Abstract
The aim of this study was to evaluate the association between resting baroreflex sensitivity (BRS) and carotid intima–media thickness (IMT), a putative marker of sub-clinical atherosclerosis. Participants were 64 men and 18 women (median age, 57 years; range, 40 to 70 years), who did not have a previous history of coronary artery disease or treatment for hypertension. Resting BRS was measured during a 9-min baseline period using the noninvasive sequence technique; carotid IMT was subsequently determined using ultrasonography. Hierarchical multiple regression analyses showed that greater IMT in the carotid bulb (an area with a high density of baroreceptors) was associated with reduced BRS. These findings remained after adjusting BRS for resting mean arterial pressure, age, body mass index, gender, and smoking history, R2 = 0.06, P = .03. In contrast, IMT in the common and internal carotid regions (areas with presumably lower baroreceptor densities) did not account for a significant proportion of the variance in BRS. These results suggest that subclinical atherosclerosis, specifically in a region with high baroreceptor density, is associated with a reduced sensitivity of the baroreflex.
Keywords: Atherosclerosis, baroreflex sensitivity, blood pressure, carotid arteries, intima–media thickness
The baroreflex regulates short-term variations in blood pressure (BP) through autonomic adjustments in heart rate, cardiac output, and peripheral resistance; decreased baroreflex sensitivity (BRS) occurs when transient changes in BP are not detected adequately and are consequently not offset effectively by these compensatory autonomic adjustments. 1 Previous research indicates that decreased BRS is associated with aging,2 hypertension,3,4 myocardial ischemia,5 exposure to psychologic stressors6, and cardiac mortality after myocardial infarction.7,8 Decreased BRS may also be associated with the structural or biochemical effects of atherosclerosis on the carotid and aortic baroreceptors.9-13 However, whereas relationships between atherosclerosis and BRS have been well documented in animal models,9-11 similar evidence in humans is both limited and indirect. Vlachakis et al,12 for example, reported that decreased BRS was associated with a greater number of risk factors for atherosclerosis (advanced age, diabetes mellitus, hyperlipidemia, smoking, and familial history of vascular disease) in both normotensive and hypertensive individuals. More recently, Katsube et al13 reported that compared to age-matched controls, BRS was lower in patients with stable coronary artery disease and that BRS was inversely related to the number of stenotic coronary vessels.
Although ultrasonographic measures of the carotid intima–media thickness (IMT) are being used increasingly to study the relation of subclinical atherosclerosis to cardiovascular risk factors, morbidity, and mortality,14,15 no studies to date other than one preliminary report16 have evaluated the possible relationship between indices of IMT and BRS. To the extent that atherosclerosis is related to decreased BRS, then greater carotid IMT may be associated with lower levels of BRS. Furthermore, IMT in specific carotid regions may show a stronger relationship with BRS, given that the baroreceptors are not uniformly distributed throughout the carotid arteries; that is, baroreceptor density is greater in the carotid bulb compared to the common and internal carotid artery segments.1,17
The aim of the present study was to evaluate the independent contributions of carotid IMT to the prediction of basal BRS in an adult sample with a moderate range of resting BP. Specifically, we examined the relationship between BRS and measures of IMT derived from the common, bulb, and internal regions of carotid arteries, after statistically controlling for factors that have previously been shown to predict BRS (age, body mass index, gender, smoking status, and resting BP).18
Methods
Subjects
Participants were 64 men and 18 women aged 40 to 70 years (median, 57 years) who were enrolled in the REActivity and Cardiovascular risk Trial (REACT), which was designed to examine the relationship between preclinical atherosclerosis, and cardiovascular reactivity to psychologic stressors among normotensive and untreated hypertensive individuals. Individuals were screened to exclude those with (1) secondary hypertension; (2) previous cerebrovascular accident or stroke; (3) coronary artery disease; (4) use of any cardiovascular medication at the time of study or medication use for more than 6 weeks in the previous two years; (5) diabetes; (6) obesity (>20% overweight as defined by Metropolitan Life Insurance tables); (7) cancer; (8) renal failure; (9) hepatitis or cirrhosis; (10) pulmonary disease; (11) angina pectoris; (12) alcoholism; and (13) a psychiatric disorder or current use of psychotropic medication. Premenopausal women and women who had undergone a hysterectomy or who were on single hormone replacement therapy (estrogen only) were also excluded. All participants provided informed consent and all procedures were approved by the University of Pittsburgh Institutional Review Board.
