Abstract
Prostate cancer is the second leading cause of cancer death among men in the United States. Standard‐of‐care chemotherapy for metastatic castration‐resistant prostate cancer is associated with significant but modest survival benefit, indicating a need for alternative and/or additional approaches. The use of therapeutic cancer vaccines for the treatment of prostate cancer represents a novel targeted therapeutic approach. Whereas vaccine strategies are being developed for the treatment of various stages of prostate cancer, this article focuses on novel vaccine strategies for castration‐resistant prostate cancer that have been translated into late‐stage clinical studies.
Keywords: prostate cancer, immunotherapy, poxviral vaccines, radiation
Therapeutic Cancer Vaccines
Therapeutic cancer vaccines constitute a potential emerging modality for the treatment of a wide range of human malignancies, including prostate cancer. Areas of intense investigation include the development and characterization of (a) tumor‐associated antigens (TAAs) or tumor‐specific antigens selectively expressed or overexpressed by tumor cells relative to normal adult tissues; (b) novel vaccine delivery systems for the induction of endogenous host antitumor immune responses; (c) cytokines and other immunostimulatory molecules to further amplify the immunogenicity of vaccine formulations; and (d) approaches employing dendritic cells (DCs) expressing relevant tumor‐rejection antigens or effector cells which activate or mediate antitumor responses, respectively.
Rationale for Vaccine Therapy in the Treatment of Prostate Cancer
Prostate cancer is the most prevalent solid tumor malignancy among men in the United States, accounting for over 192,000 new diagnoses, resulting in approximately 27,000 deaths in 2009 alone. 1 Despite local therapy, up to 40% of patients develop recurrent disease initially characterized by elevated levels of prostate‐specific antigen (PSA). Upon relapse, androgen ablation is only temporarily effective, secondary to the development of prostate cancer cells that are able to grow in spite of castrate levels of testosterone. 2 Ultimately, patients develop overt metastatic disease, with current treatment options including secondary hormone therapy, chemotherapy, and investigational agents. Docetaxel is the only chemotherapeutic agent shown to lengthen overall survival (OS) (by approximately 2–3 months) in men with metastatic castration‐resistant prostate cancer (mCRPC). 3 Although approval of docetaxel by the US Food and Drug Administration (FDA) in 2004 represented a significant milestone in the treatment of prostate cancer, alternative therapeutic strategies with more favorable toxicity profiles are highly sought after for the management of patients with disease progression. Prostate cancer patients often present with low tumor burden and good performance status and are therefore ideal candidates for vaccine therapy.
Prostate‐Specific Tumor‐Associated Antigens as Targets for Immunotherapy
The ideal targets for vaccine‐mediated immune responses are TAAs that are overexpressed in, or unique to, cancer cells relative to nonmalignant tissue. Prostate cancer cells express several of these TAAs. PSA is a 34‐kD protein uniquely expressed in prostate cancer cells and in nonessential epithelial cells within the prostate, making it the main target for numerous prostate cancer vaccines. 4 , 5 Prostate‐specific membrane antigen (PSMA) is a 100‐kD transmembrane glycoprotein mainly expressed in primary and metastatic prostate cancer cells. 6 This TAA is unique in that its expression is augmented by androgen deprivation, a fundamental treatment for prostate cancer. 7 Another potential target for vaccines is prostatic acid phosphatase (PAP), a 102‐kD TAA glycoprotein expressed on over 95% of prostate cancer cells and believed to be associated with disease progression. 8 , 9
Vaccine Strategies
At this writing, no therapeutic cancer vaccine has been approved by the FDA. However, recent evidence indicates patient benefit with the use of several new cancer vaccines and vaccine strategies for prostate cancer, either as monotherapy or combined with conventional therapeutic regimens. 10 , 11 , 12 Promising results in several clinical studies with various vaccine platforms in prostate cancer have led to ongoing or planned phase III trials ( Table 1 ).
Table 1.
Prostate cancer vaccines in late‐stage clinical trials.
