Abstract
Objective
To evaluate the therapeutic effect of treatment with a combination of the monoclonal antibodies to the VEGFR (DC101) and the EGFR (cetuximab) in an orthotopic nude mouse model of metastatic squamous cell carcinoma of oral tongue (SCCOT).
Design
In vivo study.
Subjects
8- to 12- week-old male athymic nude mice.
Intervention
To develop orthotopic nude mouse models of SCCOT, OSC-19 cells or luciferase-expressing human OSC-19-luc and JMAR-luc cells were injected into the tongues of nude mice. Animals were randomly divided into 4 groups: DC101 alone, cetuximab alone, DC101 plus cetuximab, or placebo, all given twice per week for 4 weeks. The in vivo anti-tumor activity was monitored noninvasively by bioluminescence imaging. Tumors were resected at necropsy, and immunohistochemical and immunoflourescent staining were performed.
Results
At the conclusion of the treatment period, the average tumor volumes in the cetuximab alone and the DC101 plus cextuximab treatment groups had decreased significantly compared with those that received control (68%, P=0.002 and 84%, P=0.0003 respectively). Significant effects of the treatment were also observed in bioluminescence imaging. Mice treated with DC101 plus cetuximab also lived longer and had a lower incidence of neck lymph node metastases compared with the control group (P=0.003).
Conclusion
Treatment with DC101 plus cetuximab inhibited the growth of SCCOT and decreased the incidence of the neck lymph node metastases in vivo. These results suggest that this combination treatment may be an effective stategy against metastatic SCCOT and warrants further pre-clinical trials.
Keywords: squamous cell carcinoma of the oral tongue (SCCOT), cervical lymph node metastasis, EGFR, VEGFR-2, angiogenesis
Introduction
Oral cavity cancer consistently ranks as one of 10 most frequently diagnosed cancers in the world and accounts for 34,000 new diagnoses and 7,500 deaths in 2007 in the United States.1 Squamous cell carcinoma of oral tongue (SCCOT) is the most common tumors of the oral cavity. Despite advances in surgery and radiation therapy, the five-year survival rate for oral cancer has not improved significantly over the past several decades and remains at 50–55 %.2 This is primarily because patients continue to die from metastatic disease, despite some improvement in local control. Although metastasis to cervical lymph nodes is the most reliable predictor of failure of SCCOT treatment,3 the cellular and molecular mechanisms of metastasis in SCCOT are poorly understood. Therefore, the development of new systemic adjuvant strategies for treatment of the SCCOT primary tumor and its metastatic lesions is necessary to provide improved disease control and survival.
Extensive efforts have been made to develop targeted molecular therapies designed to inhibit key signaling pathways involved in tumor growth and dissemination to metastatic sites, and some promising results have been demonstrated in the use of these therapies against various malignant tumors in preclinical and clinical studies.4 Combining these targeted therapies with the goal of increasing efficacy and reducing the toxicity profile is a rapidly emerging therapeutic strategy. One such approach that is showing promise is combined inhibition of the vascular endothelial growth factor (VEGF) and the epidermal growth factor receptor (EGFR).5
VEGF signaling plays a key role in tumor angiogenesis, which is crucial for the progression and metastasis of many types of human cancers, including induction of endothelial cell proliferation, migration, survival, and capillary tube formation.6 Several studies have reported that overexpression of VEGF is associated with poor prognosis and metastases in SCCOT, as VEGF correlates with regional lymph node metastases in SCCOT7–10 and its receptor (VEGFR) is upregulated in tumor development in squamous cell carcinoma of head and neck (SCCHN).11 These findings suggest that the inhibition of angiogenic signaling is an intriguing potential therapeutic target in SCCOT.
The EGFR plays a key role in promoting cellular proliferation and survival.12 Activation of the EGFR also regulates many processes associated with metastasis,13 and its ligand, EGF, has been shown to increase motility, in vitro invasion, and metastatic potential in several different tumor cells.14 EGFR signaling inhibitors have shown significant inhibition of tumor growth in numerous preclinical models.15–17 Overexpression of the EGFR has ranged from 34% to 80% in SCCHN, and this overexpression is associated with poor disease control and decreased survival.18, 19 Pre-clinical studies have provided evidence of the significant therapeutic effects of EGFR inhibitors in SCCHN.20 Furthermore, Bonner et al showed that cetuximab, a monoclonal antibody to EGFR, with radiotherapy improved local-regional control and survival of patients with locoregionally advanced SCCHN compared with treatment with radiotherapy alone in phase III clinical trail.21
Despite the beneficial effects of cetuximab in the treatment of SCCHN, local-regional and distant failure rates remain high in the published clinical trials. Therefore, investigators are trying to identify ways to improve the results of treatment of SCCHN via EGFR inhibition. Some studies have reported the synergistic anti-tumor effects of a targeted therapy combining EGFR and VEGFR.5 In addition, clinical trails combining inhibitors of EGFR and VEGF are already showing promise in non-small cell lung cancer.22 Therefore, the inhibitory effect produced when these 2 signaling pathways are combined may result in greater anti-tumor outcomes against metastatic SCCOT than would be produced by either pathway alone.
Although we have studied the effect of inhibition against EGFR and VEGFR signaling with a small molecule tyrosine kinase inhibitor (TKI) on an orthotopic nude mouse model of SCCOT,20 we have not previously used antibodies to target the combination of EGFR and VEGFR-2. In this study, we hypothesized that inhibition of the EGFR and VEGFR-2 signaling pathways using monoclonal antibodies to the 2 receptors would inhibit the signaling of both, tumor growth, and metastasis in an orthotopic nude mouse model of SCCOT. To test this hypothesis, we investigated the preclinical efficacy of DC101, an anti-mouse monoclonal VEGFR-2 antibody, alone and in combination with cetuximab, an anti-EGFR monoclonal antibody, against established invasive and metastatic SCCOT tumors in an orthotopic nude mice model using a bioluminescence image system.
