Abstract
Although individual differences in fear and anxiety modulate the pain response and may even cause more suffering than the initiating physical stimulus, little is known about the neural systems mediating this relationship. The present study provided the first examination of the neural correlates of individual differences in the tendency to (1) feel anxious about the potentially negative implications of physical sensations, as measured by the anxiety sensitivity index (ASI), and (2) fear various types of physical pain, as indexed by the fear of pain questionnaire (FPQ). In separate sessions, participants completed these questionnaires and experienced alternating blocks of noxious thermal stimulation (45–50 °C) and neutral thermal stimulation (38 °C) during the collection of whole-brain fMRI data. Regression analyses demonstrated that during the experience of pain, ASI scores predicted activation of a medial prefrontal region associated with self-focused attention, whereas FPQ scores predicted activation of a ventral lateral frontal region associated with response regulation and anterior and posterior cingulate regions associated with monitoring and evaluation of affective responses. These functional relationships cannot be wholly explained by generalized anxiety (indexed by STAI-T scores), which did not significantly correlate with activation of any regions. The present findings may help clarify both the impact of individual differences in emotion on the neural correlates of pain, and the roles in anxiety, fear, and pain processing played by medial and orbitofrontal systems.
Keywords: Anxiety sensitivity, Fear of pain, Pain, Medial prefrontal cortex, Orbitofrontal cortex, Anxiety, Brain imaging, Fear, Pain behavior
1. Introduction
As debilitating and disruptive as the objective physical component of pain may be, the nature of one’s subjective emotional response to pain may play a more important role in determining how much suffering one experiences (Crombez et al., 1999). Indeed, an individual’s tendency towards anxiety about, and fear of, painful sensations, predicts physical complaints and treatment outcomes in chronic pain sufferers (McCracken et al., 1998, 1999), which may result in part from biased attention to pain-related cues (Asmundsen et al., 1997; Keogh, Dillon et al., 2001). Although the behavioral and clinical correlates of pain-related anxiety and fear have received increasing attention, to date, no studies have examined the neural systems mediating these relationships. The goal of the present study was to address this issue using functional magnetic resonance imaging (fMRI).
Individual differences in pain-related fear and anxiety are commonly assessed using the fear of pain questionnaire (FPQ III, McNeil & Rainwater, 1998) and anxiety sensitivity index (ASI, Reiss et al., 1986). The FPQ assesses fear of different types of physical insult (e.g. breaking one’s leg or having a tooth pulled) and provides an overall score representing general fear of physical pain. The ASI assesses fear of and worry about the implications of anxiety-related symptoms and sensations, such as being scared by a rapid heartbeat, feeling faint, or being nervous. We used both the FPQ and ASI to assess pain-related affective tendencies in participants who experienced experimentally induced heat pain. To assess and control for general tendencies to experience anxiety unrelated to physical sensations, participants also completed the STAI-T (Spielberger et al., 1983), which may measure general anxiety conceptually distinct from anxiety sensitivity (McNally, 1997; Reiss et al., 1986).
It was hypothesized that fear of pain and anxiety sensitivity could modulate pain response in two ways. On one hand, they might influence pain response by modulating responses in pain processing systems. If this hypothesis is correct, then FPQ and ASI scores should correlate with activity in regions typically identified in contrasts of painful heat and non-painful warmth, such as the thalamus, cingulate, and insula (Coghill et al., 1994; Davis et al., 1997, 2000; Peyron et al., 2000; Rainville et al., 2002). Each of these regions has been associated with related, albeit distinct, functions related to pain, and the association of each region with pain related fear or anxiety may have different functional implications. On the other hand, pain-related fear and anxiety might influence pain response by modulating attentional and emotional processes that may be different than those identified in the overall contrast of painful heat and non-painful warmth (Asmundsen et al., 1997; Ellery et al., 2001; Keogh et al., 2001; Keogh, McNeil & Rainwater, 1998). If this hypothesis is correct, then regressions of FPQ and ASI scores against activation during pain should predict activation of distinct regions of insular, cingulate, medial prefrontal, and orbitofrontal cortices implicated in emotion, self-monitoring, and emotion regulation (Bechara et al., 2000; Bush et al., 1999, 2000; Lane et al., 1999; Ochsner and Feldman Barrett, 2001; Ochsner and Gross, 2004, 2005; O’Doherty et al., 2003).
2. Methods
2.1. Participants
13 Caucasian participants (seven female, mean age=29.5±7.0 years, range 19–42) with no prior history of chronic pain were recruited specifically for participation in this study in compliance with human subjects regulations of Stanford University Medical School. Participants were screened for good general health and the absence of any pain-related disorders (physical or psychiatric, such as anxiety or depression) using standard self-report forms used at the Lucas Center for functional imaging at Stanford University to screen participants for neurological and psychiatric disorders.