| Vaccines as monotherapy | ||||||
|---|---|---|---|---|---|---|
| Cancer stage | Vaccine type | Comment | Trial phase | n | Outcomes | Ref. |
| Metastatic CRPC | Dendritic cell | Autologous PBMC activated with a PAP‐GM‐CSF fusion protein | III | 512 | 25.8‐month median survival on vaccine arm vs. 21.7 months on placebo arm. Overall 22.5% reduction in risk of death (HR 0.775, p= 0.032). | 43 |
| Metastatic CRPC | Dendritic cell | Autologous PBMC activated with a PAP‐GM‐CSF fusion protein | III | 127 | Median survival of 25.9 months on vaccine arm vs. 21.4 months on placebo arm (p= 0.01). | 16 |
| Metastatic CRPC | Allogeneic whole tumor cell | Two irradiated prostate cancer cell lines engineered to express GM‐CSF | III | 626 | Overall survival of patients receiving vaccine was similar to patients receiving standard of care docetaxel. | 20 |
| Metastatic CRPC | Viral vector | rV/rF‐PSA‐TRICOM | II | 125 | Median survival of 25.1 months on vaccine arm vs. 16.6 months on placebo arm (HR 0.56, p= 0.0061) | 12 |
| Metastatic CRPC | Viral vector | rV/rF‐PSA‐TRICOM | II | 32 | 26.6‐month median survival with vaccine vs. 17.4‐month predicted survival | 36 |
| CRPC | Allogeneic whole tumor cell | Three irradiated prostate cancer cell lines with BCG adjuvant | II | 52 | Patients without bone metastases (n= 26): Those with declining PSAV post‐vaccination (42%) had median TTP of 58 weeks vs. historical 20‐29 weeks. Patients with bone metastases (n= 13): Those with declining PSA post‐vaccination (23%) had a median TTP of 23 weeks vs. historical 9‐16 weeks. | 17, 65 |
| Metastatic CRPC or localized recurrent disease | Dendritic cell | Autologous dendritic cells pulsed with 2 PSMA peptides | II | 62 | Patients with clinical response (30%) had median TTP of 144 days (metastatic cohort) and 184 days (local recurrence cohort). | 66 , 67 |
| Vaccines in combination with conventional therapies | ||||||
|---|---|---|---|---|---|---|
| Cancer stage | Vaccine | Conventional therapy | Trial phase | n | Outcomes | Ref. |
| Metastatic CRPC | Dendritic cell: autologous PBMC activated with a PAP‐GM‐CSF fusion protein | Docetaxel | III | 225 | Integrated analysis of patients receiving docetaxel in two identical phase III trials. Median survival with docetaxel post‐progression on vaccine (n= 51) was 34.5 months vs. 25.4 months with docetaxel post‐progression on placebo (n= 31) (HR 1.9, p= 0.023). | 44 |
| Metastatic CRPC | Viral vector: rV‐PSA/rV‐B7‐1 prime/ rF‐PSA boost | Docetaxel | II | 28 | Equivalent increase in PSA‐specific T cells post‐vaccination with and without docetaxel. Patients treated with docetaxel after progression on vaccine (n= 11) showed longer TTP on docetaxel (6.1 months) vs. historical controls (3.7 months). | 45 |
| Biochemical failure post prostatectomy | Liposome MUC‐1 | Cyclophosphamide | I | 16 | 50% of patients had stabilization of PSA | 68 |
| Localized prostate cancer | Viral vector: rV‐PSA/rV‐B7‐1 prime/ rF‐PSA boost | Radiotherapy | II | 30 | 76.5% of patients in the combination therapy arm showed >3‐fold increase in PSA‐specific T cells vs. 0% with radiotherapy alone (p < 0.0005). | 61 |
| Non‐metastatic CRPC | Viral vector: rV‐PSA/rV‐B7‐1 prime/ rF‐PSA boost | Nilutamide | II | 42 | Median overall survival for patients on nilutamide after progression on vaccine was 6.2 years vs. 3.7 years for patients on vaccine following progression on nilutamide (p= 0.045). | 11 |
PBMC = peripheral blood mononuclear cell; PSAV = prostate‐specific antigen velocity.
Vaccine as Monotherapy
An innovative approach to vaccine therapy relies on antitumor responses elicited by autologous DCs following ex vivo loading and stimulation with an antigen. The DC vaccine sipuleucel‐T (Dendreon Corp., Seattle, WA, USA) consists of autologous DCs activated in vitro with a fusion of the TAA PAP and granulocyte‐macrophage colony‐stimulating factor (GM‐CSF) following leukapheresis. Three phase I/II studies have demonstrated the safety and efficacy of this strategy in metastatic and nonmetastatic CRPC. 13 , 14 , 15 An initial phase III placebo‐controlled study was completed in patients with mCRPC with time to progression (TTP) as primary endpoint ( Table 1 ). Results indicated a trend to improved TTP in patients treated with vaccine relative to placebo (p= 0.052). Although this trial failed to meet its primary endpoint, there was statistically significant improvement in median OS with vaccine (25.9 months in the treatment arm vs. 21.4 months on placebo; p= 0.01). 16 The results of a defnitive randomized, controlled study with sipuleucel‐T were recently presented. 17 In this study, there was a statistically significant and clinically meaningful improvement in OS (25.8 months vs. 21.7 months; p= 0.032) in patients receiving vaccine compared with those receiving placebo.