Procedure
Participants attended two screening appointments during which measures of height, weight, and resting BP were obtained. After the screening sessions, eligible individuals with BP ≤130/85 mm Hg (n = 65) or ≥140/90 mm Hg (n = 25) subsequently underwent carotid ultrasonography procedures for the measurement of IMT and were exposed to a battery of cognitive, psychomotor, and interpersonal challenges while cardiovascular measures were recorded.
Participants were instructed to refrain from drinking alcohol and taking nonessential medication for 12 hours before testing and to refrain from drinking beverages containing caffeine, smoking, eating, and exercising for 3 h before testing. After electrode application, the participant was seated upright and completed a series of psychologic stress tasks during which cardiovascular measures were obtained. The data reported here include only the BRS data obtained during a 9-min vanilla baseline period, during which each participant performed a simple color identification task.19 This nonevaluative baseline task is designed to reduce psychologic anticipation of upcoming stressors by maintaining a consistent, but low, level of mental activity; the vanilla baseline is not reported to be unpleasant or stressful, and cardiovascular variables (BP and heart rate) assessed during the vanilla baseline do not differ from those obtained during a resting baseline.19
Physiologic Recording
Beat-to-beat changes in systolic (SBP) and diastolic (DBP) BP were monitored from the third finger of the dominant hand, positioned at the level of the heart, using a continuous BP tracking device (FIN-A-PRES 2300, Ohmeda; Englewood, CO, automatic servoadjustment setting deactivated). The electrocardiogram (EKG) was obtained from three Ag/AgCl electrodes that were positioned in a modified lead II configuration. The EKG was digitized (12 bit), sampled at 1000 Hz, and stored for offline processing.
Baroreflex Sensitivity Quantification
The sequence method20 was used to derive estimates of BRS from continuous recordings of SBP (derived from the FIN-A-PRES) and cardiac interbeat intervals (IBI; derived from the time in milliseconds between sequential R spikes in the EKG). Specifically, individual IBI values were first transformed into heart rate for compatibility with the BRS scoring software,6 then, series of three or more successive cardiac cycles for which there was an increase in SBP followed by a decrease in the heart rate of the subsequent heart beat (up sequence) or a decrease in SBP followed by a increase in the heart rate of the subsequent heart beat (down sequence) were identified. Only those sequences for which SBP changed by at least 1 mm Hg and the subsequent heart rate changed by at least 0.3 beats/min were evaluated. Given the range of resting heart rates in this sample (54 to 98 beats/min), a criterion of 0.3 beats/min corresponded to an interbeat interval criterion ranging from 3 to 6 msec, which falls within the range of the criteria used by previous validation studies (1 to 6 msec).4,21,22
After sequence identification, individual heart rate values were reexpressed as interbeat intervals and linear regression functions were then fit to each sequence, which yielded an unstandardized slope (ie, the BRS estimate) that reflected the change in IBI (in milliseconds) per mm Hg change in SBP. Only those sequences for which the correlation between SBP and IBI exceeded r = 0.80 were included in the calculation of BRS estimates. The average BRS derived from up sequences (M = 11.08 ± SE = 0.46 msec/mm Hg) did not differ significantly from the average BRS derived from down sequences (11.76 ± .57 msec/mm Hg), P > .10; thus, slopes were averaged across all sequences that were identified for the entire 9-min baseline period (average BRS across all participants = 11.55 ± 0.47 msec/mm Hg; average number of BRS sequences = 12.46 ± 1.13). Average BRS values were approximately normally distributed.