2.2. Behavioral paradigm
Participants experienced noxious thermal and neutral (non-noxious) thermal stimulation on their right distal lateral forearm delivered by a computer controlled thermal stimulator with an MRI compatible 3 cm×3 cm Peltier probe (TSA-2001, Medoc, Chapel Hill, NC). In a block design, 20 s of noxious thermal stimuli (45–50 °C) alternated with 30 s of neutral stimuli (38 °C) five times. Stimuli were ramped up and down at a rate of 3 °C/s. Prior to completion of a pre-scan threshold-setting session participants had never been exposed to the thermode. Temperatures used for the noxious thermal blocks were determined in this pre-scanning session to elicit the maximum level of pain without causing movement. This corresponded to a subject-defined seven out of 10 on a verbal rating scale (0=no pain, 10=worst pain imaginable). The neutral thermal stimulus (38 °C) was chosen to represent a warm sensation. Participants were instructed to attend to the stimulus throughout the task. Upon exiting the scanner, participants used verbal rating scales to rate the unpleasantness (0=not unpleasant, 10=most unpleasant experience imaginable) and the intensity (0=not at all intense, 10=most intense imaginable) of the noxious stimulation they had received. In a separate session immediately following scanning, participants completed the FPQ III (McNeil & Rainwater, 1998), ASI (Reiss et al., 1986), and STAI-T (Sawamoto et al., 2000).
2.3. MRI data acquisition
Whole brain fMRI data (32 axial slices, 3.5 mm thick) were collected at 3 T (GE Signa LX Horizon Echospeed scanner) with a T2*-sensitive gradient echo spiral-out pulse sequence (30 ms TE, 2000 ms TR, two interleaves, 60° flip angle, 24 cm field of view, 64×64 data acquisition matrix). T2-weighted flow-compensated spin-echo scans were acquired for anatomical reference using the same slice prescription (2000 ms TR; 85 ms TE), and high order shimming was performed before functional scans (Glover, 1999).
2.4. Data analysis
SPM99 (Wellcome Department of Cognitive Neurology) was used to slice time and motion-correct functional images, which were normalized using parameters derived from the normalization of coregistered anatomical images to a standard template brain. Functional images were interpolated to 2×2×2 mm3 voxels and smoothed with a Gaussian filter (6 mm full width-half maximum). For the noxious thermal and neutral warmth blocks, fixed-effects for each participant were modeled as a boxcar regressor convolved with the canonical hemodynamic response, which was then correlated voxelwise with activation using the general linear model implemented in SPM99. Contrast images for each participant summarized differences between block types, which were used to create second level group average SPM{t} maps of regions more active for noxious heat than for non-noxious warmth. These images were thresholded at P<0.005 uncorrected for multiple comparisons, with an extent threshold of 20 voxels. This corresponds to an overall alpha level of P<0.05 corrected for multiple comparisons as calculated by the Monte Carlo simulation and method of Forman et al. (1995) implemented in AFNI, which has been employed in numerous prior studies (e.g. Konishi et al., 1998; Poldrack et al., 1999; Ochsner et al., 2004a; Wagner, 1999; Wood et al., 2003). Two different analyses were used to identify regions whose activation correlated with fear of pain and anxiety sensitivity. First, parameter estimates were extracted from regions identified in the pain>warmth contrast and then correlated with FPQ, ASI, and STAI-T scores. Second, because regions whose magnitude of activation co-varies with individual differences may not always appear in overall group contrasts, an additional analysis was performed. SPM99’s simple regression function was used to search for additional regions whose magnitude of activation in the pain>warmth contrast was correlated with FPQ, ASI, or STAI-T scores. This analysis was restricted to region regions of a priori interest, including cingulate, medial prefrontal, insular, and orbitofrontal cortex. In these regions, the resulting correlation maps were thresholded at P<0.001 uncorrected for multiple comparisons, with an extent threshold of 10 voxels. This method has been used in prior studies that have used whole brain correlational analyses to examine individual differences (e.g. Bunge et al., 2002; Ray et al., 2005).
3. Results
3.1. Behavioral results
Post-scan ratings of pain unpleasantness (mean=6.73, SD=1.86) and intensity (mean=6.81, SD=1.09) confirmed the experience of pain. Scores on the ASI (mean=12.69, SD=5.74), FPQ III (mean=82.2, SD=13.16), and STAI-T (mean=35.69, SD=9.09) were within the range of responses typically given by healthy normal participants (McNeil & Rainwater, 1998; Reiss et al., 1986; Speilberger et al., 1983). ASI scores were not significantly correlated with either FPQ (r=.42, P<.16) or STAI-T (r=−.32, P<.30) scores, and FPQ and STAI-T scores also did not correlate significantly (r=−.41, P<.20). Because anxiety and emotion are known to co-vary with gender and may impact pain experience (e.g. Keogh & Herdenfeldt, 2002; Keogh et al., 2004; Kring & Gordon, 1998), mean pain ratings and score on all individual difference measures were compared (using Bonferroni-corrected post-hoc t-tests) for male and female participants. No significant gender differences were observed (all t<1, P>.3).