METHODS
Animals and maintenance
Male athymic nude mice, age 8 to 12 weeks, were purchased from the animal production area of the National Cancer Institute-Frederick Cancer Research and Development Center (Frederick, MD). The mice were housed and maintained in laminar flow cabinets under specific pathogen-free conditions in facilities approved by the American Association for Accreditation of Laboratory Animal Care in accordance with current regulations and standards of the U.S. Department of Agriculture, the U. S. Department of Health and Human Services, and the National Institutes of Health. The mice were used in accordance with the Animal Care and Use Guidelines of M. D. Anderson Cancer Center under a protocol approved by the Institutional Animal Care Use Committee.
Cell lines and culture conditions
For these studies, we used the invasive oral SCC line JMAR23 and the metastatic oral SCC line OSC-19. The OSC-19 line was obtained from the laboratory of Dr. Faye Johnson (M. D. Anderson). This cell line was established in Japan with cells from a patient with a well-differentiated SCCOT that metastasized to a cervical lymph node.24 The OSC-19 and JMAR cells were retrovirally infected with the green fluorescent protein (GFP) and the luciferease gene. For construction of the retroviral luciferase vector, a polymerase chain reaction product of luciferase cDNA was amplified from PGL3 vector (Promega, Madison, WI) and cloned into pBMN-I-GFP (Dr. Garry. P. Nolan, Stanford University) to generate the pBMN-I-Luc-GFP. The pBMN-I-Luc-GFP vector was transfected into Phoenix cells to generate a Luc-expressing retrovirus that was subsequently used to infect OSC-19 and JMAR cells. Luc-transduced stable OSC-19 and JMAR cells were obtained by sorting GFP-positive cells for green fluorescence by FACSscan (Becton Dickinson, Franklin Lakes, NJ). All cell lines were grown in vitro in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, L-glutamine, sodium pyruvate, nonessential amino acids, and a 2-fold vitamin solution (Life Technologies, Inc., Grand Island, NY). Adherent monolayer cultures were maintained on plastic and incubated at 37°C in 5% carbon dioxide and 95% air. The cultures were free of Mycoplasma species and were maintained for no longer than 12 weeks after recovery from frozen stocks.
Reagents
The monoclonal rat antimouse VEGFR-2 antibody DC101 was provided by Imclone (New York, NY).25 For in vivo administration, DC101 was provided undiluted at a concentration of 6.53 mg/mL and cetuximab (Imclone) was provided undiluted at a concentration of 2 mg/mL. Previous studies have shown that nonspecific immunoglobulin G (IgG) antibody developed in a similar fashion had no effect on tumor growth, similar to the effect of solvent, phosphate-buffered saline (PBS).25 The following antibodies were purchased for immunohistochemical analysis: rabbit polyclonal anti-EGFR and anti-VEGFR-2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), rabbit polyclonal anti-phospho–VEGFR-2 (pVEGFR-2; Oncogene, Cambridge, MA), rabbit polyclonal anti–phospho-EGFR (pEGFR; Biosource International, Camarillo, CA), mouse anti–proliferating cell nuclear antigen (PCNA) clone PC-10 (DAKO A/S, Copenhagen, Denmark), rat monoclonal anti-CD31/platelet/endothelial cell adhesion molecule 1 (CD31/PECAM-1) (PharMingen, SanDiego, CA), peroxidase-conjugated goat anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), peroxidase-conjugated rat anti-mouse IgG2a (Serotec; Harlan Bioproducts for Science, Inc., Indianapolis, IN), peroxidase-conjugated goat anti-rat IgG1 (Jackson Research Laboratories), and Alexa Fluor 594–conjugated goat anti-rat IgG and Alexa Fluor 488–conjugated anti-rabbit IgG (Molecular Probes, Eugene, OR).
Establishment of an orthotopic nude mouse model of SCCOT
OSC-19-luc, OSC-19, and JMAR-luc cells were harvested from subconfluent cultures by trypsinization and washed. The orthotopic nude mouse model of SCCOT was established by injecting OSC19-luc (3 × 104), OSC-19 (3 × 104), or JMAR-luc (1 × 105) cells suspended in 30 μL of serum-free Dulbecco’s modified Eagle’s medium into the mouse tongue as described previously.26 Eleven to 13 days after the injection of SCCOT cells, when tumors were already established, mice of similar tumor size and approximately equivalent tumor bioluminescence were randomized into 4 groups according to treatment (6–9 mice per group): placebo control, cetuximab alone, DC101 alone, and DC101 plus cetuximab. Drugs were administered intraperitoneally twice a week in the following doses: 1) 500μL phosphate-buffered saline (placebo (control), 2) 800 μg DC101, 3) 1 mg cetuximab, and 4) 800 μg DC101 plus 1 mg cetuximab. The mice were treated for 4 weeks. They were examined twice a week for weight loss and euthanized by carbon dioxide asphyxiation if they lost more than 20% of their preinjection body weight or became moribund. The remaining mice were sacrificed at 28 days post-treatment. Mice were necropsied, with removal of tongue tumors and cervical lymph nodes. Half of the tumors were fixed in formalin and embedded in paraffin for immunohistochemistry and hematoxylin-eosin (H&E) staining. The other half were embedded in OCT compound (Miles, Inc., Elkhart, IN), rapidly frozen in liquid nitrogen, and stored at −80°C. The cervical lymph nodes were also embedded in paraffin and sectioned, stained with H&E, and evaluated for the presence of metastases. At the time of the necropsy, the tumor sizes were measured with microcalipers. Tumor volume (V) was calculated using the formula “V = (A)(B2)π/6,” where A is the longest dimension of the tumor and B is the dimension of the tumor perpendicular to A. The percentage of tumor inhibition was calculated using the formula “[1 - (T/C)] ×100,” where T and C represent the mean tumor volumes of the treatment group and the control group, respectively.
