Abstract
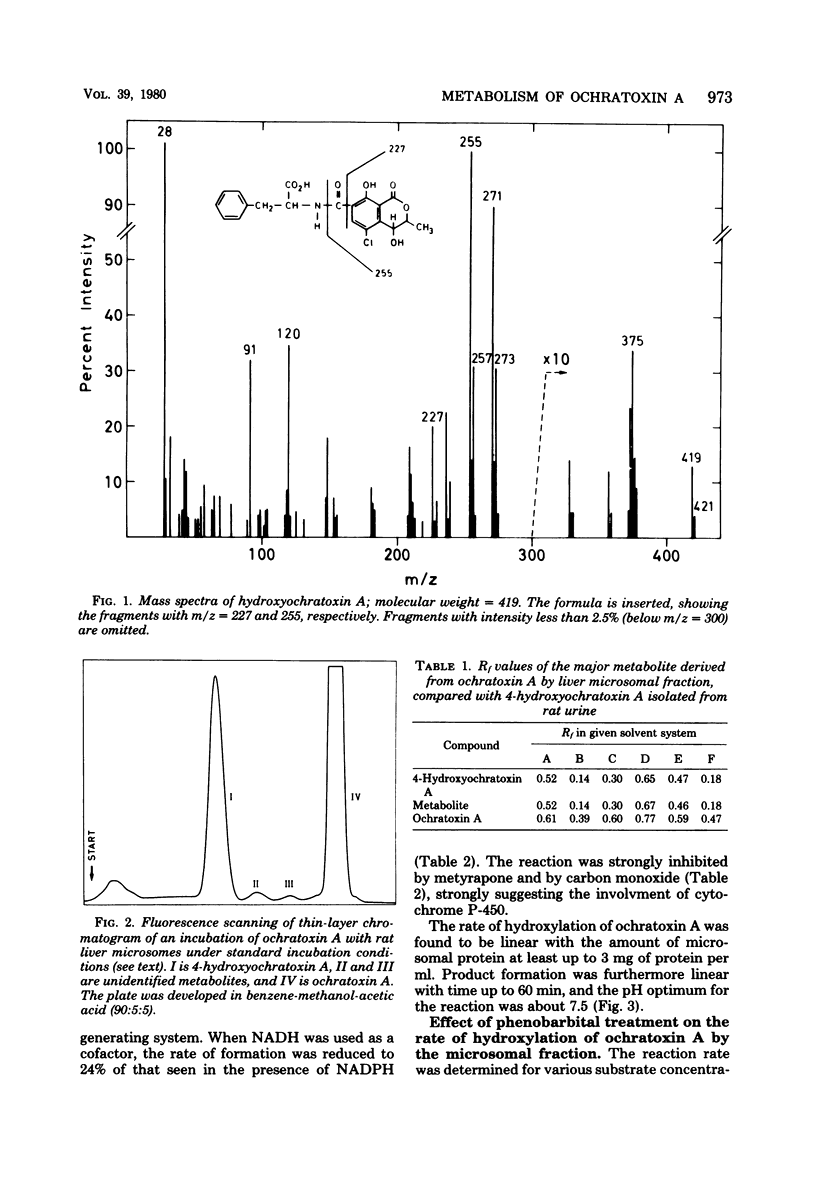
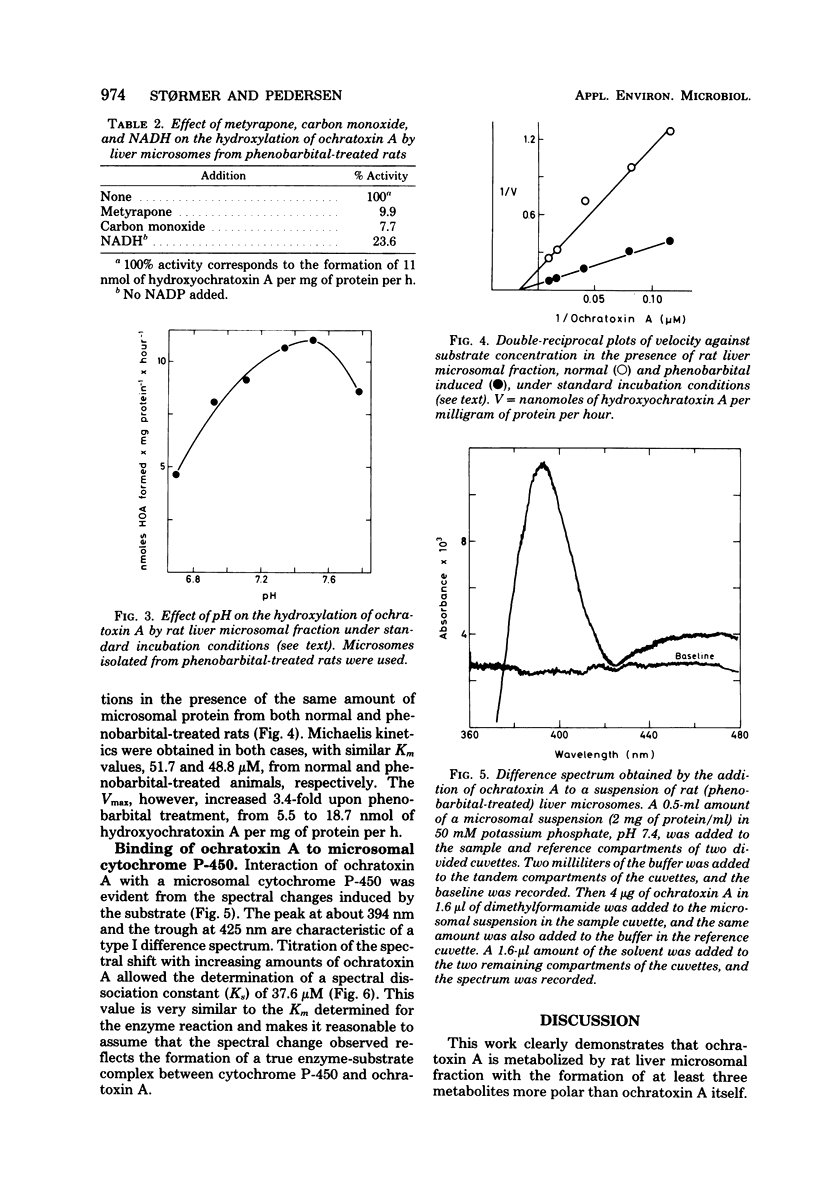
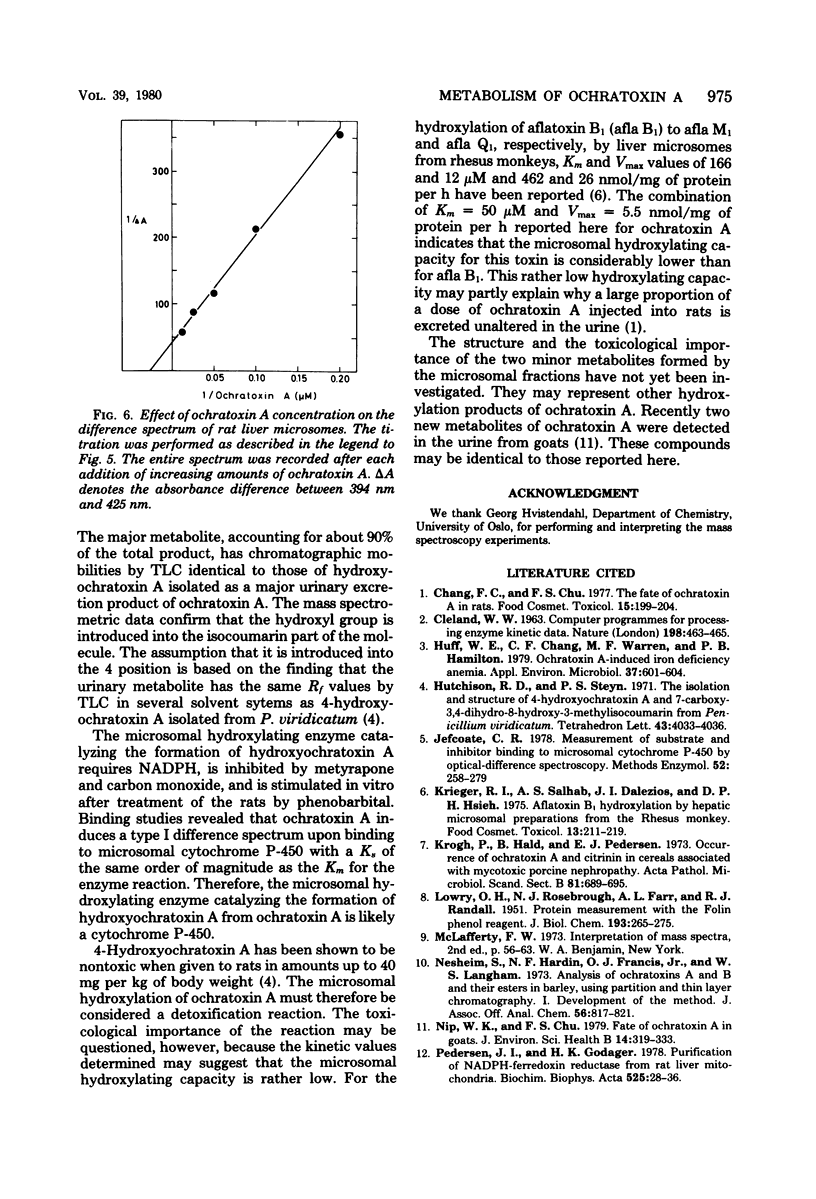
Hydroxyochratoxin A was isolated and identified from the urine of rats after injection with ochratoxin A. By incubating ochratoxin A with rat liver microsomes and reduced nicotinamide adenine dinucleotide phosphate, one major (90%) and two minor metabolites, more polar than ochratoxin A, were formed. Thin-layer chromatography revealed that the major metabolite had Rf values identical to those of hydroxyochratoxin A in six different solvent systems. Formation of the metabolites in vitro was inhibited by carbon monoxide and by metyrapone, and the rate of formation increased after pretreatment of the rats with phenobarbital. A type I spectrum appeared upon binding of ochratoxin A to microsomes with a spectral dissociation constant (Ks) of 37.6 microM. These findings strongly suggest the involvement of a cytochrome P-450 in the hydroxylation