IMT Measurement
B-mode ultrasonographic scans of the right and left common carotid artery, carotid bifurcation, and the first centimeter of the internal carotid artery were performed using a Toshiba SSA-270 scanner (Tustin, CA), which was equipped with a 5-Mhz linear array imaging probe. The right and left carotid arteries were imaged in multiple planes until the best image of the echogenic lumen–intima and media–adventitia interface was found. These images were then digitized for later offline IMT scoring. From the digitized images, the average IMT across 1-cm segments of the near and far walls of the right and left distal common carotid artery (1 cm proximal to the carotid bulb), the far wall of the carotid bulb (starting at the point at which the near and far walls of the common carotid artery are no longer parallel and ending at the flow divider), and the far wall of the internal carotid artery (from the flow divider to the first centimeter distal to this point) were derived. The IMT measures were then averaged across the right and left sides for each region, yielding an average IMT for the common (IMTCCA), bulb (IMTBulb), and internal (IMTICA) carotid regions. For reliability of REACT IMT estimates, see Thompson et al.23
Statistical Analysis
Consistent with population-based norms,24 the distributions of all IMT measures were skewed. To compare BRS between individuals in the upper and lower halves of the IMT distributions, we divided the sample at the median IMT for each carotid region (IMTCCA median = 0.81 mm; IMTBulb median = 0.90 mm; IMTICA median = 0.72 mm). This procedure yielded a dichotomous IMT status measure for each region (1 = low IMT, 2 = high IMT). Using t tests and χ2 analyses, we first evaluated univariate differences between individuals with high and low levels of IMT in the following variables: age, body mass index (in kilogram/squaremeter), smoking status (1 = current smoker, 2 = nonsmoker), gender (1 = male, 2 = female), SBP, DBP, and mean arterial pressure (MAP = DBP + (SBP − DBP)/3).
Hierarchical linear regression analyses25 were then used to predict BRS from dichotomous IMT measures after controlling for these covariates. For these analyses, BRS was regressed hierarchically on three sets of independent variables, which were entered in the following order: step 1 = age, body mass index, gender, and smoking status; step 2 = MAP; and step 3 = IMT status. A separate hierarchical regression was performed for each carotid IMT measure (IMTCCA, IMTBulb, IMTICA). We evaluated the proportion of variance in BRS accounted for by the first set of predictors (R2), and the increment in the proportion of unique variance accounted for by the second and third set of predictors (ΔR2). We also examined the standardized regression coefficient (β) and zero order (r) and partial (pr) correlation coefficients for each variable. The partial correlation reflects the correlation between a dependent measure and a given predictor after removing the linear relationship between the dependent measure and other predictors in the model.25 Point-biserial correlations (rpb) were used when one variable was dichotomous and one was continuous.
Eight individuals had an insufficient number of cardiac cycles for which BRS could be determined; these individuals did not differ significantly from those with complete data in age, body mass index, SBP, DBP, MAP, or IMT at any of the carotid regions. A type I error rate of α = 0.05 was adopted.
Results
IMT Group Comparisons
Table 1 shows descriptive characteristics of individuals classified as having low and high levels of IMT in the common, bulb, and internal carotid regions. Overall, individuals with higher IMT at each carotid region were older than those individuals with lower levels of IMT; however, this difference was marginal between IMTICA groups (P = .07). Individuals with higher levels of IMT across the carotid regions also had a higher body mass index compared to individuals with lower levels of IMT, but this difference did not reach statistical significance when IMTICA groups were compared (P > .50). Although individuals with higher IMT at the common carotid artery had a higher resting SBP than individuals with lower levels of IMT at this region, no statistically significant differences between the remaining IMT groups were found for gender or smoking frequencies, nor were group differences found for DBP or MAP (P > .30).
Table 1.