Imaging of orthotopic tumors
Bioluminescence of the tongue tumors through standardized regions of interest was quantified using Living Images (Xenogen, Alameda, CA). Animals were anesthetized with 2% isoflurane (Abbott, Abbott Park, IL) before and during imaging. An aqueous solution of luciferin (Xenogen) at 150 mg/kg in a volume of 0.1 mL27 was injected intraperitoneally 5 min prior to imaging. Animals were imaged at a peak time of 10 min after luciferin injection via a IVIS 200 imaging system (Xenogen). The photons emitted from the luciferase-expressing cells within the animal were quantified using the software program Living Image (Xenogen) as an overlay on Igor (Wavemetrics, Seattle, WA). The photon flux was calculated for each mouse using a rectangular region of interest encompassing the head and neck region of the mouse in a dorsal position. Animals were imaged after xenografting immediately (day 0) and on an almost weekly basis. Before use in vivo, engineered OSC-19-luc and JMAR-luc cells were confirmed in vitro to homogeneously express high levels of luciferase as monitored by the IVIS imaging system.
Immunohistochemical-immunofluoresence analysis and immunofluorescence double-staining analysis
To examine the activity of the treatment, tumor specimens of 4 or more mice from each group were processed for routine histologic and immunohistochemical analyses. Paraffin-embedded tissues were prepared for detection of PCNA. Frozen tissues were used for detection of CD31/PECAM-1 and terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). Slides were prepared as previously described.20 Immunostaining for PCNA (1:50) and CD31/PECAM-1 (1:400) was performed using the methods previously described.20 The TUNEL staining assay was carried out using an apoptosis detection kit (Promega, Madison, WI). For CD31-TUNEL double staining, TUNEL staining was completed on slides already labeled with anti-CD31 antibody, as described above. Double staining for CD31/EGFR (1:200), CD31/activated-EGFR (1:50), CD31/VEGFR-2 (1:200), and CD31/activated VEGFR-2 (1:200) was done as previously described.20 Immunofluorescence microscopy was carried out using a Leica DMLA microscope (Leica Microsystems, Bannockburn, IL) equipped with a 100-watt HBO mercury bulb and filter set (Chroma Inc., Brattleboro, VT) to individually capture red and blue fluorescent images. Images were captured using a cooled charged-coupled device Hamamatsu 5810 camera (Hamamatsu Corp., Bridgewater, NJ) and ImagePro Plus 6.0 software (Media Cybernnetics, Silver Spring, MD). Images at the stained sections were produced in the same microscope equipped with a three-chip-charged couple device color video camera (model DXC990; Sony Corp., Tokyo, Japan). Photomontages were prepared using Adobe Photoshop software (Adobe Systems, Inc., San Jose, CA). Photomontages were printed on a Sony digital color printer (model UP-D7000).
Quantification of PCNA, microvessel density, apoptotic tumor, and endothelial cells
For the quantification analysis, 4 slides were prepared for each group, and 3 areas were selected on each slide. The percentage of stained cells among the total number of cells in each area and the average proportion of stained cells in each group were calculated and compared. For quantification of TUNEL and PCNA expression, the cells positively stained were counted in 10 random 0.04-mm2 fields at ×200 magnification per slide. To quantify microvessel density (MVD), areas containing higher numbers of tumor-associated blood vessels were identified at low microscopic power (×100). Vessels completely stained with anti-CD31 antibodies were counted in 10 random 0.04-mm2 fields at ×200 magnification per slide. Quantification of apoptotic endothelial cells was expressed as the average of the ratios of apoptotic endothelial cells to the total number of endothelial cells in 10 random 0.04-mm2 fields at ×200 magnification.
Statistical analysis
The Wilcoxon rank-sum test was used to compare the differences in mice tumor volume, bioluminescence, and mice weight between the control and treatment groups on each day, with a significance level of P < 0.01. Associations between treatment groups and incidence of neck lymph node metastases were analyzed using the Fisher’s exact test. Survival was analyzed by the Kaplan-Meier method and compared with log-rank tests. The quantification of the immunohistochemical expression of MVD, TUNEL, PCNA, and CD31/TUNEL were compared using the Wilcoxon rank-sum test, with a significance level of P < 0.01.
Results
Inhibition of tumor growth in an orthotopic nude mouse model of SCCOT
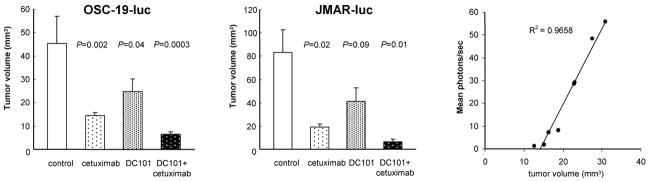
As shown in Figures 1A and 1B (respectively), at the end of treatment day 42, there was a significant decrease in the size of tumors treated with DC101 plus cetuximab and with cetuximab alone compared with tumors injected with placebo (control group; P=0.0003 and P=0.002, respectively). The median tumor volume of mice treated with DC101 alone was also lower than that of mice in the control group, but the difference was not statistically significant (P=0.04). DC101 alone, cetuximab alone, and the combination treatment led to a 45%, 68%, and 84% decreases, respectively, in the tumor volumes of xenografts nude mice models of SCCOT generated with an OSC-19-luc cell line. We also confirmed the anti-tumor effect of these treatments in the orthotopic nude mice model of SCCOT generated by the OSC-19 and JMAR-luc cell lines. The combination of DC101 and cetuximab produced significant inhibition of tumor growth in orthotopically implanted OSC-19 JMAR-luc cells as shown Figure 1B (OSC-19; data not shown).
Figure 1.
DC101, cetuximab, and combination therapy (DC101 plus cetuximab) inhibited the growth of SCCOT xenografts in nude mice.
A, OSC-19-luc cells were injected into the tongues of nude mice. Eleven days later, mice were randomized and treated with placebo, DC101 (800 ug), cetuximab (1 mg), and DC101 (800 ug) plus cetuximab (1 mg) given via intraperitoneal injection twice a week. Tumors were measured at the end of treatment (day 42). Columns, mean tumor volume; bars, SE.
B, JAMR-19-luc cells were injected into the tongues of nude mice. Eleven days later, mice were randomized and treated with placebo, DC101 (800 ug), cetuximab (1 mg), and DC101 (800 ug) + cetuximab (1 mg) given via intraperitoneal injection twice a week. Tumors were measured at the end of treatment. Columns, mean tumor volume; bars, SE.
C, Linear correlation between mean photons per second measured by the IVIS imaging system and tumor volume measured by the caliper.