of ochratoxin A by rat liver microsomes. Apparent Km and Vmax values for the formation of hydroxyochratoxin A were determined to 50 microM and 5.5 nmol/mg of protein per h, respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLELAND W. W. Computer programmes for processing enzyme kinetic data. Nature. 1963 May 4;198:463–465. doi: 10.1038/198463a0. [DOI] [PubMed] [Google Scholar]
- Chang F. C., Chu F. S. The fate of ochratoxin A in rats. Food Cosmet Toxicol. 1977 Jun;15(3):199–204. doi: 10.1016/s0015-6264(77)80390-8. [DOI] [PubMed] [Google Scholar]
- Huff W. E., Chang C. F., Warren M. F., Hamilton P. B. Ochratoxin A-induced iron deficiency anemia. Appl Environ Microbiol. 1979 Mar;37(3):601–604. doi: 10.1128/aem.37.3.601-604.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefcoate C. R. Measurement of substrate and inhibitor binding to microsomal cytochrome P-450 by optical-difference spectroscopy. Methods Enzymol. 1978;52:258–279. doi: 10.1016/s0076-6879(78)52029-6. [DOI] [PubMed] [Google Scholar]
- Krieger R. I., Salhab A. S., Dalezios J. I., Hsieh D. P. Aflaxation B1 hydroxylation by hepatic microsomal preparations from the rhesus monkey. Food Cosmet Toxicol. 1975 Apr;13(2):211–219. doi: 10.1016/s0015-6264(75)80006-x. [DOI] [PubMed] [Google Scholar]
- Krogh P., Hald B., Pedersen E. J. Occurrence of ochratoxin A and citrinin in cereals associated with mycotoxic porcine nephropathy. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Dec;81(6):689–695. doi: 10.1111/j.1699-0463.1973.tb02261.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Nip W. K., Chu F. S. Fate of ochratoxin A in goats. J Environ Sci Health B. 1979;14(3):319–333. doi: 10.1080/03601237909372131. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I., Godager H. K. Purification of NADPH-ferredoxin reductase from rat liver mitochondria. Biochim Biophys Acta. 1978 Jul 7;525(1):28–36. doi: 10.1016/0005-2744(78)90196-1. [DOI] [PubMed] [Google Scholar]


