Abstract
Purpose
This study aims to analyze the relationship between trinucleotide repeat length and reproductive outcome in a large cohort of DM1 patients undergoing ICSI and PGD.
Methods
Prospective cohort study. The effect of trinucleotide repeat length on reproductive outcome per patient was analyzed using bivariate analysis (T-test) and multivariate analysis using Kaplan-Meier and Cox regression analysis.
Results
Between 1995 and 2005, 205 cycles of ICSI and PGD were carried out for DM1 in 78 couples. The number of trinucleotide repeats does not have an influence on reproductive outcome when adjusted for age, BMI, basal FSH values, parity, infertility status and male or female affected. Cox regression analysis indicates that cumulative live birth rate is not influenced by the number of trinucleotide repeats. The only factor with a significant effect is age (p < 0.05).
Conclusion
There is no evidence of an effect of trinucleotide repeat length on reproductive outcome in patients undergoing ICSI and PGD.
Keywords: Myotonic dystrophy, Repeats, Reproductive outcome, ICSI, PGD
Introduction
Myotonic dystrophy type 1 (syn: dystrophia myotonica type 1; DM1; Steinert’s disease; OMIM #160900) is an autosomal dominant disorder, with an incidence of 1/8000 worldwide. The genetic defect is an unstable expansion of the CTG trinucleotide repeat at the 3′ end of the dystrophia myotonica proteine kinase (DMPK) gene. The gene is located on chromosome 19q13.3 and was cloned in 1992 [1, 2]. Expansion of an unstable CTG trinucleotide repeat is frequently observed after parent-to-child transmission in DM1 patients. This explains anticipation, that is an increase in severity of the disease in successive generations and the potential occurrence of the severe, often lethal, congenital form (almost exclusively) in the offspring of affected women [3].
DM1 patients are progressively affected to various degrees by myotonic dysfunction, cardiorespiratory and ophtalmological (cataract) problems [4, 5]. The clinical severity and prognosis of DM1 is highly variable, and is positively correlated with the size of the CTG tract in DM1 alleles [6] (Table 1). Male carriers of DM1 have been shown to be at increased risk of fertility problems, with reduced sperm quality as a result of gonadal atrophy [7]. Reproductive outcome in affected females is less well documented [8].
Table 1.
Clinical classification of myotonic dystrophy
| Type | Number of CTG repeats | Symptoms |
|---|---|---|
| congenital | 1000–5000 | polyhydramnios |
| premature birth | ||
| hypotonia | ||
| respiratory distress | ||
| joint contractures | ||
| severe developmental delay | ||
| childhood | 500–2000 | hypotonia |
| developmental delay | ||
| adult | 100–1000 | myotonia |
| muscle weakness | ||
| cataract | ||
| mild | 50–100 | mild muscle weakness |
| cataract |
While the size of CTG repeat expansion is largely related to clinical symptomatology, it is unclear whether this is also true in relation to the fecundity of the female DM1 patient. This study aims to analyse the effect of the size of CTG repeat expansion present on clinical reproductive outcome in female DM1 patients undergoing intracytoplasmic sperm injection (ICSI) and preimplantation genetic diagnosis (PGD), both in terms of success rates per treatment cycle as well as in terms of cumulative reproductive outcome.
Materials and methods
The study design was a prospective cohort study at a tertiary referral centre for ICSI and PGD.
Patients
78 consecutive couples of which 54 with affected females and 24 with affected males at risk of transmitting DM1 to their offspring were treated between 1995 and 2005. Baseline characteristics such as age, body mass index (BMI), fertility status, parity as well as reproductive outcome data were registered by a dedicated team of research nurses.
DNA fragment sizing
Direct analysis of the (CTG)n expansions with PCR or Southern Blot techniques, allows the accurate detection of all mutations at the DM1 locus with a high sensitivity and specificity. Southern blot analysis using a chemiluminescently labelled probe system (Digoxigenin labelled probe system, Roche Diagnostics) was performed on 10 μg of total genomic DNA extracted from peripheral blood leukocytes of the patients and digested with EcoRI or SacI. The probe was PCR synthesized using the following forward primer 50-TAGGTGGGGACAGAC30 and reverse primer 50-GGGATCACAGACCATTTCTTTCT-30 (NT_011109.15/Hs19_11266; positions 18541243-18541262 and 18541678-18541656). The CAG repeat size was measured in comparison to a molecular marker.
