risks, benefits, and alternatives to sHLA-G testing; however, because it was not considered an experimental component of the treatment, a specific institutional review board was not sought. However, for the purpose of this PhD study, ethical approval was obtained from the Ethical committee of the University of Stellenbosch (N06/07/119). Furthermore, all clinical research conducted was in full compliance with guidelines of the American Society of Reproductive Medicine and met ethical principles involving human subjects as defined by the Declaration of Helsinki in 1964.
Results
In the prospective data analysis, no statistical significance was found between the two treatment groups in regards to age, total number of oocytes, mature oocytes, number of oocytes fertilized, number of embryos, or number of embryos transferred and implantation rates. However, significance was reached for biochemical (39.3% vs. 56.1%), clinical (32.7% vs. 48.6%), and ongoing pregnancy (18.7% vs. 48.6%) rates between Group A and Group B, respectively (Table 2). In this study all embryos underwent sHLA-G testing. However, in Group A, sHLA-G results were blinded (therefore, embryos were selected without knowing the sHLA-G results prior to transfer).
A total of 770 embryos were transferred in both groups:
In Group A, 353 embryos were transferred into 107 patients, 42/107 (39.2%) positive β-hCG resulted. The 6 week ultrasound revealed that 35/42 (83.3%) pregnancies continued since β-hCG testing was performed. The breakdown included 23 singletons, eight sets of twins, three sets of triplets and one set of quadruplets, resulting into 52 fetuses with heartbeats confirming an implantation rate of 14.7%. Furthermore, the 10–12 week ultrasound revealed that 20/35 (57.1%) pregnancies continued since 6 week ultrasound was performed. The breakdown included 13 singletons, seven sets of twins, zero triplets zero quadruplets, resulting into 27 fetuses with heartbeats. The miscarriage rate (fetal loss) between biochemical and clinical pregnancies were 7/42 (16.7%) and between clinical and ongoing pregnancies were 15/35 (42.9%). Furthermore, in Group A the total pregnancy loss between biochemical and ongoing pregnancies were 20/42 (total number of pregnancy losses)/(initial number of biochemical pregnancies) (47.6%). Furthermore, there was a loss of 25 fetuses between 6 week and the 10–12 week ultrasound.
In Group B, 417 embryos were transferred into 107 patients, 60/107 (56.1%) patients had positive β-hCG results. The 6 week ultrasound revealed that 52/60 (86.7%) pregnancies continued since β-hCG testing was performed. The breakdown included 36 singletons, 12 sets of twins, three sets of triplets and one set of quadruplets, resulting into 73 fetuses with heartbeats confirming an implantation rate of 17.5%. Furthermore, the 10–12 week ultrasound revealed that 52/60 (86.7%) pregnancies continued since 6 week ultrasound was performed. The breakdown included 38 singletons, ten sets of twins, four triplets zero quadruplets, resulting into 70 fetuses with heartbeats. The miscarriage rate between biochemical and clinical pregnancies were 8/60 (13%) and between clinical and ongoing pregnancies were 0/52 (0%). Furthermore, in Group B the total pregnancy loss between biochemical and ongoing pregnancies were 8/60 (total number of pregnancy losses)/(initial number of biochemical pregnancies) (13.3%). Furthermore, there was a loss of three fetuses between 6 week and the 10–12 week ultrasound.
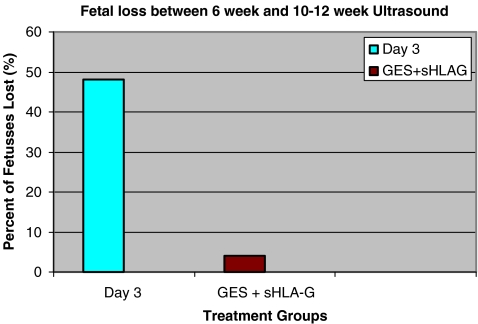
Implantation rates per embryo transferred were not significantly different at 52/353 (14.7%) and 73/417 (17.5%) for Group A and Group B, respectively (p > 0.05). However, the pregnancy loss rates between biochemical and ongoing pregnancies were significantly different at 22/42 (52.3%) and 8/60 (13.3%) for Group A and Group B, respectively (p < 0.05). Fetal loss between treatment groups were 25/52 (48.1%) and 3/73 (4.3%) for Group A and Group B, respectively and was statistically significant (p < 0.05) (Fig. 2).