Reduction of bioluminescence in orthotopic SCCOT tumors
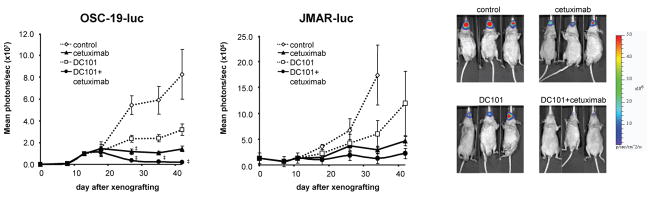
To see the effects of the treatment, we monitored the bioluminescence intensity of OSC-19-luc cells. Prior to this, a pilot experiment was performed with 8 untreated mice to optimize the system and to establish the relationship between tumor volume and mean photons per second. At the end of the experiment, the 7 remaining mice were imaged and euthanized on the same day, and their tongue tumors were excised. Figure 1C shows the relationship between mean photons per second and tumor volume (as measured by calipers). The results show that photons per second are a relative measure of tumor volume in our SCCOT orthotopic nude mice in this system. As shown in Figure 2A, in this bioluminescence imaging system, a significant reduction of bioluminescence was detected at the end of treatment (day 42) in mice treated with DC101 plus cetuximab (mean light reduction 97%, P=0.0007). The mean bioluminescence intensities of mice in the DC101 group and the cetximab group were also lower than those of mice in the control group; however, the differences were not statistically significant (mean light reduction 62% [P=0.09] and 83% [P=0.02], respectively). A significant reduction of bioluminescence was detected on the final day.
Figure 2.
The anti-tumor effects of the combination of EGFR plus VEGFR-2 targeted therapy against SCCOT xenograft mouse model monitored by bioluminescence imaging.
A, The effects of treatment on OSC-19-luc orthotopic tumor were followed by bioluminescence imaging. Points, mean (n = 8–9 animals per group); bars, SE. ‡, P < 0.05, compared with the control groups by Student’s t test.
B, The effects of treatment on JMAR-luc orthotopic tumor were followed by bioluminescence imaging. Points, mean (n = 6 animals per group); bars, SE. ‡, P < 0.05, compared with the control groups by Student’s t test. Photon counts were calculated from the imaging data using the IVIS Living Image software.
C, Representative bioluminescence images corresponding to OSC-19-luc tumors from each treatment group, at the end of the study (day 42).
Similarly, these anti-tumor effects of cetuximab alone and DC101 alone were also observed in the JMAR-luc orthotopic model (Figure 2B). Bioluminescence intensity was lower in the combination treatmentd group than in the control group at all points; however, the difference did not reach significance. Each therapy was well tolerated by the animals without significant side effects, as determined by the maintenance of body weight (data not shown).
Improvement in survival in an orthotopic nude mouse model of SCCOT
Animals injected with JMAR-luc cells were kept alive until they met some or all criteria for sacrifice (i.e., large tumor volume, significant weight loss, hunched posture, and ruffled coat). All of the mice in the control group in the survival study met the criteria for sacrifice by day 35, mostly due to weight loss. The median survival periods for the control, DC101, cetuximab, and combination treatment groups were 28, 42, 40, and 42 days, respectively. The differences in survival between the groups were statistically significant by log-rank test (P < 0.001). All treatment group animals survived longer than the control group animals. No significant difference was found between the cetuximab and combination treatment groups; however, both of these groups appeared to have the longest survivals without any deaths. When compared to animals treated with DC101 alone, however, the differences in survival durations did not reach statistical significance (Figure 3 and Table 1).
Figure 3.
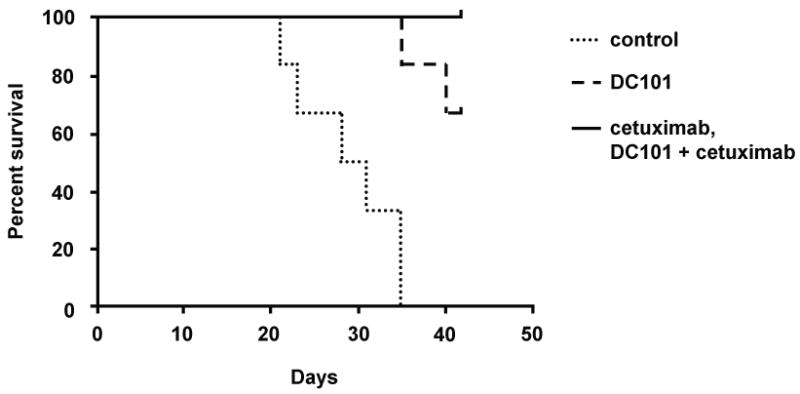
Kaplan-Meier survival curve showing the effects of EGFR plus VEGFR-2 targeted therapy on the survival of nude mice bearing orthotopic SCCOT xenografts.
P values were <0.001 for cetuximab and combination treatment groups when compared with the control groups.
Table 1.
Effects of cetuximab and DC101 on the incidence of lymph node metastasis in nude mice bearing orthotopic SCCOT xenografts
| Treatment groups | % mice with cervical lymph node metastasis | * P value vs control |
|---|---|---|
| placebo | 10/16 (62.5%) | - |
| cetuximab | 5/16 (31.3%) | 0.07 |
| DC101 | 5/16 (31.3%) | 0.07 |
| DC101 + cetuximab | 1/16 (6.3%) | 0.003 |
The combination treatment inhibited the incidence of cervical lymph node metastases markedly, with only one mouse, and the difference between combination-treatment group and the control group was highly significant (P=0.003). Although there were no significant differences, treatment with DC101 plus cetuximab resulted in a lower rate of local-regional metastases compared with the other treatment groups (P=0.07 and 0.07 for the cetuximab and DC101 groups, respectively).