To determine the exact size of healthy or lower range expanded DM1 alleles, labelled PCR products were analysed by capillary electrophoresis on an ABI 3130 Genetic Analyser (Applied Biosystems, Foster City, USA).
Genetic testing with a view to performing PGD
The first single cell assay developed for PGD DM1 allowed the selection of “unaffected” embryos based on the presence in the biopsied cell of the “normal CTG repeat” of the affected partner and on the “normal CTG repeat” of the unaffected partner [9, 10]. Initially only fully informative couples i.e. when the healthy allele of the affected parent differs from the two alleles of the healthy parent were eligible for PGD. Later on, with the development of the TP-PCR based test, half informative couples (i.e. the affected parent and healthy parent share one healthy allele, the second allele of the healthy parent is different) and non informative couples (the healthy allele of the affected parent and the two alleles of the unaffected parent are the same) could be helped as well [11, 12]. It was therefore necessary to analyse the CTG repeat size of the normal alleles in both partners prior to PGD.
Ovarian stimulation and oocyte retrieval
Controlled ovarian stimulation (COS) was carried out in an agonist protocol, using GnRH analogues for pituitary desensitisation (buserelin, Suprefact; Hoechst, Frankfurt, Germany), combined with human menopausal gonadotrophins (hMG) (Menopur, Ferring Pharmaceuticals A/S, Copenhagen, Denmark) or recombinant FSH (Puregon, Schering-Plough, Oss, The Netherlands) [13], or an antagonist protocol using recombinant FSH combined with a GnRH antagonist (ganirelix, Orgalutran, Schering-Plough) [14]. The starting dose of gonadotrophins was determined according to the patient’s age and/or previous response to ovarian stimulation (range 75–450 IU). Human chorionic gonadotrophin (hCG) (10000 IU, Pregnyl; NV Organon or Profasi, Serono, Geneva, Switzerland) was administered when at least three follicles of more than 17 mm mean diameter were seen at transvaginal ultrasound scan. Transvaginal ultrasound-guided oocyte collection was scheduled 36 h after hCG administration. Oocyte collection (OC) was carried out under premedication with pethidine 1 mg/kg IM and paracervical block with mepivacaine hydrochloride [13, 14].
ICSI and embryo biopsy procedure
The details of the in vitro fertilization (IVF) and ICSI procedure have been described previously [15]. Regardless of the sperm quality, ICSI was the method of choice rather than classical IVF to prevent DNA contamination with sperm DNA in PCR-based PGD [16].
Genetic diagnosis in blastomeres
The PCR procedures were performed as previously described [12]. Direct analysis of the CTG repeat size of the DM1 allele was used for fully informative couples. For semi-informative couples or not informative couples the Triplet-Primed PCR (TP-PCR) protocol was applied. A conclusive diagnosis of an unaffected embryo was assigned only if and when two blastomeres gave the same unaffected result for the disorder [10, 12].
Embryo transfer procedure
If available one or more unaffected embryos were transferred into the uterus on day three to five post insemination. As in regular IVF cycles, the age of the patient, the rank of trial and embryo quality determined the number of embryos transferred. For Belgian patients, the number of embryos for transfer was restricted according to age and rules laid out by federal law from July 2003 onwards. Supernumerary unaffected embryos were cryopreserved subject to consent by the patient.
Luteal phase supplementation consisted of intravaginal administration of 600 mg of natural micronized progesterone daily (Utrogestan, Besins, Brussels, Belgium).
Outcome measures
The embryological data and reproductive outcome (mean number of oocytes, mean number of embryos for biopsy, mean number of embryos transferred, clinical pregnancy rate (CPR), cumulative delivery rate) were assessed on a per patient basis.