3.2. Imaging results
Regions activated during the experience of pain were identified in the pain>warmth contrast. These regions are shown in Table 1 and Fig. 1, and included regions of anterior cingulate and insular cortex commonly identified in studies of pain, as well as regions of thalamus, lateral prefrontal cortex, and parietal cortex that also have been identified in pain studies (Peyron et al., 2000). Correlations of individual difference scores with parameter estimates for regions activated in this contrast revealed (1) that FPQ scores correlated with activation of the anterior and posterior cingulate cortex, and (2) the ASI and STAI-T scores significantly correlated with none of the regions identified in the overall group contrast (Table 3).
Table 1.
Group activations for pain (noxious heat)>warmth (non-noxious heat) contrast
| Region of activation | Broadmann | Coordinates |
Z score | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Medial frontal gyrus | 6 | 0 | −12 | 68 | 2.97 | 224 |
| Middle frontal gyrus | R46/45 | 44 | 32 | 24 | 3.73 | 1144 |
| Middle frontal gyrus | R6 | 54 | 4 | 52 | 3.37 | 608 |
| Inferior frontal gyrus | R9 | 48 | 8 | 36 | 3.55 | 1160 |
| Inferior frontal gyrus | R10 | 38 | 42 | 2 | 3.48 | 328 |
| Anterior cingulate gyrus* | 24 | 6 | 8 | 36 | 3.36 | 184 |
| Posterior cingulate gyrus* | 23 | −2 | −28 | 34 | 3.47 | 424 |
| Insula/claustrum* | R13 | 28 | 16 | 0 | 4.30 | 2568 |
| Insula* | R13 | 42 | 0 | 10 | 2.94 | 248 |
| Insula* | R13 | 38 | −22 | 12 | 2.92 | 208 |
| Insula/Inferior Parietal | R13 | 44 | −32 | 26 | 3.93 | 360 |
| Superior temporal gyrus | L22 | −70 | 2 | 2 | 3.15 | 312 |
| Superior temporal gyrus | R41/42 | 54 | −38 | 12 | 3.21 | 160 |
| Inferior parietal lobe | R40 | 54 | −36 | 32 | 2.99 | 200 |
| Inferior parietal lobe | R40 | 66 | −46 | 40 | 3.16 | 168 |
| Inferior parietal lobe | L39 | −42 | −68 | 40 | 3.42 | 344 |
| Putamen/caudate | L | −20 | 0 | 14 | 3.45 | 240 |
| Thalamus (anterior)* | L | −8 | −2 | 6 | 3.42 | 192 |
| Thalamas (ventral anterior)* | R | 16 | −6 | 14 | 3.55 | 528 |
R and L hemisphere is not designated for maxima within 6 mm of midline. Coordinates are in MNI space.
Designates regions selected for correlations with ASI and FPQ scores in Table 3.
Fig. 1.
Regions of anterior and posterior cingulate cortex (A), posterior, mid and anterior insular cortex (B), and bilateral thalamus (C) activated in the pain> warmth contrast. Cingulate, insula, and thalamic regions activated in this contrast were hypothesized on a priori grounds to be related to ASI or FPQ scores (see Table 3). Those highlighted in red circles showed the strongest correlations, but only with FPQ scores. Scatter-plots illustrate these correlations.
Table 3.
Correlations of ASI scores and FPQ scores with activation of peak voxels in functionally defined regions of interest identified in regression and overall contrast analyses
| Region of interest | Measure |
|
|---|---|---|
| ASI | FPQ | |
| Identified in ASI regression | ||
| MPFC | .890* (.804) | .533**(.203) |
| Identified in FPQ regression | ||
| Right lateral OFC | .583***(.463) | .912* (.869) |
| Identified in overall pain>warm contrast | ||
| Anterior cingulate | .336 | .610***(.486) |
| Posterior cingulate | .137 | .533**(.603) |
| Insula (anterior) | .167 | .394 |
| Insula (mid) | −.076 | .122 |
| Insula (posterior) | −.242 | −.173 |
| Left thalamus | −.116 | −.083 |
| Right thalamus | .263 | .359 |
P<.001;
P<.10,
P<.05;.
All others not marked, P>.15. ASI, anxiety sensitivity index; FPQ, fear of pain questionnaire. Regions identified in the pain>warmth contrast were selected from those listed in Table 1 (and shown in Fig. 1) as representative of those commonly activated in studies of pain (Peyron et al., 2000). Values in parentheses are correlations partialling out individual differences in pain threshold temperatures.