Decreased incidence of cervical lymph node metastases in an orthotopic nude mouse model of SCCOT
In the groups of animals with OSC-19-luc and OSC-19 tumors, cervical lymph nodes were harvested and examined histologically to identify cervical lymph node metastases. As shown in Figure 3 and Table 1, the cervical lymph node metastases were detected in 10 (62.5%) of the 16 control mice, 5 (31.3%) of the 16 cetuximab-treated mice, 5(31.3%) of the 16 DC101-treated mice, and 1 (6.3%) of the 16 mice in the combination therapy group. Thus, combination treatment inhibited the development of cervical lymph node metastases markedly, and the differences in outcomes in the combination-therapy and control groups were significant (P=0.003). The difference in metastasis in the control group and that in the DC101-and cetuximab-treated groups did not reach statistical significance (Figure 4). The difference in metastasis in the combination-treatment group and the DC101- and cetuximab-treated groups also did not reach statistical significance. In the groups of animals with JMAR-luc tumors, only 1 neck lymph node metastasis was found in the group treated with DC101 (data not shown).
Figure 4.
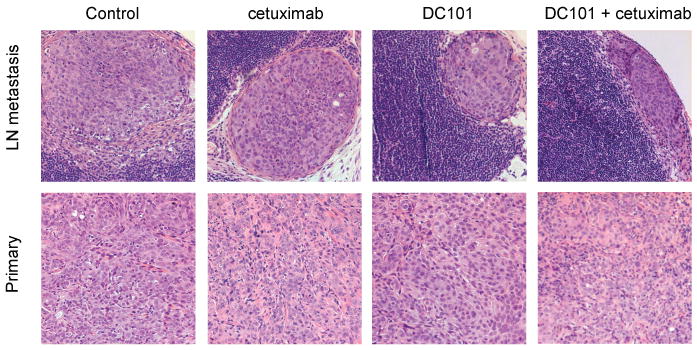
Cervical lymph node of SCCOT xenografts with subcapsular metastasis (*).
Significant decrease in MVD, inhibition of cell proliferation, and induction of apoptosis of tumor cells and in tumor-associated endothelial cells with combination treatment
To clarifiy the mechanism of the antiangiogenic effects of DC101 and cetuximab, we stained tumor sections for CD31-specific antibodies and determined the MVD by the number of CD31-positive microvessels (Figure 5). OSC-19 cells were injected into the tongues of nude mice. Eleven days later, mice were randomized and treatment was started with placebo, DC101 (800 ug), cetuximab (1 mg), or DC101 (800 ug) + cetuximab (1 mg), Mice were sacrificed after 14 days of treatment. Primary tumors were harvested and stained with specific antibodies. As shown in Table 2, the MVD was highest in the control group. The tumors of mice treated with DC101 and with DC101 in combination with cetuximab showed significantly lower MVDs compared to controls (41% and 64%, respectively; P <0.01). Treatment with cetuximab decreased MVD by 30%; however, the difference compared with that of control was not significant. To examine in vivo cell proliferation and apoptosis, antibodies were used against PCNA and the TUNEL assay, respectively. PCNA-positive cells were abundant in the control group and decreased in treated tumors (Figure 5). As shown in Table 2, the mean percentage of PCNA-positive tumor cells in the control group was 75.2 ± 10.2. Compared with the controls, significantly lower percentages of PCNA-positive tumor cells (P < 0.01) were detected in the DC101-treated group (58.3 ± 12.5), cetuximab-treated group (53.4 ± 10.4) and DC101 plus cetuximab–treated group (41.0 ± 11.6). Although TUNEL-positive cells were rarely detected in tumors from the control mice, a progressive increase in the green fluorescent apoptotic cells was found in the tumors from the treated mice (Figure 5). The percentage of TUNEL positive cells in the control group was 1.6 ± 0.1. Compared with the controls, significantly higher percentages of TUNEL-positive tumor cells were detected in the DC101-treated group (8.7 ± 3.0), cetuximab-treated group (16.5 ± 9.1), and DC101 plus cetuximab–treated group (35.0 ± 13.4) (P <0.01; Table 2). Finally, double staining for CD31 (red staining)/TUNEL (green staining) revealed that the percentage of apoptotic endothelial cells (yellow staining) was significantly higher in the tumors of mice treated with cetuximab alone, DC101 alone, and DC101 plus cetuximab than in the control group (P <0.01; Table 2; Figure 5).
Figure 5.
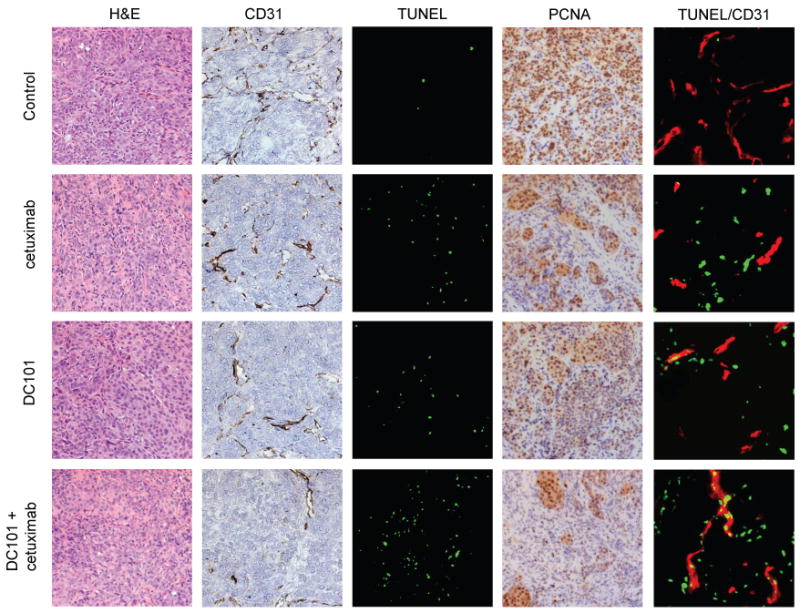
Immunohistochemical analyses of OSC-19 tumors in xenograft nude mice.
Tumors were harvested after 14 days of treatment and representative sections obtained from OSC-19 tumors were stained for H&E and immunostained for expression of CD31 (endothelial cell marker), PCNA (tumor cell proliferation), and TUNEL (tumor cell apoptosis). Magnification, ×100. Double staining for CD31 (red)/TUNEL (green) was also performed to reveal induction of apoptosis in tumor-associated endothelial cells. Apoptotic endothelial cells were identified as a merge of red and green fluorescence. Magnification, ×200.