A biochemical pregnancy was defined as two consecutive positive rising (>15 IU/ml) serum hCG levels. A clinical pregnancy was defined as a gestational sac seen at transvaginal ultrasound scan at least 5 weeks after embryo transfer. An ongoing pregnancy (OPR) was defined as a clinical pregnancy with a fetal heartbeat at ≥12 weeks [17]. In these cases, and without any exception, the couple was advised to undergo prenatal diagnosis in order to confirm the preimplantation diagnosis and to exclude a false negative diagnosis.
A miscarriage was defined as a pregnancy loss before 20 weeks of gestation. A stillbirth was an intra-uterine or intrapartum death of a child born at a gestation of ≥20 weeks or with a birthweight of ≥500 g. A premature delivery was defined as a delivery before 34 weeks’ gestation. A preterm delivery was defined as a delivery at 34 weeks’ gestation or later, and before 37 weeks’ gestation [17]. The live birth delivery rate was defined as the number of live birth deliveries expressed per 100 initiated cycles, oocyte collection cycles (OCC) or embryo transfer (ET) cycles [18]. The reproductive outcome parameters in this study are reported per oocyte retrieval (OR) and per embryo transfer (ET). The real cumulative delivery rate is the observed number of deliveries born at a gestation of ≥20 weeks or with a birthweight of ≥500 g, over a maximum of 6 treatment cycles per patient. The expected cumulative delivery rate using Kaplan-Meier analysis takes into account drop out patients over a maximum of 6 treatment cycles, and calculates the cumulative delivery rate had they not discontinued the treatment [19, 20].
Statistical analysis
The effect of the size of the unstable CTG trinucleotide repeat tract on each reproductive outcome measure (ie in turn, clinical pregnancy, ongoing pregnancy, and live birth delivery) was analyzed using bivariate (t tests; χ² tests) and multivariate (logistic regression) analyses simultaneously adjusted for age, body mass index (BMI), fertility status, parity and CTG trinucleotide repeat size. The effect of these variables including CTG trinucleotide repeat size on cumulative live birth rate was performed with Kaplan-Meier and Cox regression analysis. Cumulative delivery rates are expressed as cumulative percentage probabilities with 95% confidence intervals (95% CI). The maximum number of cycles per patient included was six. Transfers of frozen-thawed embryos were not included in the analysis. SPSS 16.0 for Windows (SPSS Inc, Chicago, Illinois) was used for statistical analysis.
Results
Patient characteristics
Between 1995 and 2005, 205 cycles of ICSI and PGD were carried out for DM1 in 78 couples. In 54 couples the female partner was affected (group A), versus 24 couples with the male partner affected (group B). The patient characteristics did not differ significantly between the two groups. The mean number of CTG repeats overall, taking into account the approximate measurement characteristic to the Southern blotting technique, was 410 (SD 390; range 1280 (min 50 - max 1330)) and did not differ between the female affected and the male affected group (Table 2).
Table 2.
Patient characteristics
| Characteristics | DM1 group | |||
|---|---|---|---|---|
| Total | Group A | Group B | ||
| n | 78 | 54 | 24 | |
| age (mean) | affected female | 31.2 | 31.6 | 30.3 |
| affected male | 33.8 | 34.3 | 32.7 | |
| CTG triplet repeats | 410 (SD 290) | 430 (SD 310) | 350 (SD 215) | |
| reported infertility | 45.90% | 43.40% | 47.80% | |
Bivariate analysis did not reveal a significant difference in BMI, infertility status, male or female affected status or expanded CTG trinucleotide repeat size between the groups with a positive or negative clinical pregnancy, ongoing pregnancy and live birth delivery outcome per treatment cycle with oocyte retrieval (Table 3). The ratio of patients with documented infertility was significantly lower in the group with a positive outcome. According to logistic regression analysis the size of the expanded CTG trinucleotide repeats does not have an influence on reproductive outcome per treatment cycle when adjusted by logistic regression analysis for age, BMI, parity, infertility status and male versus female affected status. Age as an independent factor has a significant influence on CPR (OR 0.83, 95%CI 0.71–0.97) and OPR (OR 0.83, 95%CI 0.71–0.97), as well as on LBDR (OR 0.84, 95%CI 0.72–0.98). Parity as an independent factor has a significant influence on LBDR per treatment cycle (OR 4.42, 95% CI 1.05–18.61) (Table 4).