Fig. 2.
Fetal loss rates
Prospective data for Group B indicated that ninety three out of one hundred and seven (93/107) patients (86.9%) received a cohort of embryos for transfer that contained at least one sHLA-G positive embryo combined with the highest available GES-score. This “sHLA-G positive sub-group” resulted into 55/93 (59.1%) (biochemical), 50/93 (53.8%) clinical) and 50/93 (53.8%) (ongoing pregnancy), respectively. Furthermore, fourteen out of one hundred and seven (14/107) patients (13.1%) received embryos for transfer that contained ALL sHLA-G negative embryo combined with the highest available GES-score. This “sHLA-G negative sub-group” resulted into 5/14 (35.7%) (biochemical), 2/14 (14.2%) clinical) and 2/14 (14.2%) (ongoing pregnancy), respectively.
Retrospective data for Group A and un-blinding of sHLA-G results revealed that twenty seven out of one hundred and seven (27/107) patients (25.2%) received a cohort of embryos for transfer that contained at least one sHLA-G positive embryo combined with the highest available GES-score. This “sHLA-G positive sub-group” resulted into 17/27 (63.0%) (biochemical), 14/27 (51.9%) clinical) and 14/27 (51.9%) (ongoing pregnancy), respectively. Furthermore, eighty out of one hundred and seven (80/107) patients (74.8%) received embryos for transfer that contained ALL sHLA-G negative embryo combined with the highest available GES-score. This “sHLA-G negative sub-group” resulted into 25/80 (31.2%) (biochemical), 21/80 (26.3%) clinical) and 6/80 (7.5%) (ongoing pregnancy), respectively.
Combined data regarding the s-HLA-G status from group A and B revealed that 120/214 (56.1%) of patients received a cohort that included at least one sHLA-G-positive embryo. This subgroup resulted in 72/120 (60.0%) biochemical, 64/120 (53.3%) clinical and 64/120 (53.3%) ongoing pregnancies, respectively. Furthermore, 94/214 (43.9%) of patients received a cohort that included ALL sHLA-G-negative embryos. This subgroup resulted in 30/94 (31.9%) biochemical, 23/94 (24.5%) clinical and 8/94 (8.5%) ongoing pregnancies.
Summarizing: pregnancy outcome for patients that received sHLA-G-positive cohorts for transfer versus those that received sHLA-G negative cohorts for transfer were 60.0% vs. 31.9% (biochemical), 53.3% vs. 24.5% (clinical) and 53.3% vs. 8.5%, respectively and was significant (p < 0.05).
Discussion
The aim of this prospective randomized study was to compare a traditional embryo selection method (Day 3 morphology) with a novel GES-score plus sHLA-G expression and its impact on implantation, pregnancy and miscarriage rates. To find non-invasive techniques to improve pregnancy outcome and also reduce high order multiple has been an ongoing challenge since the start of IVF in 1988. Earlier studies have outlined different criteria for embryo selection, focusing on a variety of parameters. Pronuclear morphology such as orientation, surrounding halo, number of nucleoli and its arrangement at the juxta-junction and the role of precursor bodies, early cleavage, and day 3 blastomere morphology including number of blastomeres, their shape and size, and the percentage of fragmentation provided better understanding of embryo development pattern. Whether to transfer embryos at the cleaved or the blastocyst stage is still an ongoing argument.
All the above approaches has been based on morphology, however, the detection of sHLA-G in culture medium surrounding clusters of developing embryos [18, 19, 22] sparked a new interest in the search for non invasive markers, or so-called proteomics, to assist in the identification of the “competent” embryos for transfer. In the study presented here, a traditional Day 3 morphology compared to the sHLA-G expression plus a GES-score in order to identify embryos for transfer resulting into favorable clinical and ongoing pregnancy rates. These findings are supported by a number of researchers [20, 23–26]. Furthermore, we reported a decrease of fetal loss, in the sHLA-G positive group, between 6 week and 12 week ultrasound, a finding supported by Rebmann et al. [27].