Table 2.
Quantitative immunohistochemical analysis of OSC-19 tumors in the tongues of nude mice
| Variable | Treatment group |
|||
|---|---|---|---|---|
| # tumor cells, mean±SD | control | cetuximab | DC101 | DC101 + cetuximab |
| TUNEL (%)† | 1.6 ± 0.1 | 16.5 ± 9.1‡ | 8.7 ± 3.0‡ | 35.0 ± 13.4‡ |
| PCNA (%)† | 75.2 ± 10.2 | 53.4 ± 10.4‡ | 58.3 ± 12.5‡ | 41.0 ± 11.6‡ |
| Endothelial cells, mean±SD | ||||
| MVD|| | 38.2 ± 9.9 | 26.9 ± 4.2 | 22.5 ± 7.3‡ | 13.9 ± 5.5‡ |
| CD31/TUNEL (%)** | 0.3 ± 0.3 | 6.1 ± 2.0‡ | 15.1 ± 7.8‡ | 24.9 ± 10.8‡ |
PCNA and TUNEL positivity was quantitated as the ratio of positively stained cells/total cells in 10 random 0.04-mm2 fields at ×200 magnification per slide
MVD was determined by measuring the number of completely stained blood vessels in 10 random 0.159-mm2 fields at ×100 magnification.
CD31/TUNEL positivity was quantitated as the ratio of CD31/TUNEL–positive cells/total endothelial cells in in 10 random 0.04-mm2 fields at ×200 magnification per slide.
P < 0.01 as compared with controls
Inhibition of EGFR and VEGFR-2 phosphorylation with DC101 plus cetuximab
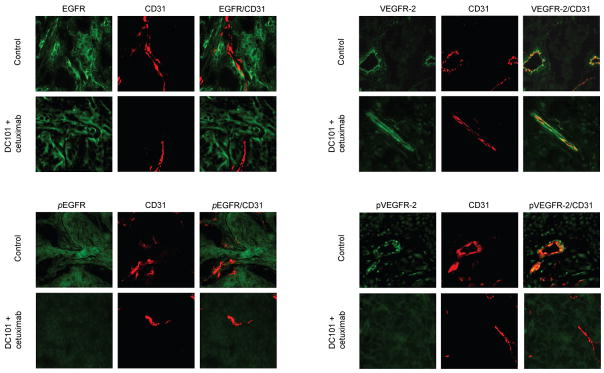
To determine whether treatment with DC101 plus cetuximab inhibits phosphorylation of the targeted receptors EGFR and VEGFR-2, double staining for CD31/activated EGFR and CD31/EGFR, which was done with CD31 (red staining)/total EGFR and activated EGFR (green staining), CD31/total VEGFR-2 and activated VEGFR-2 (green staining) was performed. Tumors from the control group or the DC101 plus cetuximab–group showed similar levels of EGFR expression (Figure 6A), whereas only tumors from control mice or mice treated with DC101 stained positive for phosphorylated EGFR, a finding consistent with inhibition of EGFR autophosphorylation in vivo. In addition, the status of EGFR activation in endothelial cells (double staining: yellow color) was also significantly suppressed in OSC-19 tumors of mice treated with DC101 plus cetuximab (Figure 6B). The level of expression of VEGFR-2 on endothelial cells showed double staining (yellow color) of fluorescent CD31 staining (red) specific for endothelial cells with fluorescent green staining of VEGFR-2 and did not vary significantly among the tumor and endothelial cells from mice in all 4 treatment groups (Figure 6C); however, treatment with the combination of DC101 plus cetuximab decreased double staining (yellow color) for these markers, a finding consistent with reduced signaling through VEGFR-2 in tumor endothelial (Figure 6D).
Figure 6.
Double immunofluorescent staining of OSC-19 tumors in xenograft nude mice.
Tumors were harvested from control group or from the DC101 plus cetuximab group. The sections were immunostained for expression of A, CD31(endothelial cells marker, red)/EGFR: B, CD31/activated EGFR; C, CD31/VEGFR-2; and D, CD31/activated VEGFR-2. Magnification, ×200.
DISCUSSION
In this study, we found that blockade of the EGFR and VEGFR-2 pathways by DC101 and cetuximab inhibits not only orthotopic tumor growth of SCCOT but also the incidence of cervical lymph node metastases in nude mice. Our data also demonstrated that the combination of DC101 and cetuximab prolonged survival and led to significant suppression of proliferation, vascularity, and phosphorylation of these 2 receptors in vivo and significant enhancement of apoptotic cells in both tumor and endothelial cells in our SCCOT orthotopic nude mouse model.
The presence of cervical lymph node metastasis is a critical event for patients with SCCOT, as this is the most accurate predictor of poor treatment outcome. More than 30% of patients with SCCOT can be expected to have cervical lymph node metastases, even if abnormal lymph node metastases are not detected clinically.28 The best way to manage cervical lymph node metastases remains controversial, and we cannot always predict cervical lymph node metastasis from the size and extent of invasion of the primary tumors. Therefore, the development of effective therapies in metastatic SCCOT is required. We developed an orthotopic nude model of SCCOT by injecting metastatic SCCOT cells (OSC-19 and OSC19-luc) and invasive SCCOT cells (JMAR-luc) into the tongues of athymic mice. Our SCCOT mouse model follows the metastatic pattern of human tumors that we have reported previously,26 Using this tongue tumor model, we were able to evaluate the effect of targeted systemic agents on cervical lymph node metastases.