Table 3.
Bivariate (crude) analysis per cycle with oocyte retrieval of parameters affecting reproductive outcome in female DM1 patients
| Variables | Live birth delivery | No live birth delivery | |
|---|---|---|---|
| n = 41 | n = 104 | ||
| mean age | 31.1 (SD 4.5) | 32.7 (SD 4.0) | P = 0.09 |
| mean body mass index | 23.2 (SD 1.3) | 23.8 (SD 1.1) | P = 0.72 |
| mean number of CTG repeats | 380 (SD 50) | 420 (SD 55) | P = 0.58 |
| mean reported infertility rate | 22 / 40 (55.0%) | 11 / 35 (31.4%) | P < 0.05 |
NS not significant
Table 4.
Logistic regression analysis of patient characteristics potentially affecting reproductive outcome
| Outcome (dependent) variables | |||
|---|---|---|---|
| Predictor (independent) | |||
| Variables | Clinical pregnancy | Ongoing pregnancy | Live birth delivery |
| age | OR 0.83 (95%CI 0.71–0.97) | OR 0.83 (95%CI 0.71–0.97) | OR 0.84 (95%CI 0.72–0.98) |
| infertility | OR 0.50 (95%CI 0.15–1.67) | OR 0.50 (95%CI 0.15–1.67) | OR 0.62 (95%CI 0.19–2.00) |
| multiparity | OR 3.66 (95%CI 0.85–15.7) | OR 3.66 (95%CI 0.85–15.7) | OR 4.42 (95%CI 1.05–18.61) |
| CTG repeats | OR 0.99 (95%CI 0.99–1.0) | OR 0.99 (95%CI 0.99–1.0) | OR 0.99 (95%CI 0.99–1.00) |
| affected status | OR 1.10 (95%CI 0.30–4.00) | OR 1.10 (95%CI 0.30–4.00) | OR 1.05 (95%CI 0.29–3.81) |
OR odds ratio
Significant values are in bold print
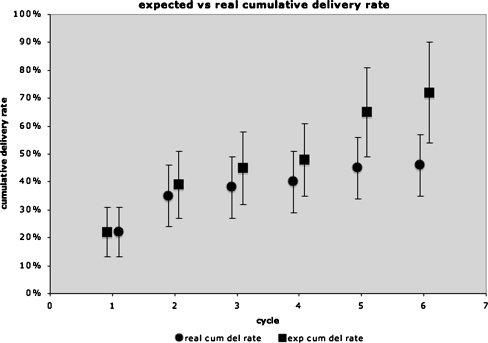
The observed cumulative delivery rate (max 6 cycles) as analysed by Kaplan-Meier overall was 46%. The expected cumulative delivery rate was 72% (Fig. 1). The real and expected cumulative delivery rate in group A was 41% and 81% respectively, compared to 58% and 72% respectively in group B. Cumulative live birth rate is not influenced by the number of trinucleotide repeats as analysed by Cox regression analysis, with simultaneous adjustments for age, multiparity, BMI, infertility status and male or female affected status (p = 0.362).
Fig. 1.
Expected (∎) vs real (●) cumulative delivery rate (percentage) study group total (female + male affected Steinert patients) (n = 78)
The CPR, OPR and LBDR overall (group A + B) per OR and per ET are illustrated in Table 5. The CPR, OPR and LBDR were not significantly different between group A and B. Forty-one deliveries led to the birth of 49 children overall (group A + B), 31 in group A and 18 in group B. The twin pregnancy rate was 19.5% overall, 19.2% in group A and 20.0% in group B. The mean gestational age overall (group A + B) was 38.0 weeks (SD 2.2) with a mean birth weight of 3029.4 g (SD 472.0). The mean gestational age at delivery for singletons and twins was 38.7 (SD 1.8) weeks and 36.5 (SD 2.3) weeks respectively. The mean birth weight for singletons and twins was 3293.9 g (SD 472.0) and 2483.8 g (SD 340.3) respectively.
Table 5.