Comparison of individuals with higher and lower levels of intima-media thickness (IMT) at the common (CCA), bulb, and internal (ICA) carotid artery regions
| IMTCCA |
IMTBulb |
IMTICA |
||||
|---|---|---|---|---|---|---|
| <0.81 mm (n = 45) | >0.81 mm (n = 45) | <0.90 mm (n = 45) | >0.90 mm (n = 45) | <0.72 mm (n = 45) | >0.72 mm (n = 45) | |
| Age (y) | 51.8 (8.5) | 57.8 (8.3)* | 52.4 (8.5) | 57.2 (8.7)* | 53.1 (8.4) | 56.5 (9.1)† |
| BMI (kg/m2) | 26.4 (3.1) | 27.9 (3.5)* | 26.4 (3.2) | 28.0 (3.4)* | 27.1 (3.0) | 27.3 (3.8) |
| Smokers (n) | 10 | 9 | 11 | 8 | 9 | 10 |
| Gender (M/F) | 36/9 | 35/10 | 34/11 | 37/8 | 36/9 | 35/10 |
| SBP (mm Hg) | 125.8 (12.4) | 132.2 (15.7)* | 127.9 (15.6) | 130.2 (13.3) | 129.2 (14.3) | 128.9 (14.7) |
| DBP (mm Hg) | 84.4 (8.5) | 81.6 (9.9) | 83.5 (9.7) | 82.5 (8.9) | 84.3 (10.6) | 81.7 (7.6) |
| MAP (mm Hg) | 98.2 (9.2) | 98.5 (10.8) | 98.3 (10.8) | 98.4 (9.1) | 99.3 (10.8) | 97.4 (9.0) |
| BRS (msec/mm Hg) | 12.1 (4.4) | 11.0 (4.1) | 12.7 (4.3) | 10.2 (4.0)* | 11.9 (4.9) | 11.2 (3.4) |
| BRS sequences | 14.43 (12.0) | 10.63 (8.0) | 13.63 (12.4) | 11.31 (7.6) | 13.24 (10.7) | 11.69 (9.9) |
BMI = body mass index; M/F = male/female; SBP = systolic blood pressure; DBP = diastolic blood pressure; MAP = mean arterial pressure; BRS = baroreflex sensitivity.
Values in parentheses indicate standard deviation.
P < .05;
P = .07.
Baroreflex Sensitivity and Carotid IMT
Table 2 shows the hierarchical regression results predicting BRS from three sets of predictors. Within the first set, no statistically significant relationships were observed between BRS and age, gender, smoking status, or body mass index, step 1 R2 = 0.04, F (4, 74) < 1. In the second step, however, MAP accounted for a significant proportion of the remaining variance in BRS, step 2 ΔR2 = 0.08, F (1, 73) = 6.31, P = .01. Specifically, replicating prior findings, 2-4 increased MAP was associated with decreased BRS. After controlling for age, gender, smoking status, body mass index, and MAP in the first two steps of the hierarchical regression analysis, the IMTBulb measure accounted for a unique proportion of the remaining variance in BRS, step 3 ΔR2 = 0.06, F (1, 72) = 5.01, P = .03. As shown in Table 1 and Fig. 1, BRS was lower among those individuals with higher IMTBulb levels compared to individuals with lower IMTBulb levels. In contrast to the findings for the IMTBulb measure, IMTCCA(ΔR2 = 0.004) and IMTICA (ΔR2 = 0.003) did not account for a significant proportion of the variance in BRS, step 3 F (1, 72) < 1.
Table 2.
Hierarchical regression analysis predicting baroreflex sensitivity
| Variable | β | pr | r (rpb) | t |
|---|---|---|---|---|
| Step 1 | ||||
| Age | −0.16 | −0.15 | −0.14 | 1.26 |
| Sex | 0.04 | 0.04 | −0.02 | 0.35 |
| BMI | −0.11 | −0.11 | 0.11 | 0.96 |
| Smoking status | −0.11 | −0.10 | −0.05 | 0.89 |
| Step 2 | ||||
| MAP | −0.28 | −0.28 | −0.28 | 2.51* |
| Step 3 | ||||
| IMTCCA | −0.07 | −0.07 | −0.13 | 0.56 |
| IMTBulb | −0.25 | −0.26 | −0.27 | 2.24* |
| IMTICA | −0.05 | −0.05 | −0.07 | 0.46 |
Step 1 R2 = 0.04, P > .5; step 2 ΔR2 = 0.08, P = .01; step 3 IMTCCA ΔR2 = 0.004, P > .5; step 3 IMTBulb ΔR2 = 0.06, P = .03; step 3 IMTICA ΔR2 = 0.003, P > .7.
A separate regression analysis was performed with each IMT measure for step 3.
Bulb = carotid bulb; other abbreviations as in Table 1.
P < .05.
FIG. 1.