We also showed that the combination with DC101 plus cetuximab inhibits tumor growth of OSC-19-luc cells and JMAR-luc cells compared to control in vivo. This finding is consistent with reports of this treatment in other pre-clinical models5, 29 and with our reports that treatment of SCCOT with small-molecule TKIs inhibited EGFR and VEGFR-2 signaling in our SCCOT ortothopic model.20
Combination treatment was found to inhibit the development of cervical lymph node metastasis in the orthotopic nude mouse model. Our findings are consistent with a study that reported an inhibitory effect of DC101 plus paclitaxel on metastasis of bladder cancer.30 The process of metastasis is complex, and the genetic and biochemical determinants remain incompletely understood in most cancers, including SCCOT. However, angiogenesis is thought to play an important role in the proliferation of primary tumors by maintaining a supply of oxygen and nutrients that support tumor growth and metastasis.31 On the other hand, the EGFR signaling pathway is one of the major pathways regulating tumor proliferation, which is required at the secondary site to establish metastasis. Several prior studies have reported that overexpression of VEGF, which plays a major role in angiogenesis, is associated with poor prognosis and metastases in SCCOT.7–10 In addition, EGFR overexpression is a strong predictor of decreased survival in SCCHN.19 Moreover, the VEGF and EGFR pathways seem to be closely related, particularly with respect to angiogenesis in many tumors. The EGFR pathway increases angiogenesis by upregulating VEGF or other key mediators in the angiogenic process,32 and EGFR blockade results in downregulation of pro-angiogenic mediators in preclinical models,33 In the present study, combination therapy with DC101 plus cetuximab potently inhibited the phosphorylation of these 3 receptors, significantly induced both endothelial and tumor apoptosis, and decreased tumor MVD and proliferation in OSC-19 cells in vivo. These findings are consistent with other reports.20, 29
Lastly, the combination treatment significantly prolonged survival in the orthotopic nude mouse model of SCCOT. At the same time, treatment with cetuximab alone also showed a significant effect on the survival rate in the SCCOT model. According to this survival analysis and the inhibitory effect of tumor growth, it is possible that treatment with only cetuximab may be enough to treat our SCCOT model, whereas treatment with DC101 alone showed only limited anti-tumor effects. However, in our findings, cetuximab combined with blockade of VEGFR-2 signaling was very useful in preventing the incidence of cervical lymph node metastases. Treatment with DC101 alone showed an inhibitory effect on the incidence of cervical lymph node metastases, and the combination of cetuximab and DC101 showed marked inhibition, whereas the effect of cetuximab treatment against the incidence of cervical lymph node metastases did not reach significance.
On the basis of promising preclinical results, angiogenesis inhibitors such as bevacizumab, a humanized anti-VEGF antibody, and small-molecule TKI of VEGFR-2 have been studied extensively in pre-clinical models and clinical trials, including SCCHN,34 and cetuximab demonstrated a significant improvement in median overall survival compared with radiotherapy alone in a phase III trial against SCCHN.21 Clinical trials with EGFR and VEGFR-2 inhibitors, such as vandetanib, are also underway in SCCHN.
In summary, targeted therapy combining the EGFR and VEGFR pathways with monoclonal antibodies showed significant anti-tumor activity against an orthotopic mouse model of SCCOT and also inhibited the incidence of cervical lymph node metastases in vivo. This treatment blocked these receptors’ phosphorylation, inducing both endothelial apoptosis and tumor apotosis and decreasing tumor MVD and proliferation. These results suggest that this combination treatment may be an effective strategy against metastatic SCCOT and warrants further pre-clinical trials.
Acknowledgments
This work was supported by the M. D. Anderson Cancer Center SPORE in Head and Neck Cancer Grant P50 CA097007A, ImClone, The University of Texas M. D. Anderson Cancer Center PANTHEON Program, Award Number T32CA060374 from the National Cancer Institute, and the NIH Cancer Center Support Grant CA16672.
We thank Carol M. Johnston for her technical assistance with the immunohistochemical stainings and Vickie J. Williams for her critical editorial review of the manuscript.
Footnotes
This work was presented at the 7th International Conference on Head and Neck Cancer, July 19-23, 2008, San Francisco, CA
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007 Jan-Feb;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Silverman S., Jr Demographics and occurrence of oral and pharyngeal cancers. The outcomes, the trends, the challenge. J Am Dent Assoc. 2001 Nov;132( Suppl):7S–11S. doi: 10.14219/jada.archive.2001.0382. [DOI] [PubMed] [Google Scholar]
- 3.Grandi C, Alloisio M, Moglia D, et al. Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg. 1985 Nov-Dec;8(2):67–73. doi: 10.1002/hed.2890080202. [DOI] [PubMed] [Google Scholar]
- 4.Baselga J. Targeting tyrosine kinases in cancer: the second wave. Science. 2006 May 26;312(5777):1175–1178. doi: 10.1126/science.1125951. [DOI] [PubMed] [Google Scholar]
- 5.Tonra JR, Deevi DS, Corcoran E, et al. Synergistic antitumor effects of combined epidermal growth factor receptor and vascular endothelial growth factor receptor-2 targeted therapy. Clin Cancer Res. 2006 Apr 1;12(7 Pt 1):2197–2207. doi: 10.1158/1078-0432.CCR-05-1682. [DOI] [PubMed] [Google Scholar]
- 6.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003 Jun;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 7.Smith BD, Smith GL, Carter D, Sasaki CT, Haffty BG. Prognostic significance of vascular endothelial growth factor protein levels in oral and oropharyngeal squamous cell carcinoma. J Clin Oncol. 2000 May;18(10):2046–2052. doi: 10.1200/JCO.2000.18.10.2046. [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto K, Sasaki A, Yoshihama Y, Mese H, Tsukamoto G, Matsumura T. Expression of vascular endothelial growth factor-C predicts regional lymph node metastasis in early oral squamous cell carcinoma. Oral Oncol. 2003 Jun;39(4):391–396. doi: 10.1016/s1368-8375(02)00143-4. [DOI] [PubMed] [Google Scholar]