Allogeneic whole tumor cell vaccines are vaccines utilizing tumor cell lines, of en engineered to express cytokines and thus become more immunogenic. GVAX (Cell Genesys, Inc., San Francisco, CA, USA) consists of two prostate cancer cell lines (LNCaP and PC‐3) engineered to express GM‐CSF at the vaccine site ( Table 1 ). In a phase II study, the median survival for patients with metastatic disease (n= 34) was 26.2 months, more than 6 months longer than the median survival predicted for each group by the Halabi nomogram. 18 Based on these encouraging data, a multicenter, randomized, controlled phase III trial (VITAL‐1) was designed to compare OS of asymptomatic metastatic prostate cancer patients treated with GVAX to those treated with docetaxel plus prednisone. VITAL‐1 completed recruitment of 626 patients in July 2007. Possibly because of the trial design (randomized potentially to chemotherapy), the patients enrolled on this study had more advanced disease than those enrolled on the phase II studies. 19 Because of data obtained from another study, the company did a non‐pre‐specified futility analysis and found that the OS of patients treated with GVAX was similar to those patients treated with docetaxel (hazard ratio [HR] 1.03), and with significantly less toxicity associated with vaccine. In addition, in the subgroup of patients with a Halabi nomogram predicted survival of > 18 months, there was a trend toward improved survival in patients randomized to treatment with vaccine compared to patients treated with docetaxel (HR 0.9). 20 As VITAL‐1 was not designed to show noninferiority, the trial was terminated in 2008 after a futility analysis showed only a 30% chance of demonstrating a survival benefit. Since these data are relatively immature with only 371 deaths having occurred in 621 enrolled patients at time of initial reporting, further follow‐up is planned. 20 Because patients with a longer predicted survival tended to have a greater improvement in survival compared with chemotherapy alone, it is possible that further follow‐up may show even more advantage to vaccine.
An alternative approach consists of vaccine strategies to enhance the host immune response to TAAs. Recent advances in these strategies have enhanced vaccine therapeutic efficacy and include (a) identification of new TAAs or modification of known TAAs to optimize epitope presentation to cytotoxic T lymphocytes (CTLs); (b) concurrent delivery of multiple costimulatory signals to augment the generation of tumor‐specific T‐cell responses; (c) use of diversified prime‐and‐boost regimens where patients are primed with a recombinant TAA encoded by recombinant vaccinia (rV) virus and boosted multiple times with a related nonreplicating recombinant fowlpox (rF) virus; and (d) combination of cancer vaccines with conventional therapies.
Early studies with rV‐PSA demonstrated that poxvirus vaccines enhance PSA‐specific immune responses. 21 , 22 , 23 , 24 Further, immune responses were augmented by incorporation of both vaccinia and avian poxviruses in a diversified prime‐and‐boost regimen. 22 , 24 , 25 These preclinical findings provided the basis for the use of vaccinia prime/fowlpox boost strategies in current clinical trials.
A second critical aspect of vaccine design is the delivery of strong costimulatory signals for effective T‐cell activation. Preclinical studies 26 , 27 , 28 , 29 , 30 have demonstrated the advantage of using poxvirus vaccines containing the transgenes for three T‐cell costimulatory molecules (TRICOM) along with the TAA transgene, compared to vaccines containing either one or no costimulatory transgenes ( Figure 1 ). This advantage was defined by both increases in T‐cell responses to the TAA and by antitumor responses. Furthermore, increasing costimulatory signals via TRICOM generates higher‐avidity CTLs and memory T cells. 30 , 31 , 32 , 33 Preclinical studies in tumor‐bearing mice transgenic for the human carcinoembryonic antigen (CEA‐Tg) treated with rV/rF‐CEA‐TRICOM showed significantly improved survival relative to mice receiving rV/rF‐CEA or to control animals. 29
Figure 1.
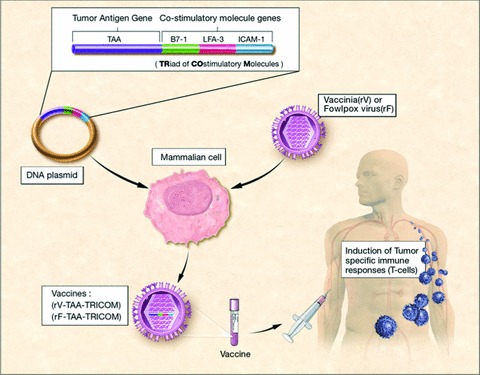
Recombinant vaccinia (rV) or recombinant fowlpox (rF) engineered to express both TAAs and a TRIad of COstimulatory Molecules (B7‐1, ICAM‐1, and LFA‐3) (TAA‐TRICOM) can induce tumor‐specific immune responses in both preclinical and clinical settings.