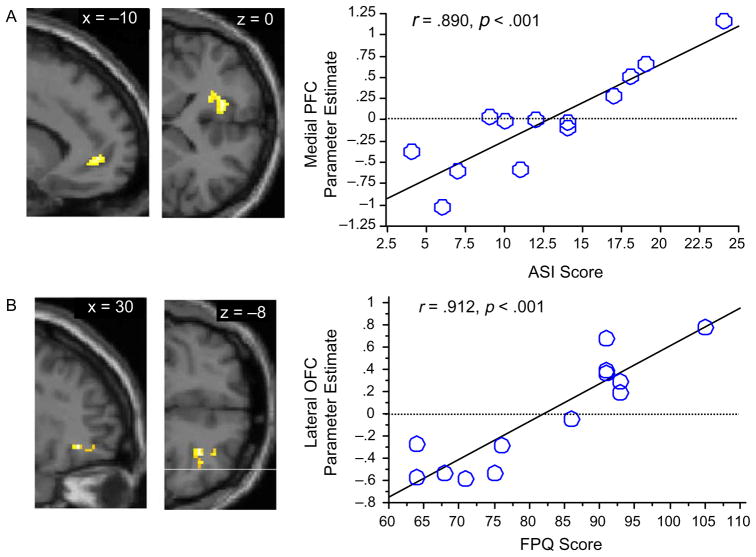
To identify regions whose activation correlated with fear of pain, anxiety sensitivity, or general trait anxiety, but were not identified in the overall group contrast, FPQ, ASI, and STAI-T scores were regressed against brain activation in the pain>warmth contrast (Table 2, Fig. 2). These analyses revealed (1) that activation of a single region of right lateral orbital prefrontal cortex was correlated with FPQ scores, (2) that activation of a single region of left medial prefrontal cortex at the junction of Broadmann areas 32 and 10 was correlated with ASI scores, and (3) that activation did not significantly correlate with STAI-T scores for any regions (Table 3).
Table 2.
Group activations for ASI and FPQ regressions
| Region of activation | Broadmann | Coordinates |
Z score | Volume (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| ASI | ||||||
| ACC/Medial frontal gyrus | 32/10 | −10 | 48 | 0 | 4.08 | 808 |
| ACC | 32 | −14 | 40 | 0 | 3.61 | (L) |
| FPQ | ||||||
| Middle frontal gyrus | R11/47 | 30 | 40 | −8 | 4.35 | 88 |
| Middle FG | R11/47 | 38 | 42 | −8 | 3.65 | (L) |
| Middle FG | R11/47 | 32 | 48 | −10 | 3.53 | (L) |
Local maxima for these clusters are denoted with (L). R and L hemisphere is not designated for maxima within 6 mm of midline. Coordinates are in MNI space. These regions are shown in Fig. 1.
Fig. 2.
Regions identified in regression analyses (see Tables 2 and 3) whose activation during pain correlates P<.001 with individual differences in either anxiety sensitivity or fear of pain shown on canonical T1 anatomical images: (A) Sagittal (left) and axial (right) views of left MPFC region correlated (r=.890) with ASI scores; (B) Sagittal (left) and axial (right) views of right lateral orbital frontal region correlated (r=.912) with FPQ scores. Scatter-plots illustrate these correlations. See Table 3 for correlations of these regions with STAI scores.
Because temperatures used to elicit pain (rated seven out of 10 where 10 is the worst pain imaginable) differed across participants, it was important to examine the relationship between temperature, ASI and FPQ scores, and brain activation to determine whether brain-behavior correlations are significantly influenced by the absolute magnitude of the noxious thermal stimulation experienced. Although scores on both questionnaires were significantly inversely correlated with temperature thresholds (FPQ: r=−.577, P< 0.05; ASI: r=−.640, P>0.05), it does not appear that differences in thresholded temperature can account for correlations between ASI and FPQ scores and activation of lateral orbitofrontal, medial prefrontal, and cingulate systems. As shown in Table 3, when the effects of temperature thresholds were partialled out of these relationships, the relationships to anxiety sensitivity, fear of pain, and brain activation were not appreciably diminished.
Because behavioral responses to pain (Keogh & Herdenfeldt, 2002; Keogh et al., 2004) and neural responses to affect arousing stimuli may co-vary with gender (Hamann & Canli, 2004), parameter estimates for regions of activation identified in the group contrasts were compared (using Bonferroni-corrected post-hoc t-tests) for male and female participants. No significant gender differences were observed (all t<2, P>.1).
4. Discussion
This is the first study to examine the neural correlates of individual differences in pain-related anxiety and fear. Three important relationships between individual variability in fear of pain, anxiety sensitivity, and brain activation were revealed.
First, activation in two pain processing regions identified in the overall contrast of painful heat and non-painful warmth—the anterior and posterior cingulate—correlated with FPQ scores, whereas ASI scores did not correlate with activation of any regions identified in this contrast. Anterior cingulate has been associated with pain and pain affect (Coghill et al., 1999; Peyron et al., 2000; Rainville et al., 2002) and with evaluation of emotional stimuli more generally (Davidson & Irwin, 1999; Ochsner and Feldman Barrett, 2001; Phillips et al., 2003), and is thought to monitor ongoing processing to signal when something is ‘wrong’ enough to require an alteration in behavior (Botvinick et al., 2001; Eisenberger & Lieberman, 2004; Ochsner and Feldman Barrett, 2001). In this context, pain may be a primitive signal for behavioral change—although more complex circumstances may elicit the signal as well (Botvinick et al., 2001; Eisenberger & Lieberman, 2004; Ochsner et al., 2001, 2002). The posterior cingulate has been associated with evaluating the valence of external and potentially threatening stimuli (Maddock et al., 1997Maddock et al., 2003). Recruitment of anterior and posterior cingulate cortices may suggest that individuals high in fear of pain closely monitor and evaluate the potential threat value of a painful stimulus.