Reproductive outcome per oocyte retrieval (OR) and per embryo transfer (ET)
| Group A | Group B | |
|---|---|---|
| patients | 54 | 24 |
| cycles with oocyte retrieval (OR) | 145 | 60 |
| cycles with embryo transfer (ET) | 109 | 42 |
| clinical pregnancy rate | 32 | 16 |
| per OR | 22,1% | 26,7% |
| per ET | 29.4% | 38,1% |
| ongoing pregnancy rate | 28 | 15 |
| per OR | 19,3% | 25,0% |
| per ET | 25.7% | 35,7% |
| live birth delivery rate | 26 | 15 |
| per OR | 17,9% | 25,0% |
| per ET | 23.8% | 35,7% |
Group A: female partner affected
Group B: male partner affected
There are no significant differences between any of these results
Discussion
The main finding of this study is that the size of the expanded CTG trinucleotide repeat fragment in DM1 affected females does not affect reproductive outcome per treatment cycle of ICSI and PGD, nor does it affect cumulative live birth delivery rate. The growing numbers of patients undergoing PGD make it increasingly important for accurate outcome data to be generated and assessed, in order to correctly counsel patients and their partner on the reproductive outcome prognosis, and guide them in their decision whether or not to proceed to PGD treatment. PGD patients often do not suffer with infertility (in this study 45.9% documented infertility) and could therefore also opt for spontaneous pregnancy. The results of this study suggest that regardless of the size of the expanded CTG repeat PGD treatment is justified in DM1 patients, as far as reproductive function is concerned and not taking into account the physical status of the patient that may well contraindicate reproductive treatment. The symptomatic condition of DM1 patients and the associated risk of developing cardiac and respiratory insufficiency are largely related to the genetic status, resulting in congenital and childhood types being at a much higher risk of severe morbidity and mortality [21, 22]. Our centre adheres to a policy of pre-treatment assessment of the cardiological and neurological status of all female DM1 patients, in order to anticipate any periconceptional and obstetrical risk, and advize against pregnancy if and when necessary. So far, none of the patients presenting at our clinic have been advized against treatment on the basis of the size of the expanded CTG repeat fragment or their symptomatic status.
Recognizing the limitations of the current study in terms of a potential by-pass effect of ovarian stimulation on the one hand, and in vitro fertilisation (IVF) by ICSI on the other, our regression analysis does not support the hypothesis of impaired fecundity relative to the size of the expanded CTG tracts in female DM1 patients. The pituitary-ovarian axis function and fertility in general in DM1 affected women has commonly been accepted as normal, although oligomenorrhea, abortions and early menopause have been reported [23]. In contrast with this, Feyereisen and colleagues suggested in a recent small series of patients that ovarian response is lower than in controls based on an observed significant delay in day of hCG administration and a higher prevalence of poor quality embryos in the DM1 group [24]. Another small study by Sahu et al similarly suggested a reduced ovarian reserve and ovarian response [25]. A larger study at our centre [8] however could not demonstrate any evidence of gonadal dysfunction in terms of ovarian response to stimulation and cumulative or per cycle reproductive outcome in affected female DM1 patients undergoing ovarian stimulation, ICSI and PGD [8]. Male DM1 patients on the other hand are known to have an increased risk of fertility problems, due to reduced sperm quality as a result of gonadal atrophy. The major lesion in men is believed to be that of seminiferous tubular destruction. Secondary sexual development is usually normal [7].
On the basis that age as an independent factor significantly affects reproductive outcome of IVF/ICSI with PGD for DM1, consideration should be given to counseling DM1 affected females negatively with increasing age. Previous studies have clearly established the inverse correlation between age and reproductive outcome in IVF/ICSI [26]. Health-economic studies should establish the justification of offering reimbursement for those couples requesting PGD where the female partner is over a certain age; until then, the data of the current study provide a means to counsel couples on the expected reproductive outcome and offer them alternatives if and when appropriate. Multiparity does, as expected, improve the chances of having a live birth delivery.