These preclinical findings provided the rationale for clinical translation of rV/rF‐PSA‐TRICOM, a prostate cancer vaccine now in late‐stage clinical studies. rV/rF‐PSA‐TRICOM is a vector‐based vaccine delivered in a heterologous prime‐and‐boost regimen as an initial priming dose of vaccinia recombinant for PSA‐TRICOM (rV‐PSA‐TRICOM) followed by boosts with rF expressing the same four transgenes (rF‐PSA‐TRICOM). 12 , 34 Two concurrent phase II clinical studies have demonstrated the clinical potential of rV/rF‐PSA‐TRICOM in patients with metastatic prostate cancer ( Table 1 ). One recent study was a randomized, placebo (empty vector)‐controlled, multicenter trial in patients (n= 125) with progressive metastatic disease despite castrate testosterone levels and a Gleason score of ≤7. 12 , 35 Patients with visceral disease or a history of chemotherapy or narcotic use were excluded. Although the study did not meet its primary endpoint of progression‐free survival, vaccinated patients had a greater 3‐year OS rate than control patients (30% vs. 17%), and a longer median OS (25.1 vs. 16.6 months; estimate d HR 0.56; 95% CI 0.37–0.85) ( Figure 2 ). The vaccine was well tolerated and associated with a 44% reduction in the death rate and an 8.5‐month increase in median OS over patients treated with placebo (p= 0.0061). 12
Figure 2.
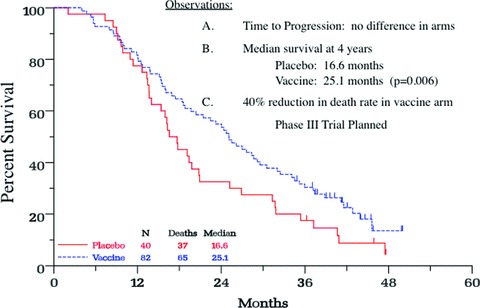
Overall survival (OS) analysis of a phase II, randomized, controlled trial of a poxviral‐based PSA‐targeted immunotherapy in mCRPC. Graphs indicate the Kaplan‐Meier estimator for vaccine (blue) and control (red) arms. Vertical ticks indicate censoring times. Taken from Reference 12.
A concurrent multicenter, randomized phase II trial employing rV/rF‐PSA‐TRICOM vaccine also provided evidence of enhanced median OS (p= 0.0061) in patients with mCRPC. 36 In this study, 32 patients were vaccinated with rV/ rF‐PSA‐TRICOM in a diversified prime‐and‐boost regimen ( Figure 3 ). No exclusion criteria for visceral disease or narcotic use were applied. While Gleason score has traditionally been a main prognostic indicator for localized prostate cancer, this trial stratified patient responses retrospectively using the Halabi nomogram, 37 a tool that can be used to predict OS in patients with mCRPC. The Halabi nomogram is a pretreatment prognostic model derived from results of six separate Cancer and Leukemia Group B (CALGB) trials of 1,101 patients with mCRPC, which predicts OS based on seven baseline prognostic factors: lactate dehydrogenase, PSA, alkaline phosphatase, Gleason score, Eastern Cooperative Oncology Group (ECOG) performance status, hemoglobin, and presence of visceral disease. Following vaccination with rV/rF‐PSA‐TRICOM, declining serum PSA and index lesion size were observed in 37.5% and 16.7% of patients, respectively. The median OS was 26.6 months (vs. 17.4 month median survival predicted by the Halabi nomogram) with a trend toward increased survival in those with a greater than six‐fold increase in PSA‐specific T cells (p= 0.055). Stratification of the results by Halabi predicted survival showed the most benefit in patients with more indolent disease ( Table 2 ). Patients with a predicted survival of less than 18 months (median 12.3 months) had an actual median OS of 14.6 months, while those with predicted survival of 18 months or more (median 20.9 months) will reach at least 37.3 months median OS (p= 0.035). 36
Figure 3.
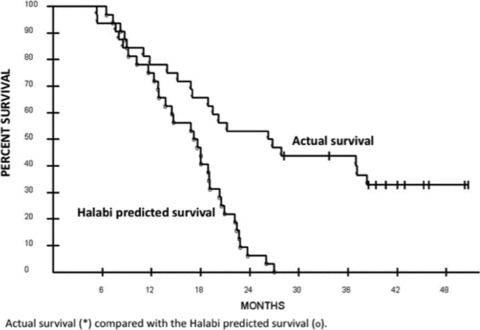
Overall survival analysis of a phase II study of PSA‐TRICOM in the treatment of mCRPC. The Kaplan‐Meier curve for all 32 patients enrolled demonstrates a median survival of 26.6 months and a median Halabi predicted survival of 17.4 months. Taken from Reference 36.