Second, a regression analysis identified additional regions that correlated even more strongly with ASI and FPQ scores. This analysis correlated questionnaire scores with actvation in the pain>warmth contrast for all voxels in cingulate and frontal regions of interest. Such analyses allow identification of regions whose activation co-varies with individual difference measures, but may be lost in overall group contrasts that average activations for high and low scorers.
FPQ scores predicted activation of right lateral orbital prefrontal cortex (OFC). In prior work, activation of ventrolateral/orbitofrontal cortex has been observed during pain (Lorenz et al., 2003; Lotze et al., 2001; Rolls et al., 2003; Tracey et al., 2000) and during affective states more generally—including induced depressed mood (Baker et al., 1997), anger (Kimbrell et al., 1999), when sensing pleasant tastes (O’Doherty, et al., 2001, 2001), and when receiving rewards (Rogers et al., 1999; O’Doherty et al., 2001) or punishments (O’Doherty, Kringelbach et al., 2001,O’Doherty, Kringelbach et al., 2003) for choices in a computerized game. Other work suggests that this region may track changes in the motivational value of stimuli and guide response selection accordingly. Thus, OFC lesions impair alteration or inhibition of a prepotent response to a previously reinforced stimulus (Rolls et al., 1994), and activation of this region is found when participants must inhibit or select among competing responses (Barch et al., 2001; Cunningham et al., 2004; Kiehl et al., 2000; Nobre et al., 1999; O’Doherty et al., 2003), including the down-regulation of negative emotion via cognitive reappraisal (Ochsner et al., 2004b) or pain via cognitive distraction (Bantick et al., 2002; Petrovic et al., 2000), or placebo (Lieberman et al., 2004; Petrovic et al., 2002; Wager et al., 2004). Right lateral OFC activity may therefore reflect attempts by fearful individuals to evaluate and/or regulate possible responses to the painful stimulus (Ochsner & Gross, 2005). Anterior and posterior cingulate cortex, discussed above, may signal the presence of threatening levels of pain and trigger regulatory processes implemented in OFC (Eisenberger & Lieberman, 2004; Ochsner & Gross, 2005).
ASI scores, which index the tendency to feel anxious about the negative implications of bodily sensations, predicted activation of medial prefrontal cortex (MPFC). In prior work, this region of MPFC has been implicated in self-reflective and self-regulatory processes that might be related to the kinds of self-focused attention and anxiety measured by the ASI. Thus, this region of MPFC has been implicated in judging how well trait words describe one’s self (Kelley et al., 2002) and/or others (Mitchell et al., 2002; Ochsner et al., in press), processing emotional cues (Bush at al, 1997; Mohanty et al., 2005), and motivating responses (Wager et al., 2005), judging (Gusnard et al., 2001; Ochsner et al., 2004a) or becoming distant from (Ochsner et al., 2004b) one’s emotional state, rumination (Ray et al., 2005), depression (Drevets, 2000), and has been hypothesized to support a default state of self-monitoring present when participants are not explicitly directed to engage in a specific task (Gusnard & Raichle, 2001). Notably, ASI scores for our participants were somewhat low compared to previously observed means for non-clinical undergraduate populations (Reiss et al., 1986). This might suggest that even at low overall levels of anxiety sensitivity, higher ASI scores may predict engagement of mechanisms supporting self-focused attention and monitoring of one’s internal state. It remains for future work, however, to determine whether the same findings will be observed in individuals with higher mean ASI scores, although the association of MPFC with induced anxiety (Simpson et al., 2001) and PTSD (e.g. Bremner et al., 1999) suggests that it might be likely. Taken together, the present and prior findings dovetail to suggest that MPFC supports self-focused elaboration of the negative personal implications of pain that may characterize individuals high in anxiety sensitivity (Reiss et al., 1986).
Third and last, the relationships of ASI and FPQ scores to brain activation cannot be explained wholly by differences in either of two potential confounding factors. The first is generalized trait anxiety as indexed by STAI-T scores (Spielberger et al., 1983). Critically, STAI-T scores were not correlated with activation of any brain regions. The second concerned the fact that, across participants, different temperatures were used to obtain equal levels of subjectively experienced thermal pain. This raises the possibility that differences in the absolute magnitude of thermal stimulation could at least partially underlie the brain-behavior relationships reported here. However, we found that the significant relationships reported in Table 3 were not substantially impacted when differences in temperature thresholds were removed statistically. This suggests that the psychological interpretation or appraisal of pain—which presumably results in the subjective experience of pain—is what may be associated with pain-related fear and anxiety.