The exact mechanism by which sHLA-G improved the ongoing pregnancy outcome is unknown; however, whether it is due to good communication (cross-talk) [28], or the suppression/masking of the natural immune response [29, 30], the presence of sHLA-G has been postulated to protect the conceptus from destruction by the maternal immune response [31]. The first two cell cycles of human embryogenesis (that is embryonic divisions up to the 4-cell stage) are regulated by the maternal genome [32, 33] Activation of the embryonic genome takes place between the 4-cell and 8-cell stages post fertilization in humans and is essential for zygote protein synthesis and subsequent embryo development. With these assumptions, we suggest that collecting the culture medium surrounding individually cultured embryos at 44–46 h post ICSI and subsequently analyzing it for the presence of sHLA-G will help to identify embryos with a “healthy” maternal genome, which should convert into a properly activated embryonic genome, resulting into a competent developing embryo.
In a recent meta-analysis it was concluded that the presence of sHLA-G in the supernatants of cultured embryos is moderately helpful to predict the ability to achieve pregnancy in women undergoing IVF treatment [34]. Additionally they suggested it to be more beneficial if the embryos are of good quality. In our study we used both the expression of sHLA-G and applied a novel, cumulative embryo score (GES), providing detailed information regarding each embryo’s morphology and biochemical competency The implantation rates in the groups studied, did not differ significantly but the fetal loss rate was significantly lower in the sHLA-G-positive group. Furthermore, data revealed a significant increase in the biochemical, clinical and ongoing pregnancies in patients that received cohorts containing at least one sHLA-G-positive embryo vs. cohorts containing all sHLA-G-negative embryos for transfer. This finding highlights a further benefit in performing sHLA-G in an IVF program. This knowledge prior to embryo transfer will assist in selecting the better embryo.
This study is a first prospective randomized study comparing the traditional way of embryo evaluation versus sHLA-G expression plus a cumulative GES-score on day 3 as selection criteria. It was performed by the same physician, using the same stimulation drug, the same embryologist performed the embryology on all the patients and sHLA-G ELISA was performed by the same lab in order to minimize as many variables as possible.
We conclude that by combining a positive sHLA-G expression with the highest GES-score on Day 3 resulted into significantly improved ART outcome. Furthermore, that the power of sHLA-G status is a very important criterion to identify prior to embryo transfer.
Contributor Information
Dirk J. Kotze, Email: dkotze@sherinstitute.com
Thinus Kruger, Phone: +27-21-9389209, FAX: +27-21-9322455, Email: mms1@sun.ac.za.
References
- 1.Cummins J, Breen T, Harrison K. A formula for scoring human embryo growth rates in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J In Vitro Fert Embryo Transf. 1986;3:284–95. doi: 10.1007/BF01133388. [DOI] [PubMed] [Google Scholar]
- 2.Puissant F, Rysselberge M, Barlow P. Embryo scoring as a prognostic tool in IVF treatment. Hum Reprod. 1987;2:705–8. doi: 10.1093/oxfordjournals.humrep.a136618. [DOI] [PubMed] [Google Scholar]
- 3.Staessens C, Camus M, Bollen N. The relationship between embryo quality and the occurrence of multiple pregnancies. Fertil Steril. 1992;57:626–30. [PubMed] [Google Scholar]
- 4.Steer C, Mills C, Tan S. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer program. Hum Reprod. 1992;7:117–9. doi: 10.1093/oxfordjournals.humrep.a137542. [DOI] [PubMed] [Google Scholar]
- 5.Roseboom T, Vermeiden J. Evaluation of embryo scoring systems and their value in predicting in vitro fertilization outcome. Assist Reprod Rev. 1995;5:53–9. [Google Scholar]