Table 2.
Vaccination of patients with PROSTVAC‐V/F. Survival predicted by Halabi nomogram versus actual overall survival. Taken from Reference 36.
| All patients (n= 32) | Patients with Halabi predicted survival < 18 months | Patients with Halabi predicted survival ≥ 18 months | |
|---|---|---|---|
| Predicted survival by Halabi score (months) | 17.4 | 12.3 | 20.9 |
| Actual median overall survival (months) | 26.6 | 14.6 | Not reached (≥ 37.3 months) |
| Difference (months) | 9.2 | 2.3 | ≥ 16.4 |
| Patients, survival longer than predicted by Halabi nomogram | 22 of 32 (69%) | 10 of 17 (59%) | 12 of 15 (80%) p = 0.035 |
These provocative studies provide preliminary evidence of clinically meaningful benefit and have resulted in the design of a confirmatory randomized, double‐blind, placebo‐controlled phase III trial in patients with mCRPC to begin in 2010.
Vaccine and Chemotherapy
Docetaxel is among the most widely used chemotherapeutic agents for carcinoma therapy and is the only FDA‐approved agent for metastatic prostate cancer. 3 The traditional goal of chemotherapeutic regimens has been to induce direct cytotoxicity and tumor cell death. However, certain chemotherapeutic agents including docetaxel have been shown to also modulate the expression of immune‐relevant proteins in the tumor cell as well components of the host immune system which can be advantageous for vaccine‐mediated antitumor activity. 38 , 39 , 40 , 41 , 42 Recent evidence from two placebo controlled randomized phase III trials with Sipuleucel‐T suggest clinical benefit for patients receiving docetaxel post‐vaccination. 10 , 16 , 43 Patients with asymptomatic mCRPC were randomly assigned in a 2 to 1 ratio to receive vaccine (n= 147) or placebo (n= 78). Although the primary endpoint of these studies—progression‐free survival— did not achieve statistical significance, of great interest, however, is the finding that patients who subsequently received docetaxel chemotherapy showed increased survival if they had received prior vaccine therapy rather than placebo. 17 , 44
In the preclinical setting, docetaxel has been shown to modulate CD4+, CD8+, CD19+, NK, and Treg populations in nontumor bearing mice. 40 Further, these studies demonstrated that docetaxel provides optimal enhancement of antigen‐specific T‐cell responses to recombinant viral vaccines when administered after vaccination, resulting in increased tumor‐derived cascade antigen responses and reduced tumor burden. These studies have translated into clinical trials in patients with metastatic CRPC and rising PSA using rV‐ and rF‐PSA. A randomized phase II study combining standard docetaxel chemotherapy with rV‐PSA and a vector containing a single T‐cell costimulatory molecule (rV‐B7‐1) followed by 7 monthly boosts with rF‐PSA in patients with mCRPC has been completed. Patients received either vaccine alone or vaccine plus docetaxel. 45 Chemotherapy did not appear to blunt the immune response, as both cohorts demonstrated similar increases in PSA‐specific T‐cell precursors. Recent poxviral studies have added multiple costimulatory molecules to both priming and boosting vaccines. 46 A multicenter randomized Phase II trial has now been approved by ECOG and CTEP (NCI) in patients with metastatic prostate cancer. Patients will be randomized to either docetaxel, or rV/rF‐PSA‐TRICOM (PROSTVAC) vaccine followed by docetaxel. These PSA‐TRICOM vaccines are showing evidence of clinical benefit in terms of enhanced survival compared with predicted survival in patients with metastatic CRPC. 47
Vaccine and Radiation
Radiation is standard therapy for prostate cancer, primarily for local tumor control via direct cytotoxicity. Although local control of the primary tumor can usually prevent development of subsequent systemic metastases, tumor radiation fails to control pre‐existing systemic disease, which may be present only as micrometastatic (and therefore undetectable) deposits. Combining radiation therapy with immunotherapy allows one to exploit two broad areas: (a) radiation‐induced tumor‐cell death as a potential source of tumor antigens for immunotherapy, and (b) post‐radiation tumor‐cell modulation that allows more efficient immune‐cell access and increased sensitivity to T‐cell killing ( Figure 4 ). Preclinical studies and early clinical trials have demonstrated that radiation may enhance the efficacy of therapeutic cancer vaccines, which provided the rationale for current clinical trials employing both modalities combined.
Figure 4.