- 9.Uehara M, Sano K, Ikeda H, et al. Expression of vascular endothelial growth factor and prognosis of oral squamous cell carcinoma. Oral Oncol. 2004 Mar;40(3):321–325. doi: 10.1016/j.oraloncology.2003.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Tanigaki Y, Nagashima Y, Kitamura Y, Matsuda H, Mikami Y, Tsukuda M. The expression of vascular endothelial growth factor-A and -C, and receptors 1 and 3: correlation with lymph node metastasis and prognosis in tongue squamous cell carcinoma. Int J Mol Med. 2004 Sep;14(3):389–395. [PubMed] [Google Scholar]
- 11.Neuchrist C, Erovic BM, Handisurya A, et al. Vascular endothelial growth factor receptor 2 (VEGFR2) expression in squamous cell carcinomas of the head and neck. Laryngoscope. 2001 Oct;111(10):1834–1841. doi: 10.1097/00005537-200110000-00031. [DOI] [PubMed] [Google Scholar]
- 12.Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- 13.El-Rayes BF, LoRusso PM. Targeting the epidermal growth factor receptor. Br J Cancer. 2004 Aug 2;91(3):418–424. doi: 10.1038/sj.bjc.6601921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khazaie K, Schirrmacher V, Lichtner RB. EGF receptor in neoplasia and metastasis. Cancer Metastasis Rev. 1993 Sep;12(3–4):255–274. doi: 10.1007/BF00665957. [DOI] [PubMed] [Google Scholar]
- 15.Bruns CJ, Harbison MT, Davis DW, et al. Epidermal growth factor receptor blockade with C225 plus gemcitabine results in regression of human pancreatic carcinoma growing orthotopically in nude mice by antiangiogenic mechanisms. Clin Cancer Res. 2000 May;6(5):1936–1948. [PubMed] [Google Scholar]
- 16.Overholser JP, Prewett MC, Hooper AT, Waksal HW, Hicklin DJ. Epidermal growth factor receptor blockade by antibody IMC-C225 inhibits growth of a human pancreatic carcinoma xenograft in nude mice. Cancer. 2000 Jul 1;89(1):74–82. [PubMed] [Google Scholar]
- 17.Prewett MC, Hooper AT, Bassi R, Ellis LM, Waksal HW, Hicklin DJ. Enhanced antitumor activity of anti-epidermal growth factor receptor monoclonal antibody IMC-C225 in combination with irinotecan (CPT-11) against human colorectal tumor xenografts. Clin Cancer Res. 2002 May;8(5):994–1003. [PubMed] [Google Scholar]
- 18.Xia W, Lau YK, Zhang HZ, et al. Combination of EGFR, HER-2/neu, and HER-3 is a stronger predictor for the outcome of oral squamous cell carcinoma than any individual family members. Clin Cancer Res. 1999 Dec;5(12):4164–4174. [PubMed] [Google Scholar]
- 19.Ulanovski D, Stern Y, Roizman P, Shpitzer T, Popovtzer A, Feinmesser R. Expression of EGFR and Cerb-B2 as prognostic factors in cancer of the tongue. Oral Oncol. 2004 May;40(5):532–537. doi: 10.1016/j.oraloncology.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Yigitbasi OG, Younes MN, Doan D, et al. Tumor cell and endothelial cell therapy of oral cancer by dual tyrosine kinase receptor blockade. Cancer Res. 2004 Nov 1;64(21):7977–7984. doi: 10.1158/0008-5472.CAN-04-1477. [DOI] [PubMed] [Google Scholar]
- 21.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006 Feb 9;354(6):567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 22.Herbst RS, Johnson DH, Mininberg E, et al. Phase I/II trial evaluating the anti-vascular endothelial growth factor monoclonal antibody bevacizumab in combination with the HER-1/epidermal growth factor receptor tyrosine kinase inhibitor erlotinib for patients with recurrent non-small-cell lung cancer. J Clin Oncol. 2005 Apr 10;23(11):2544–2555. doi: 10.1200/JCO.2005.02.477. [DOI] [PubMed] [Google Scholar]
- 23.Swan EA, Jasser SA, Holsinger FC, Doan D, Bucana C, Myers JN. Acquisition of anoikis resistance is a critical step in the progression of oral tongue cancer. Oral Oncol. 2003 Oct;39(7):648–655. doi: 10.1016/s1368-8375(03)00049-6. [DOI] [PubMed] [Google Scholar]
- 24.Yokoi T, Yamaguchi A, Odajima T, Furukawa K. Establishment and characterization of a human cell line derived from a squamous cell carcinoma of the tongue. Tum Res. 1988;23:43–57. [Google Scholar]
- 25.Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999 Oct 15;59(20):5209–5218. [PubMed] [Google Scholar]
- 26.Myers JN, Holsinger FC, Jasser SA, Bekele BN, Fidler IJ. An orthotopic nude mouse model of oral tongue squamous cell carcinoma. Clin Cancer Res. 2002 Jan;8(1):293–298. [PubMed] [Google Scholar]
- 27.Jenkins DE, Yu SF, Hornig YS, Purchio T, Contag PR. In vivo monitoring of tumor relapse and metastasis using bioluminescent PC-3M-luc-C6 cells in murine models of human prostate cancer. Clin Exp Metastasis. 2003;20(8):745–756. doi: 10.1023/b:clin.0000006817.25962.87. [DOI] [PubMed] [Google Scholar]
- 28.Shah JP, Andersen PE. Evolving role of modifications in neck dissection for oral squamous carcinoma. Br J Oral Maxillofac Surg. 1995 Feb;33(1):3–8. doi: 10.1016/0266-4356(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 29.Jung YD, Mansfield PF, Akagi M, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002 May;38(8):1133–1140. doi: 10.1016/s0959-8049(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 30.Inoue K, Slaton JW, Davis DW, et al. Treatment of human metastatic transitional cell carcinoma of the bladder in a murine model with the anti-vascular endothelial growth factor receptor monoclonal antibody DC101 and paclitaxel. Clin Cancer Res. 2000 Jul;6(7):2635–2643. [PubMed] [Google Scholar]
- 31.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000 Mar;21(3):505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 32.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999 Feb;5(2):257–265. [PubMed] [Google Scholar]
- 33.Ellis LM. Epidermal growth factor receptor in tumor angiogenesis. Hematol Oncol Clin North Am. 2004 Oct;18(5):1007–1021. viii. doi: 10.1016/j.hoc.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Savvides P, Greskovich J, Bokar J, et al. Phase II study of bevacizumab in combination with docetaxel and radiation in locally advanced squamous cell cancer of the head and neck (SCCHN) Journal of Clinical Oncology. 2007;25(18S):6068. [Google Scholar]