- 6.Ziebe S, Petersen K, Lindenberg S. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Reprod. 1997;12:1545–9. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]
- 7.Visser D, Fourie F. The applicability of the cumulative embryo score system for embryo selection and quality control in an in-vitro fertilization/embryo transfer program. Hum Reprod. 1993;8(1):719–1722. doi: 10.1093/oxfordjournals.humrep.a137922. [DOI] [PubMed] [Google Scholar]
- 8.Desai N, Goldstein J, Rowland Y, Goldfarb M. Morphology evaluation of human embryos and derivation of and embryo quality scoring system specific for day 3 embryos: a preliminary study. Hum Reprod. 2000;15(10):2190–6. doi: 10.1093/humrep/15.10.2190. [DOI] [PubMed] [Google Scholar]
- 9.Fisch J, Rodriguez H, Ross R, Overby G, Sher G. The graduate embryo scoring system (GES) predicts blastocyst formation and pregnancy rates from cleaved—stage embryos. Hum Reprod. 2001;16(9):1970–5. doi: 10.1093/humrep/16.9.1970. [DOI] [PubMed] [Google Scholar]
- 10.Alikani M, Cohen J, Tomkin G, Garrisi J, Mack C, Scott RT. Human embryo fragmentation in vitro and its implications for pregnancy and implantation. Fertil Steril. 1999;71:836–42. doi: 10.1016/S0015-0282(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 11.Sakkas D, Shoukir Y, Chardonnens D, Bianchi PG, Campana A. Early cleavage of human embryos to the two-cell stage after intracytoplasmic sperm injection as an indicator of embryo viability. Hum Reprod. 1999;13(1):182–7. doi: 10.1093/humrep/13.1.182. [DOI] [PubMed] [Google Scholar]
- 12.Royen E, Mangelschots K, Neubourgh D, Valkenburg M, Meerssche RG, Eestermans W, et al. Characterization of a top quality embryo, a step towards single-embryo transfer. Hum Reprod. 1999;14(9):2345–9. doi: 10.1093/humrep/14.9.2345. [DOI] [PubMed] [Google Scholar]
- 13.Scott L, Alvero R, Leondires M, Miller B. The morphology of human pronuclear embryos is positively related to blastocyst development and implantation. Hum Reprod. 2000;15(11):2394–403. doi: 10.1093/humrep/15.11.2394. [DOI] [PubMed] [Google Scholar]
- 14.Coskun S, Hollanders J, Al-Sufyan H, Al-Mayman H, Jaroudi K. Day 5 versus day 3 embryo transfer: a controlled randomized trail. Hum Reprod. 2000;15(9):1947–52. doi: 10.1093/humrep/15.9.1947. [DOI] [PubMed] [Google Scholar]
- 15.Kovacic B, Vlaisavljevic V, Reljic M, Lovrec VG. Clinical outcome of day 2 versus day 5 transfer with one or two developed embryos. Fertil Steril. 2002;77(3):529–36. doi: 10.1016/S0015-0282(01)03212-5. [DOI] [PubMed] [Google Scholar]
- 16.Scholtes MC, Zeilmaker GH. A prospective, randomized study of embryos transfer results after 3 or 5 days of embryo culture in in vitro fertilization. Fertil Steril. 1996;65(6):1245–8. doi: 10.1016/s0015-0282(16)58349-6. [DOI] [PubMed] [Google Scholar]
- 17.Blake D, Proctor M, Johnson N, Olive D. Cleavage stage versus blastocyst stage embryo transfer in assisted conceptus. Cochrane Data Base System. 2002;Rev 2:CD 002118. [DOI] [PubMed]
- 18.Jurisicova A, Casper RF, MacLusky NJ, Mills GB, Librach CL. HLA-G expression during preimplantation human embryo development. Proc Natl Acad Sci USA. 1996;93:161–5. doi: 10.1073/pnas.93.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuzzi B, Rizzo R, Criscouli L, Noci I, Melchiorri L, Scarselli B, et al. HLA-G expression in early embryos is a fundamental prerequisite for the obtainment of pregnancy. Eur J Immunol. 2002;32:311–5. doi: 10.1002/1521-4141(200202)32:2<311::AID-IMMU311>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 20.Sher G, Keskintepe L, Fisch J, Acacio B, Ahlering J, Batzofin J. Soluble leukocyte antigen G (sHLA-G) expression in the Phase 1 culture media at 46 hours post fertilization predicts pregnancy and implantation from day 3 embryo transfer. Fertil Steril. 2004; RBM-online article. [DOI] [PubMed]
- 21.Veeck L. Abnormal morphology of human oocytes and conceptus. In: Veeck L editor. Atlas of the human oocyte and early conceptus (vol. 2). 1999. pp. 151–179.