The molecular etiology of DM1 associated reproductive effects and the physiological contribution of DMPK is not entirely clear [27]. DMPK has a role in diverse cellular functions, ranging from modulation of contraction-relaxation cycle and ion handling to cytoskeletal movement and organelle localization in various muscle tissue types, where DMPK is primarily expressed [28, 29]. However little evidence exists on expression patterns in the human hypothalamus, pituitary gland and gonads. The CTG expansion associated with DM1 causes transcriptional silencing of the flanking SIX5 gene. A decrease in Six5 gene expression has been associated with deficient spermatogenesis and a progressive decrease in testicular mass with age. Based on elevated FSH levels, with normal LH and serum testosterone levels, it is speculated that pituitary function could be abnormal in terms of a deficient feed back inhibition of FSH secretion by testosterone and inhibin B. On the other hand, and although the number of Sertoli cells is decreased as a result of Six5 gene mutation, spermatogenesis is rather thought to be impaired by functional abnormalities in Sertoli cell-germ cell and Sertoli cell-Leydig cell interactions, resulting in increased germ cell apoptosis [30]. At present there is insufficient evidence suggesting any effect of deficient Six5 expression on female gametogenesis. It is possible, albeit unestablished, that female DM1 patients may experience infertility, but the etiology is potentially different to that of male DM1 patients and may not be related to effects of Six5 but rather to unlimited DMPK expansion in gametes.
It is known that the expanded CTG repeat is highly unstable in the disease range in both somatic tissues and in the male germline. Also taking into account a potential age effect, the measured blood allele sizes may not reflect the true germline status of the patient, and accurate predictions on transmission and anticipation cannot be made [31]. This implies that the results of this study should be interpreted with caution as far as the predictability of fertility issues in DM1 males and females is concerned. However, since the average allele size in blood shows the best correlation with disease severity and is closest to the inherited progenitor allele [31–33], it remains the best available indicator for likely disease severity and potential fertility problems.
Conclusion
This study shows that there is no evidence of a correlation between the size of the expanded trinucleotide tracts in the DMPK gene and reproductive outcome in female DM1 patients undergoing ICSI and PGD. Age and parity have a significant and independent effect on the relative and cumulative live birth delivery rate. Further studies are required to elucidate the correlation of the size of the expanded trinucleotide repeat tracts with spontaneous reproductive outcome in DM1 patients, and the molecular mechanism involved.
Acknowledgements
The authors thank the clinical, laboratory, technical, nursing and secretarial staff at the Centres for Reproductive Medicine and Medical Genetics at UZ Brussel.
Footnotes
Capsule
The number of CTG repeats in myotonic dystrophy type 1 (DM1) is not correlated with the reproductive outcome of ICSI and PGD.
References
- 1.Brook JD, McCurragh ME, Harley HG, et al. Molecular basis of myotonic dystrophy: expansion of the trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;69:385. doi: 10.1016/0092-8674(92)90418-c. [DOI] [PubMed] [Google Scholar]
- 2.Mahadevan M, Tsilfidis C, Sabourin L, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- 3.Harper PS, Harley HG, Reardon W, Shaw DJ. Review article: anticipation in myotonic dystrophy: new light on an old problem. Am J Hum Genet. 1992;51:10–16. [PMC free article] [PubMed] [Google Scholar]