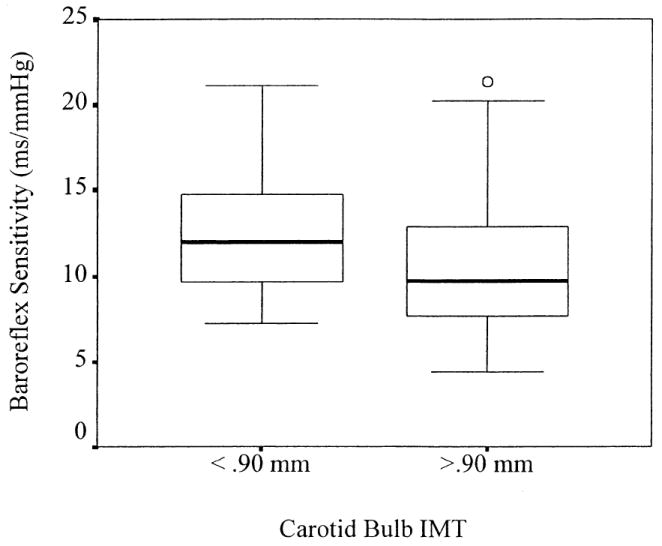
Baroreflex sensitivity (BRS) for individuals with low (<0.90 mm) and high (>0.90 mm) levels of carotid bulb intima–media thickness (IMT). In this plot, bolded horizontal lines represent the median BRS for each group; each box represents the interquartile range, containing 50% of the BRS values for each group. Bars extend from the upper and lower quartiles to the highest and lowest observed BRS values, excluding one outlier value shown as a circle; exclusion of this outlier, which was defined as any value falling between 1.5 and 3 times the interquartile range, did not alter the observed results.
Discussion
The primary finding of the present study was that greater IMT in the carotid bulb—a region with a high density of baroreceptors—was associated with reduced BRS. This association remained after adjusting BRS for resting BP and other factors that have also been shown to predict BRS. In contrast, IMT in the common and internal carotid regions—areas with presumably lower densities of baroreceptors—were not associated with BRS. Similarly, no statistically significant correlations were found between BRS and gender, body mass index, smoking status, or age; however, these particular findings are not atypical.18,26 Given that we studied a relatively healthy sample of individuals with a restricted age range and few women and smokers, and because body mass index accounts for a small percentage of the variance in BRS (~1%),18 a larger sample with fewer exclusionary criteria would likely be required to observe relationships between these variables and BRS.
To the extent that IMT is a valid marker of early, subclinical atherosclerosis,27 there may be several explanations for an inverse relationship between carotid bulb IMT and BRS. First, atherogenesis may reduce BRS by structural changes in vessel wall composition, which may contribute to increased vascular stiffness. Such structural changes may affect BRS because a higher pressure threshold would be required to distend the arterial wall, and thus activate the stretch-sensitive baroreceptors.9,28,29 Second, so-called functional mechanisms related to the progression of atherosclerosis (eg, paracrine factors) have also been shown to affect BRS in animal models.10 Most notably, accumulation of oxygen-derived free radicals during the development of atherosclerosis has been shown to reduce baroreceptor activity.11,30 However, because carotid IMT was used as an estimate of subclinical atherosclerosis in the present study, inferences regarding the relationship between these structural (ie, compliance/distensibility) or functional mechanisms and BRS are necessarily limited. For instance, increased arterial wall thickness, as indicated by greater IMT, may not necessarily reflect decreased arterial distensibility. Furthermore, because carotid bulb IMT explained a relatively moderate amount of the variance in BRS, it is likely that other factors that were not assessed in the present study (eg, estimates of autonomic cardiac control, myocardial ischemia) may account for a significant percentage of the remaining variance in BRS. Finally, because correlational methods were used to evaluate the relationship between BRS and IMT, the issue of whether changes in BRS precede or follow changes in IMT cannot be resolved adequately. Indeed, a number of studies indicate that BRS provides a stable index of individual differences in autonomic function, and that these individual differences are important predictors of future cardiac arrhythmias and mortality.7,8 On the other hand, previous animal studies have shown that the development of atherosclerosis precedes alterations in BRS.10 Taken together, these findings suggest the possibility that the BRS–cardiovascular disease relationship may be bidirectional. With regard to the results of the present study, future prospective studies are needed to determine the temporal relationship between changes in BRS and IMT.