The present findings may have important implications for at least four domains of research. First, the identification of regions recruited by individuals high in pain-related fear and anxiety may help explain variability in medial and orbital frontal activation across studies examining pain (Lorenz et al., 2003; Lotze et al., 2001; Peyron et al., 2000; Rolls et al., 2003) and also may help clarify abnormal patterns of cingulate, medial and orbital frontal activation in chronic pain (Grachev et al., 2002; Apkarian et al., 2001, 2003). To the extent that anxiety and worry exacerbate chronic or current pain, greater activation of these systems may be observed. More broadly, identifying neural markers for anxiety and fear may be relevant to the study of individuals with psychiatric conditions, such as panic disorder, that are characterized by anxiety about bodily sensations (McNally, 2002).
Second, the involvement of MPFC and OFC in pain-related fear and anxiety broadens our understanding of the functional roles that these brain systems play in emotion. The present study extends prior work associating medial prefrontal cortex with an anxious state during anticipation of pain (Sawamoto et al., 2000; Simpson et al., 2001) by demonstrating that trait differences in anxiety predict MPFC recruitment when a painful stimulus is present. Similarly, OFC lesions have been shown to diminish fear of the consequences of socially inappropriate behavior (Beer et al., 2003; Davidson et al., 2001), and the association of OFC with fear of pain may suggest a broader role for this region in other types of fear-related processing.
Third, the fact that anxiety sensitivity and fear of pain correlate with activation of distinct brain regions that have been associated with different self-reflective and regulatory and functions may suggest that the ASI and FPQ tap into different psychological constructs. This possibility was suggested by Keogh et al. (2001), who found that FPQ but not ASI scores predicted a failure to deflect attention away from pain-related cues.
Fourth, gender differences may influence attention to, elaboration of, and emotional responses to pin and other life events (Kring & Gordon, 1998; Keogh & Herdenfeldt, 2002; Keogh et al., 2004), which may be reflected in the differential recruitment of brain systems (Hamann & Canli, 2004; Wager & Ochsner, 2005). Although the present study did not observe significant differences in either pain behavior or brain activation—very possibly due to a small sample—it will be important for future work to examine this issue more closely.
Finally, it is important to note that although the present study provides new insights into the routes by which anxiety and fear may modulate the activity of attentional and regulatory systems during the experience of pain, it does not clarify exactly why or how this modulation occurs. Information about causal mechanisms awaits studies examining the active self-regulation of pain.
Acknowledgments
The authors wish to acknowledge support by grants from the John and Dodie Rosekranz Endowment and the Foundation for Anesthesia Education and Research (SCM), grant BCS-93679 from the National Science Foundation (KNO) and grant RR 09784 from the NIH (GHG).
References
- Apkarian A, Baliki M, Sosa Y, Parrish T, Harden R, Levy R, Chialvo D. Chronic back pain perception is mediated through orbitofrontal activity: an fMRI study of spontaneous fluctuations of ongoing pain. J Pain. 2003;4(2 Suppl):54. [Google Scholar]
- Asmundson GJ, Kuperos JL, Norton GR. Do patients with chronic pain selectively attend to pain-related information?: preliminary evidence for the mediating role of fear. Pain. 1997;72:27–32. doi: 10.1016/s0304-3959(97)00010-9. [DOI] [PubMed] [Google Scholar]
- Baker SC, Frith CD, Dolan RJ. The interaction between mood and cognitive function studied with PET. Psychol Med. 1997;27:565–78. doi: 10.1017/s0033291797004856. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–9. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Barch DM, Braver TS, Akbudak E, Conturo T, Ollinger J, Snyder A. Anterior cingulate cortex and response conflict: effects of response modality and processing domain. Cereb Cortex. 2001;11:837–48. doi: 10.1093/cercor/11.9.837. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Beer JS, Heerey EA, Keltner D, Scabini D, Knight RT. The regulatory function of self-conscious emotion: insights from patients with orbitofrontal damage. J Pers Soc Psychol. 2003;85(4):594–604. doi: 10.1037/0022-3514.85.4.594. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108:624–52. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–11. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Frazier JA, Rauch SL, Seidman LJ, Whalen PJ, Jenike MA, Rosen MA, Biederman J. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the Counting Stroop. Biol Psychiatry. 1999;45:1542–52. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Coghill RC, Talbot JD, Evans AC, Meyer E, Gjedde A, Bushnell MC, Duncan GH. Distributed processing of pain and vibration by the human brain. J Neurosci. 1994;14:4095–108. doi: 10.1523/JNEUROSCI.14-07-04095.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill RC, Sang CN, Maisog JM, Iadarola MJ. Pain intensity processing within the human brain: a bilateral, distributed mechanism. J Neurophysiol. 1999;82:1934–43. doi: 10.1152/jn.1999.82.4.1934. [DOI] [PubMed] [Google Scholar]
- Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. 1999;80:329–39. doi: 10.1016/s0304-3959(98)00229-2. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: fMRI correlates of valence, emotional intensity, and control in the processing of attitudes. J Cogn Neurosci. 2004;16:1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Toward a biology of personality and emotion. Ann NY Acad Sci. 2001;935:191–207. doi: 10.1111/j.1749-6632.2001.tb03481.x. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taylor SJ, Crawley AP, Wood ML, Mikulis DJ. Functional MRI of pain- and attention-related activations in the human cingulate cortex. J Neurophysiol. 1997;77:3370–80. doi: 10.1152/jn.1997.77.6.3370. [DOI] [PubMed] [Google Scholar]
- Davis KD, Taub E, Duffner F, Lozano AM, Tasker RR, Houle S, Dostrovsky JO. Activation of the anterior cingulate cortex by thalamic stimulation in patients with chronic pain: a positron emission tomography study. J Neurosurg. 2000;92:64–9. doi: 10.3171/jns.2000.92.1.0064. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Glover G. 3D Z-shim method for reduction of susceptibility effects in BOLD fMRI. Magn Reson Med. 1999;42:290–9. doi: 10.1002/(sici)1522-2594(199908)42:2<290::aid-mrm11>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Grachev ID, Fredrickson BE, Apkarian AV. Brain chemistry reflects dual states of pain and anxiety in chronic low back pain. J Neural Transm. 2002;109:1309–34. doi: 10.1007/s00702-002-0722-7. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–64. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann S, Canli T. Individual differences in emotion processing. Curr Opin Neurobiol. 2004;14(2):233–8. doi: 10.1016/j.conb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Keogh E, Herdenfeldt M. Gender, coping and the perception of pain. Pain. 2002;97(3):195–201. doi: 10.1016/S0304-3959(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Keogh E, Dillon C, Georgiou G, Hunt C. Selective attentional biases for physical threat in physical anxiety sensitivity. J Anxiety Disord. 2001;15:299–315. doi: 10.1016/s0887-6185(01)00065-2. [DOI] [PubMed] [Google Scholar]
- Keogh E, Ellery D, Hunt C, Hannent I. Selective attentional bias for pain-related stimuli amongst pain fearful individuals. Pain. 2001;91:91–100. doi: 10.1016/s0304-3959(00)00422-x. [DOI] [PubMed] [Google Scholar]
- Keogh E, Hamid R, Hamid S, Ellery D. Investigating the effect of anxiety sensitivity, gender and negative interpretative bias on the perception of chest pain. Pain. 2004;111(1–2):209–17. doi: 10.1016/j.pain.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–23. [PubMed] [Google Scholar]
- Kimbrell TA, George MS, Parekh PI, Ketter TA, Podell DM, Danielson AL, Repella JD, Benson BE, Willis MW, Herscovitch P, Post RM. Regional brain activity during transient self-induced anxiety and anger in healthy adults. Biol Psychiatry. 1999;46:454–65. doi: 10.1016/s0006-3223(99)00103-1. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Sekihara K, Miyashita Y. No-go dominant brain activity in human inferior prefrontal cortex revealed by functional magnetic resonance imaging. Eur J Neurosci. 1998;10:1209–13. doi: 10.1046/j.1460-9568.1998.00167.x. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: expression, experience, and physiology. J Pers Soc Psychol. 1998;74(3):686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- Lane RD, Chua PM, Dolan RJ. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia. 1999;37:989–97. doi: 10.1016/s0028-3932(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, Naliboff BD, Suyenobu BY, Mandelkern M, Mayer EA. The neural correlates of placebo effects: a disruption account. Neuroimage. 2004;22:447–55. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Lorenz J, Minoshima S, Casey KL. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain. 2003;126:1079–91. doi: 10.1093/brain/awg102. [DOI] [PubMed] [Google Scholar]
- Lotze M, Wietek B, Birbaumer N, Ehrhardt J, Grodd W, Enck P. Cerebral activation during anal and rectal stimulation. Neuroimage. 2001;14:1027–34. doi: 10.1006/nimg.2001.0901. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Faber SD, Janeck AS. Pain-related anxiety predicts non-specific physical complaints in persons with chronic pain. Behav Res Ther. 1998;36:621–30. doi: 10.1016/s0005-7967(97)10039-0. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Spertus IL, Janeck AS, Sinclair D, Wetzel FT. Behavioral dimensions of adjustment in persons with chronic pain: pain-related anxiety and acceptance. Pain. 1999;80:283–9. doi: 10.1016/s0304-3959(98)00219-x. [DOI] [PubMed] [Google Scholar]
- McNally RJ. Memory and anxiety disorders. Philos Trans R Soc Lond B Biol Sci. 1997;352:1755–9. doi: 10.1098/rstb.1997.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally RJ. Anxiety sensitivity and panic disorder. Biol Psychiatry. 2002;52:938–46. doi: 10.1016/s0006-3223(02)01475-0. [DOI] [PubMed] [Google Scholar]