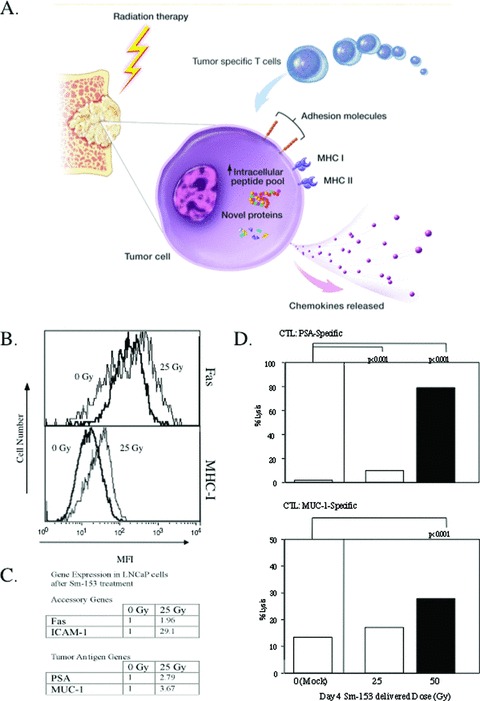
(A) Irradiation modulates tumor‐cell phenotype and increases immune recognition. Irradiation can cause (1) upregulation of chemokines and adhesion molecules, providing signals for T cells to come to areas of tumor, (2) upregulation of MHC molecules and TAAs, making it easier for T cells to recognize tumor, and (3) upregulation of Fas and downregulation of regulatory T cells, making it easier for cytotoxic tumor‐specific T cells to kill tumor. (B) Upregulation of MHC class I and Fas on prostate cancer cells after exposure to 153Sm‐EDTMP. LNCaP cells were exposed to 0 Gy (heavy line) or 25 Gy 153Sm‐EDTMP (thin line) over 4 days. Cell‐surface expression of MHC class I (bottom panel) and Fas (top panel) was measured by flow cytometry (MFI = mean fluorescence intensity). (C) Upregulation of accessory and tumor antigen genes following exposure to 153Sm‐EDTMP. LNCaP cells were treated with 0, 25, or 50 Gy 153Sm‐EDTMP. At 48 hours, RNA was extracted from treated cells and quantitative real‐time PCR was performed on indicated genes and normalized against the housekeeping gene GAPDH. Numbers depict fold increase compared with the 0 Gy sample. (D) Effect of 153Sm‐EDTMP on sensitivity of LNCaP cells to antigen‐specific CTL killing. LNCaP cells were exposed to 0, 25, or 50 Gy 153Sm‐EDTMP. Cells were harvested 72 hours after exposure and labeled with 111In. CTLs specific for PSA or MUC‐1 were incubated with labeled LNCaP cells at an E:T ratio of 30:1. After 18 hours, supernatant was harvested and specific lysis was calculated. Taken from Reference 64.
Local irradiation generates an inflammatory microenvironment in which tumor antigens released by dying tumor cells are presented to the immune system in the context of the costimulatory “danger” signals necessary for effiective TAA‐specific T‐cell activation ( Figure 4 ). 48 , 49 , 50 , 51 In addition, preclinical studies have demonstrated that sublethal doses of radiation induce phenotypic changes in tumor cells, including upregulation of many cell‐surface proteins involved in T‐cell target recognition, adhesion, and lysis, including ICAM‐1, MHC class I and II, Fas, and multiple TAAs (CEA, MUC‐1, CA125, Her2‐neu, p53, gp70, PSA, PSMA, and PAP). These phenotypic changes render tumor cells more susceptible to T‐cell‐mediated cytolysis 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 and have been specifically shown in mouse models to synergize with DNA, liposome, and poxviral‐based vaccine strategies. 50 , 56 , 57 , 59
Initial clinical data involving a first‐generation poxviral vaccine provided clinical proof of concept. Thirty patients with localized prostate cancer were treated with standard radiation therapy, and two‐thirds of these patients were randomized to receive vaccine as well. The vaccine utilized in this study was a priming dose of rV‐PSA admixed with rV‐B7‐1, followed by monthly boosts of rF‐PSA for a total of eight vaccinations. T irteen of 19 patients randomized to radiation plus vaccine had a ≥ 3‐fold increase in PSA‐specific T cells after radiation, compared to no change in T cells in patients treated with radiation alone (p= 0.0005). 61 A follow‐up study conf rmed a similar magnitude of response in a similar proportion of patients. 62
Samarium‐153 (153Sm‐EDTMP) is an FDA‐approved agent for palliation of bone‐related pain in metastatic cancer patients. 153Sm‐EDTMP is composed of radioactive samarium and a tetraphosphate chelator that binds to metastatic lesions in bone, targeting low levels of radiation to sites of disease. 63
In preclinical models, prostate cancer cells exposed to palliative doses of 153Sm‐EDTMP upregulated expression of tumor antigens and accessory molecules, rendering tumor cells more susceptible to killing by multiple antigen‐specific CTLs ( Figure 4 ). 64
These studies formed the basis for an ongoing randomized phase II study designed to evaluate whether rV/rF‐PSA‐TRICOM in combination with 153Sm‐EDTMP can improve TTP compared to 153Sm‐EDTMP alone in patients with CRPC metastatic predominantly to bone. The study will also evaluate the ability of low‐level local radiation to generate specific immunologic responses.