Acknowledgments
We thank Jeanne McCaffery and Natasha Tokowicz for their helpful comments, and Tom Debski and Melissa Smith for their assistance in data acquisition and reduction.
This research was supported by National Institutes of Health (NIH) HL40962 and by training grant NIH HL07560.
References
- 1.Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. New York: Oxford University Press; 1992. [Google Scholar]
- 2.Gribbin B, Pickering TG, Sleight P, Peto R. Effect of age and high blood pressure on baroreflex sensitivity in man. Circ Res. 1971;24:424–431. doi: 10.1161/01.res.29.4.424. [DOI] [PubMed] [Google Scholar]
- 3.Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;34:48–54. doi: 10.1161/01.cir.39.1.48. [DOI] [PubMed] [Google Scholar]
- 4.Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedotti A, Zanchetti A, Mancia G. Evaluation of the baroreceptor-heart rate reflex by 24-hour intra-arterial blood pressure monitoring in humans. Hypertension. 1988;12:214–222. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- 5.Pomidossi G, Saino A, Perondi R, Gregorini L, Alessio P, Rimini A, Omboni S, Zanchetti A, Mancia G. Impairment of the arterial baroreflex during symptomatic and silent myocardial ischemia in humans. J Am Coll Cardiol. 1993;22:1866–1872. doi: 10.1016/0735-1097(93)90771-r. [DOI] [PubMed] [Google Scholar]
- 6.Steptoe A, Sawada Y. Assessment of baroreceptor reflex function during mental stress and relaxation. Psychophysiology. 1979;26:140–147. doi: 10.1111/j.1469-8986.1989.tb03145.x. [DOI] [PubMed] [Google Scholar]
- 7.La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- 8.La Rovere MT, Specchia G, Mortara A, Schwartz PJ. Baroreflex sensitivity, clinical correlates, and cardiovascular mortality among patients with a first myocardial infarction. Circulation. 1988;78:816–824. doi: 10.1161/01.cir.78.4.816. [DOI] [PubMed] [Google Scholar]
- 9.Angell-James JE. Arterial baroreceptor activity in rabbits with experimental atherosclerosis. Circ Res. 1974;34:27–39. doi: 10.1161/01.res.40.4.27. [DOI] [PubMed] [Google Scholar]
- 10.Chapleau MW, Cunningham JT, Sullivan MJ, Wachtel RE, Abboud FM. Structural versus functional modulation of the arterial baroreflex. Hypertension. 1995;26:341–347. doi: 10.1161/01.hyp.26.2.341. [DOI] [PubMed] [Google Scholar]
- 11.Wilfert K, Drischel K, Unbehaun A, Guski H, Persson PB, Stauss HM. Vascular response to angiotensin II in atherosclerosis: Role of the baroreflex. Hypertension. 2000;35:685–690. doi: 10.1161/01.hyp.35.2.685. [DOI] [PubMed] [Google Scholar]
- 12.Vlachakis ND, Mendlowitz M, DeGuia DeGusman D. Diminished baroreceptor sensitivity in elderly hypertensives: possible role of atherosclerosis. Atherosclerosis. 1976;24:243–249. doi: 10.1016/0021-9150(76)90079-4. [DOI] [PubMed] [Google Scholar]
- 13.Katsube Y, Sato H, Naka M, Kim BH, Kinoshita N, Koretsune Y, Hori M. Decreased baroreflex sensitivity in patients with stable coronary artery disease is correlated with the severity of coronary narrowing. Am J Cardiol. 1996;78:1007–1010. doi: 10.1016/s0002-9149(96)00525-5. [DOI] [PubMed] [Google Scholar]
- 14.Aronow WS, Ahn C, Schoenfeld MR, Gutstein H. Association of extracranial carotid arterial disease and chronic atrial fibrillation with the incidence of new thromboembolic stroke in 1,846 older persons. Am J Cardiol. 1999;83:1403–1404. doi: 10.1016/s0002-9149(99)00107-1. [DOI] [PubMed] [Google Scholar]