- McNeil DW, Rainwater AJ., 3rd Development of the fear of pain questionnaire—III. J Behav Med. 1998;21:389–410. doi: 10.1023/a:1018782831217. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Heatherton TF, Macrae CN. Distinct neural systems subserve person and object knowledge. Proc Natl Acad Sci USA. 2002;99:15238–43. doi: 10.1073/pnas.232395699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Herrington JD, Koven NS, Fisher JE, Wenzel EA, Webb AG, Heller W, Banich MT, Miller GA. Neural mechanisms of affective interference in schizotypy. J Abnorm Psychol. 2005;114:16–27. doi: 10.1037/0021-843X.114.1.16. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Coull JT, Frith CD, Mesulam MM. Orbitofrontal cortex is activated during breaches of expectation in tasks of visual attention. Nat Neurosci. 1999;2:11–12. doi: 10.1038/4513. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nat Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Rolls ET, Francis S, Bowtell R, McGlone F. Representation of pleasant and aversive taste in the human brain. J Neurophysiol. 2001;85:1315–21. doi: 10.1152/jn.2001.85.3.1315. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Critchley H, Deichmann R, Dolan RJ. Dissociating valence of outcome from behavioral control in human orbital and ventral prefrontal cortices. J Neurosci. 2003;23:7931–9. doi: 10.1523/JNEUROSCI.23-21-07931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Feldman B. A multiprocess perspective on the neuroscience of emotion. In: Mayne TJ, Bonanno GA, editors. Emotions: currrent issues and future directions. New York: The Guilford Press; 2001. pp. 38–81. [Google Scholar]
- Ochsner KN, Gross JJ. Thinking makes it so: a social cognitive neuroscience approach to emotion regulation. In: Vohs K, Baumeister R, editors. The handbook of self-regulation. New Jersey: Erlbaum; 2004. [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Kosslyn SM, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, Rauch SL. Deficits in visual cognition and attention following bilateral anterior cingulotomy. Neuropsychologia. 2001;39:219–30. doi: 10.1016/s0028-3932(00)00114-7. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: an fMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow D, Hanelin J, Ramachandran T, Mackey S. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004a;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004b;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson E, Cooper J, Gabrieli JDE, Kihlstrom JF, D’Esposito M. The neural correlates of direct and reflected self-knowledge. Neuroimage. doi: 10.1016/j.neuroimage.2005.06.069. in press. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Petersson KM, Ghatan PH, Stone-Elander S, Ingvar M. Pain-related cerebral activation is altered by a distracting cognitive task. Pain. 2000;85:19–30. doi: 10.1016/s0304-3959(99)00232-8. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia—imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Neurophysiol Clin. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JD. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Rainville P, Hofbauer RK, Bushnell MC, Duncan GH, Price DD. Hypnosis modulates activity in brain structures involved in the regulation of consciousness. J Cogn Neurosci. 2002;14:887–901. doi: 10.1162/089892902760191117. [DOI] [PubMed] [Google Scholar]
- Ray RD, Ochsner KN, Cooper JC, Robertson EK, Gabrieli JDE, Gross JJ. Individual differences in trait rummation modulate neural systems supporting the cognitive regulation of emotion. Cogn Affect Behav Neurosci. 2005;5:156–68. doi: 10.3758/cabn.5.2.156. [DOI] [PubMed] [Google Scholar]
- Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Hornak J, Wade D, McGrath J. Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry. 1994;57:1518–24. doi: 10.1136/jnnp.57.12.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, O’Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13:308–17. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, Hanakawa T, Kanda M, Fukuyama H, Konishi J, Shibasaki H. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. J Neurosci. 2000;20:7438–45. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex. II. During anticipatory anxiety. Proc Natl Acad Sci USA. 2001;98:688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushemne R, Vagg PR, Jacobs GA. Manual for the state-trait anxiety inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Tracey I, Becerra L, Chang I, Breiter H, Jenkins L, Borsook D, Gonzalez RJ. Noxious hot and cold stimulation produce common patterns of brain activation in humans: a functional magnetic resonance imaging study. Neurosci Lett. 2000;288:159–62. doi: 10.1016/s0304-3940(00)01224-6. [DOI] [PubMed] [Google Scholar]
- Wager TD, Ochsner KN. Sex differences in the emotional brain. NeuroReport. 2005;16(5):85–7. doi: 10.1097/00001756-200502080-00001. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in fMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Jonides J, Smith EE. Individual differences in multiple subtypes of switching attention. Cogn Affect Behav Neurosci. 2005;5(2):127–143. doi: 10.3758/cabn.5.2.127. [DOI] [PubMed] [Google Scholar]
- Wagner AD. Working memory contributions to human learning and remembering. Neuron. 1999;22:19–22. doi: 10.1016/s0896-6273(00)80674-1. [DOI] [PubMed] [Google Scholar]
- Wood JN, Romero SG, Makale M, Grafman J. Category-specific representations of social and nonsocial knowledge in the human prefrontal cortex. J Cogn Neurosci. 2003;15:236–48. doi: 10.1162/089892903321208178. [DOI] [PubMed] [Google Scholar]