- 22.Menicucci A, Noci I, Fuzzi B, Criscuoli L, Scarselli G, Baricordi O, et al. Non-classic sHLA class I in human oocytes culture medium. Hum Immunol. 1999;60:1054–7. doi: 10.1016/S0198-8859(99)00108-1. [DOI] [PubMed] [Google Scholar]
- 23.Noci I, Fuzzi B, Rizzo R, Melchiorri L, Criscuoli L, Dabizzi S, et al. Embryonic soluble HLA-G as a marker of developmental potential in embryos. Hum Reprod. 2005;20:138–46. doi: 10.1093/humrep/deh572. [DOI] [PubMed] [Google Scholar]
- 24.Fisch J, Keskintepe L, Ginsburg M, Adamowicz M, Sher G. Graduated embryo score and soluble human leukocyte antigen-G expression improve assisted reproductive technology outcomes and suggest a basis for elective single-embryo transfer. Fertil Steril. 2007;87(4):757–63. doi: 10.1016/j.fertnstert.2006.08.088. [DOI] [PubMed] [Google Scholar]
- 25.Yie SM, Balakier H, Motamedi G, Librach CL. Secretion of human leukocyte antigen-G by human embryos is associated with a higher in vitro fertilization pregnancy rate. Fertil Steril. 2005;83(1):30–6. doi: 10.1016/j.fertnstert.2004.06.059. [DOI] [PubMed] [Google Scholar]
- 26.Desai N, Filipovits J, Goldfarb J. Secretion of soluble HLA-G by day 3 human embryos associated with higher pregnancy and implantation rates: asy of culture media using a new ELISA kit. RBM Online. 2006;13(2):272–7. doi: 10.1016/s1472-6483(10)60626-8. [DOI] [PubMed] [Google Scholar]
- 27.Rebmann V, Switala M, Eue I, Schwahn E, Merzenich M, Grosse-Wilde H. Rapid evaluation of soluble HLA-G levels in supernatants of in vitro fertilized embryos. Hum Immunol. 2007;68(4):251–8. doi: 10.1016/j.humimm.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Viganò P, Mangioni S, Pompei F, Chiodo I. Maternal-conceptus cross talk—a review. Placenta. 2003;24(2):S56–61. doi: 10.1016/S0143-4004(03)00137-1. [DOI] [PubMed] [Google Scholar]
- 29.Gal FA, Riteau B, Sedlik C, Khalil-Daher I, Menier C, Dausset J, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol. 1999;11:1351–6. doi: 10.1093/intimm/11.8.1351. [DOI] [PubMed] [Google Scholar]
- 30.Navarro F, Llano M, Garcia P, Lopez-Botet M. NK cell mediated recognition of HLA class Ib molecules: role of CD94/NKG2 receptors. J Reprod Immunol. 1999;43:167–73. doi: 10.1016/S0165-0378(99)00030-3. [DOI] [PubMed] [Google Scholar]
- 31.Hviid TV, Hylenius S, Lindhard A, Christiansen OB. Association between human leukocyte antigen-G genotype and success of in vitro fertilization and pregnancy outcome. Tissue Antigen. 2004;64:66–9. doi: 10.1111/j.1399-0039.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 32.Tesarik J, Kopecny V, Plachot M, Mandelbaum J. Activation of nucleolar and extranucleolar RNA synthesis and changes in the ribosomal content of human embryos developing in vitro. J Reprod Fert. 1986;78:463–70. doi: 10.1530/jrf.0.0780463. [DOI] [PubMed] [Google Scholar]
- 33.Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of pre-implantation development. Nature. 1988;332:459–61. doi: 10.1038/332459a0. [DOI] [PubMed] [Google Scholar]
- 34.Vercammen MJ, Verloes A, Velde H, Haentjens P. Accuracy of soluble human leukocyte antigen-G for predicting pregnancy among women undergoing infertility treatment: meta-analysis. Hum Reprod Update. 2008;14:209–18. doi: 10.1093/humupd/dmn007. [DOI] [PubMed] [Google Scholar]