Conclusion
Because standard‐of‐care chemotherapy for mCRPC is associated with only a modest survival advantage and significant toxicity, studies are ongoing to determine if therapeutic cancer vaccines can provide benefit as monotherapy or can synergize with standard therapies, such as radiation or docetaxel. Thus, there is a need for relevant preclinical and early clinical studies to evaluate these approaches. Future clinical trials will also need to incorporate more extensive monitoring of immune responses to help determine how vaccines induce effective tumor immunity, and to validate specific assays that correlate with clinical responses. Finally, nearly all of the clinical trials with therapeutic cancer vaccines have been conducted in cancer patients with advanced‐stage disease. The role of these vaccines in the more appropriate patient population with early‐stage disease and/or low tumor burden needs to be further explored.
Acknowledgments
The authors thank Bonnie L. Casey for editorial assistance in the preparation of this manuscript. This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
References
- 1. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun M. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59: 225–249. [DOI] [PubMed] [Google Scholar]
- 2. Shepard DR, Raghavan D. Innovations in the systemic therapy of prostate cancer. Nat Rev Clin Oncol. 2010; 7(1): 13–21. [DOI] [PubMed] [Google Scholar]
- 3. Tannock IF, De Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004; 351(15): 1502–1512. [DOI] [PubMed] [Google Scholar]
- 4. Madan RA, Gulley JL, Arlen PM. PSA‐based vaccines for the treatment of prostate cancer. Expert Rev Vaccines. 2006; 5(2): 199–209. [DOI] [PubMed] [Google Scholar]
- 5. Oesterling JE. Prostate specific antigen: a critical assessment of the most useful tumor marker for adenocarcinoma of the prostate. J Urol. 1991; 145(5): 907–923. [DOI] [PubMed] [Google Scholar]
- 6. Murphy GP, Elgamal AA, Su SL, Bostwick DG, Holmes EH. Current evaluation of the tissue localization and diagnostic utility of prostate specific membrane antigen. Cancer. 1998; 83(11): 2259–2269. [PubMed] [Google Scholar]
- 7. Wright GL, Jr ., Grob BM, Haley C, Grossman K, Newhall K, Petrylak D, Troyer J, Konchuba A, Schellhammer PF, Moriarty R. Upregulation of prostate‐specific membrane antigen after androgen‐deprivation therapy. Urology. 1996; 48(2): 326–334. [DOI] [PubMed] [Google Scholar]
- 8. Veeramani S, Yuan TC, Chen SJ, Lin FF, Petersen JE, Shaheduzzaman S, Srivastava S, MacDonald RG, Lin MF. Cellular prostatic acid phosphatase: a protein tyrosine phosphatase involved in androgen‐independent proliferation of prostate cancer. Endocr Relat Cancer. 2005; 12(4) 805–822. [DOI] [PubMed] [Google Scholar]
- 9. Vihko P, Virkkunen P, Henttu P, Roiko K, Solin T, Huhtala ML. Molecular cloning and sequence analysis of cDNA encoding human prostatic acid phosphatase. FEBS Lett. 1988; 236(2): 275–281. [DOI] [PubMed] [Google Scholar]
- 10. Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double‐blind, placebo‐controlled, phase 3 trials of active cellular immunotherapy with sipuleucel‐T in advanced prostate cancer. Cancer. 2009; 115(16): 3670–3679. [DOI] [PubMed] [Google Scholar]
- 11. Madan RA, Gulley JL, Schlom J, Steinberg SM, Liewehr DJ, Dahut WL, Arlen PM. Analysis of overall survival in patients with nonmetastatic castration‐resistant prostate cancer treated with vaccine, nilutamide, and combination therapy. Clin Cancer Res. 2008; 14(14): 4526–4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall survival analysis of a phase II randomized controlled trial of a poxviral‐based PSA‐targeted immunotherapy in metastatic castration‐resistant prostate cancer. J Clin Oncol. 2010; 28(7): 1099–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burch PA, Breen JK, Buckner JC, Gastineau DA, Kaur JA, Laus RL, Padley DJ, Peshwa MV, Pitot HC, Richardson RL, Smits BJ, Sopapan P, Strang G, Valone FH, Vuk‐Pavlovic S. Priming tissue‐specific cellular immunity in a phase I trial of autologous dendritic cells for prostate cancer. Clin Cancer Res. 2000; 6(6): 2175–2182. [PubMed] [Google Scholar]