- 15.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharret AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the atherosclerosis risk in communities (ARIC) study, 1987–1993. Am J Epidemiol. 1997;14:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 16.de Vries R, Lefrandt J, Terpstra W, Smit A, May J. Baroreflex function is related to intima media thickness of the carotid artery in mild to moderate hypertensive subjects (Abstract) J Hypertens. 2000;18:S204. [Google Scholar]
- 17.Muratori G. Histological observations on the structure of the carotid sinus in man and animals. In: Kezdi P, editor. Baroreceptors and Hypertension. New York: Pergamon Press; 1967. pp. 253–265. [Google Scholar]
- 18.Kardos A, Watterich G, de Menezes R, Csanady M, Casadei B, Rudas L. Determinants of spontaneous baroreflex sensitivity in a healthy working population. Hypertension. 2001;37:911–916. doi: 10.1161/01.hyp.37.3.911. [DOI] [PubMed] [Google Scholar]
- 19.Jennings JR, Kamarck T, Stewart C, Eddy M, Johnson P. Alternate cardiovascular baseline assessment techniques: vanilla or resting baseline. Psychophysiology. 1992;29:742–750. doi: 10.1111/j.1469-8986.1992.tb02052.x. [DOI] [PubMed] [Google Scholar]
- 20.Bertinieri G, di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. A new approach to analysis of the arterial baroreflex. J Hyperten. 1985;3(Suppl):S79–S81. [PubMed] [Google Scholar]
- 21.Parlow J, Viale J-P, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans: comparison with drug-induced responses. Hypertension. 1995;25:1058–1068. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]
- 22.Reyes del Paso GA, Langewitz W, Robles H, Perez N. A between-subjects comparison of respiratory sinus arrhythmia and baroreceptor cardiac reflex sensitivity as non-invasive measure of tonic para-sympathetic cardiac control. Intern J Psychophysiol. 1996;22:163–171. doi: 10.1016/0167-8760(96)00020-7. [DOI] [PubMed] [Google Scholar]
- 23.Thompson T, Sutton-Tyrrell K, Wildman R. Continuous quality assessment programs can improve carotid duplex scan quality. J Vasc Tech. 2001;25:33–39. [Google Scholar]
- 24.Howard G, Sharrett AR, Heiss G, Evans GW, Chambless LE, Riley WA, Burke GL. Carotid artery intimal–medial thickness distribution in general populations as evaluated by B-mode ultrasonography. Stroke. 1993;24:1297–1304. doi: 10.1161/01.str.24.9.1297. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J, Cohen P. Applied Multiple Regression/Correlation Analysis for the Behavioral Sciences. Hillsdale, NJ: L. Erlbaum; 1983. [Google Scholar]
- 26.Laitinen T, Hartikainen J, Van Ninen E, Niskanen L, Geelen G, Länsimies E. Age and gender dependency of baroreflex sensitivity in healthy subjects. J Appl Physiol. 1998;84:576–583. doi: 10.1152/jappl.1998.84.2.576. [DOI] [PubMed] [Google Scholar]
- 27.Bonithon-Kopp C, Touboul P-J, Berr C, Leroux C, Mainard F, Courbon D, Ducimetere P. Relation of intima–media thickness to atherosclerotic plaques in carotid arteries: the vascular aging (EVA) study. Arterioscler Thromb Vasc Biol. 1996;16:310–316. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 28.Bonyhay I, Jokkel G, Kollai M. Relation between baroreflex sensitivity and carotid artery elasticity in healthy humans. Am J Physiol. 1996;271:H1139–H1144. doi: 10.1152/ajpheart.1996.271.3.H1139. [DOI] [PubMed] [Google Scholar]
- 29.Lage SG, Polak JF, O’Leary DH, Creager MA. Relationship of arterial compliance to baroreflex function in hypertensive patients. Am J Physiol. 1993;265:H232–H237. doi: 10.1152/ajpheart.1993.265.1.H232. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Mao HZ, Abboud FM, Chapleau MW. Oxygen-derived free radicals contribute to baroreceptor dysfunction in atherosclerotic rabbits. Circ Res. 1996;79:802–811. doi: 10.1161/01.res.79.4.802. [DOI] [PubMed] [Google Scholar]