- 4.Cho DH, Tapscott SJ. Myotonic dystrophy: emerging mechanisme for DM1 and DM2. Review. Biochim Biophys Acta. 2007;1772:195–204. doi: 10.1016/j.bbadis.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Machuca-Tzili L, Brook D, Hilton-Jones D. Clinical and molecular aspects of the myotonic dystrophies: a review. Muscle Nerve. 2005;32:1–18. doi: 10.1002/mus.20301. [DOI] [PubMed] [Google Scholar]
- 6.Gennarelli M, Novelli G, Bassi A, et al. Prediction of myotonic dystrophy clinical severity based on the number of intragenic (CTG) trinucleotide repeats. Am J Med Genet. 1996;65:342–347. doi: 10.1002/(SICI)1096-8628(19961111)65:4<342::AID-AJMG18>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 7.Harper P. Myotonic dystrophy and other autosomal muscular dystrophies. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular basis of inherited disease. New York: McGraw-Hill; 1995. pp. 4227–4253. [Google Scholar]
- 8.Verpoest W, Rademaeker M, Sermon K, et al. Real and expected delivery rates of patients with myotonic dystrophy undergoing intracytoplasmic sperm injection and preimplantation genetic diagnosis. Hum Reprod. 2008;23:1654–1660. doi: 10.1093/humrep/den105. [DOI] [PubMed] [Google Scholar]
- 9.Sermon K, Lissens W, Joris H, et al. Clinical application of preimplantation diagnosis for myotonic dystrophy. Prenat Diagn. 1997;17:925–932. doi: 10.1002/(SICI)1097-0223(199710)17:10<925::AID-PD178>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Sermon K, Vos A, Velde H, et al. Fluorescent PCR and automated fragment analysis for the clinical application of preimplantation genetic diagnosis (Steinert’s disease) Mol Hum Reprod. 1998;4:791–796. doi: 10.1093/molehr/4.8.791. [DOI] [PubMed] [Google Scholar]
- 11.Warner JP, Barron LH, Goudie D, et al. A general method for the detection of large CAG repeat expansions by fluorescent PCR. J Med Genet. 1996;33:1022–1026. doi: 10.1136/jmg.33.12.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sermon K, Seneca S, Rycke M, Goossens V, et al. PGD in the lab for triplet repeat diseases-myotonic dystrophy, Huntington’s disease and Fragile-X syndrome. Mol Cell Endocrinol. 2001;183:S77–S78. doi: 10.1016/S0303-7207(01)00572-X. [DOI] [PubMed] [Google Scholar]
- 13.Velde H, Vos A, Joris H, Nagy ZP, Steirteghem AC. Effect of timing of oocyte denudation and micro-injection on survival, fertilization and embryo quality after intracytoplasmic sperm injection. Hum Reprod. 1998;13:3160–3164. doi: 10.1093/humrep/13.11.3160. [DOI] [PubMed] [Google Scholar]
- 14.Kolibianakis EM, Zikopoulos K, Verpoest W, et al. Should we advise patients undergoing IVF to start a cycle leading to a day 3 or a day 5 transfer? Hum Reprod. 2004;19:2550–2554. doi: 10.1093/humrep/deh447. [DOI] [PubMed] [Google Scholar]
- 15.Landuyt L, Vos A, Joris H, Verheyen G, Devroey P, Steirteghem A. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil Steril. 2005;83:1397–1403. doi: 10.1016/j.fertnstert.2004.10.054. [DOI] [PubMed] [Google Scholar]
- 16.Liebaers I, Sermon K, Staessen C, et al. Clinical experience with preimplantation genetic diagnosis and intracytoplasmic sperm injection. Hum Reprod. 1998;13(suppl 1):186–195. doi: 10.1093/humrep/13.suppl_1.186. [DOI] [PubMed] [Google Scholar]
- 17.Bonduelle M, Liebaers I, Deketelaere V, et al. Neonatal data on a cohort of 2889 infants born after ICSI (1991–1999) and of 2995 infants born after IVF (1983–1999) Hum Reprod. 2002;17:671–694. doi: 10.1093/humrep/17.3.671. [DOI] [PubMed] [Google Scholar]
- 18.Zegers-Hochschild F, Adamson GD, de Mouzon J, et al. International Committee for Monitoring Assisted Reproductive Technology; World Health Organization. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) Revised Glossary on ART Terminology. Hum Reprod. 2009;24:2683–2687. doi: 10.1093/humrep/dep343. [DOI] [PubMed] [Google Scholar]
- 19.Hull MG. Infertility treatment: relative effectiveness of conventional and assisted conception methods. Hum Reprod. 1992;7:785–796. doi: 10.1093/oxfordjournals.humrep.a137738. [DOI] [PubMed] [Google Scholar]
- 20.Osmanogaoglu K, Tournaye H, Camus M, Vandervorst M, Steirteghem A, Devroey P. Cumulative delivery rates after intracytoplasmic sperm injection: 5 year follow up of 498 patients. Hum Reprod. 1999;14:2651–2655. doi: 10.1093/humrep/14.10.2651. [DOI] [PubMed] [Google Scholar]
- 21.White RJ. Case report. Anaesthetic management of a patient with myotonic dystrophy. Paed Anaesth. 2001;11:494–497. doi: 10.1046/j.1460-9592.2001.00710.x. [DOI] [PubMed] [Google Scholar]
- 22.White RJ, Bass SP. Review article. Myotonic dystrophy and paediatric anaesthesia. Paed Anaesth. 2003;13:94–102. doi: 10.1046/j.1460-9592.2003.00889.x. [DOI] [PubMed] [Google Scholar]
- 23.Sagel J, Distiller LA, Morley JE, Isaacs H, Kay G, Walt A. Myotonia dystrophica: studies on gonadal function using luteinizing hormone-releasing hormone (LRH) J Clin Endocrinol Metab. 1975;40:1110–1113. doi: 10.1210/jcem-40-6-1110. [DOI] [PubMed] [Google Scholar]
- 24.Feyereisen E, Amar A, Kerbrat V, et al. Myotonic dystrophy: does it affect ovarian follicular status and responsiveness to controlled ovarian stimulation? Hum Reprod. 2006;21:175–182. doi: 10.1093/humrep/dei310. [DOI] [PubMed] [Google Scholar]
- 25.Sahu B, Ozturk O, Deo N, Fordham K, Ranierri M, Serhal P. Response to controlled ovarian stimulation and oocyte quality in women with myotonic dystrophy type I. J Assist Reprod Genet. 2008;25:1–5. doi: 10.1007/s10815-007-9193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winchester CL, Ferrier RK, Sermoni A, Clark BJ, Johnson KJ. Characterization of the expression of DMPK and SIX5 in the human eye and implications for pathogenesis in myotonic dystrophy. Hum Mol Genet. 1999;8:481–492. doi: 10.1093/hmg/8.3.481. [DOI] [PubMed] [Google Scholar]
- 27.O’Cochlain DF, Perez-Terzic C, Reyes S, et al. Transgenic overexpression of human DMPK accumulates into hypertrophic cardiomyopathy, myotonic myopathy and hypotension traits of myotonic dystrophy. Hum Mol Genet. 2004;13:2505–2518. doi: 10.1093/hmg/ddh266. [DOI] [PubMed] [Google Scholar]
- 28.Amack JD, Mahadevan MS. Myogenic defects in myotonic dystrophy. Dev Biol. 2004;265:294–301. doi: 10.1016/j.ydbio.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 29.Sarkar PS, Paul S, Han J, Reddy S. Six5 is required for spermatogenic cell survival and spermiogenesis. Hum Mol Genet. 2004;13:1421–1431. doi: 10.1093/hmg/ddh161. [DOI] [PubMed] [Google Scholar]
- 30.Martorell L, Monckton DG, Gamez J, Baiget M. Complex patterns of male germline instability and somatic mosaicism in myotonic dystrophy type 1. Eur J Hum Genet. 2000;8:423–430. doi: 10.1038/sj.ejhg.5200478. [DOI] [PubMed] [Google Scholar]
- 31.Thornton CA, Johnson KJ, Moxley RT. Myotonic dystrophy patients have larger CTG expansions in skeletal muscle than in leukocytes. Ann Neurol. 1994;31:518–520. doi: 10.1002/ana.410350116. [DOI] [PubMed] [Google Scholar]
- 32.Monckton DG, Wong L-JC, Ashizawa T, Caskey CT. Somatic mosaicsim, germline expansions, germline reversions and intergenerational reductions in myotonic dystrophy males: small pool PCR analyses. Hum Mol Genet. 1995;4:1–8. doi: 10.1093/hmg/4.1.1. [DOI] [PubMed] [Google Scholar]
- 33.Osmanogaoglu K, Tournaye H, Kolibianakis E, Camus M, Steirteghem A, Devroey P. Cumulative delivery rates after ICSI in women aged >37 years. Hum Reprod. 2002;17:940–944. doi: 10.1093/humrep/17.4.940. [DOI] [PubMed] [Google Scholar]