- 14. Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone‐refractory prostate cancer with antigen‐loaded dendritic cells. J Clin Oncol. 2000; 18(23): 3894–3903. [DOI] [PubMed] [Google Scholar]
- 15. Burch PA, Croghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, Richardson RL, Valone FH, Vuk‐Pavlovic S. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen‐independent prostate cancer: a Phase 2 trial. Prostate. 2004; 60(3): 197–204. [DOI] [PubMed] [Google Scholar]
- 16. Small EJ, Schellhammer PF, Higano CS, Redfern CH, Nemunaitis JJ, Valone FH, Verjee SS, Jones LA, Hershberg RM. Placebo‐controlled phase III trial of immunologic therapy with sipuleucel‐T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006; 24(19): 3089–3094. [DOI] [PubMed] [Google Scholar]
- 17. Michael A, Ball G, Quatan N, Wushishi F, Russell N, Whelan J, Chakraborty P, Leader D, Whelan M, Pandha H. Delayed disease progression after allogeneic cell vaccination in hormone‐resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res. 2005; 11(12): 4469–4478. [DOI] [PubMed] [Google Scholar]
- 18. Small EJ, Sacks N, Nemunaitis J, Urba WJ, Dula E, Centeno AS, Nelson WG, Ando D, Howard C, Borellini F, Nguyen M, Hege K, Simons JW. Granulocyte macrophage colony‐stimulating factor– secreting allogeneic cellular immunotherapy for hormone‐refractory prostate cancer. Clin Cancer Res. 2007; 13(13): 3883–3891. [DOI] [PubMed] [Google Scholar]
- 19. Madan RA, Mohebtash M, Schlom J, Gulley JL. Therapeutic vaccines in metastatic castration‐resistant prostate cancer: principles in clinical trial design. Expert Opin Biol Ther. 2010; 10(1): 19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Higano C, Saad F, Somer B, Curti B, Petrylak D, Drake C, Schnell F, Redfern C, Schrijvers D, Sacks N. A phase III trial of GVAX immunotherapy for prostate cancer versus docetaxel plus prednisone in asymptomatic, castration‐resistant prostate cancer (CRPC) [abstract]. ASCO Genitourinary Cancers Symposium. 2009; LBA150. [Google Scholar]
- 21. Hodge JW, Schlom J, Donohue SJ, Tomaszewski JE, Wheeler CW, Levine BS, Gritz L, Panicali D, Kantor JA. A recombinant vaccinia virus expressing human prostate‐specific antigen (PSA): safety and immunogenicity in a non‐human primate. Int J Cancer. 1995; 63(2): 231–237. [DOI] [PubMed] [Google Scholar]
- 22. Gulley J, Chen AP, Dahut W, Arlen PM, Bastian A, Steinberg SM, Tsang K, Panicali D, Poole D, Schlom J, Michael Hamilton J. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV‐PSA) in patients with metastatic androgen‐independent prostate cancer. Prostate. 2002; 53(2): 109–117. [DOI] [PubMed] [Google Scholar]
- 23. Sanda MG, Smith DC, Charles LG, Hwang C, Pienta KJ, Schlom J, Milenic D, Panicali D, Montie JE. Recombinant vaccinia‐PSA (PROSTVAC) can induce a prostate‐specific immune response in androgen‐modulated human prostate cancer. Urology. 1999; 53(2): 260–266. [DOI] [PubMed] [Google Scholar]
- 24. Eder JP, Kantoff PW, Roper K, Xu GX, Bubley GJ, Boyden J, Gritz L, Mazzara G, Oh WK, Arlen P, Tsang KY, Panicali D, Schlom J, Kufe DW. A phase I trial of a recombinant vaccinia virus expressing prostate‐specific antigen in advanced prostate cancer. Clin Cancer Res. 2000; 6(5): 1632–1638. [PubMed] [Google Scholar]
- 25. Kundig TM, Kalberer CP, Hengartner H, Zinkernagel RM. Vaccination with two different vaccinia recombinant viruses: long‐term inhibition of secondary vaccination. Vaccine. 1993; 11(11): 1154–1158. [DOI] [PubMed] [Google Scholar]
- 26. Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003; 9(5): 1837–1849. [PubMed] [Google Scholar]
- 27. Hodge JW, Sabzevari H, Yafal AG, Gritz L, Lorenz MG, Schlom J. A triad of costimulatory molecules synergize to amplify T‐cell activation. Cancer Res. 1999; 59(22) 5800–5807. [PubMed] [Google Scholar]
- 28. Hodge JW, Chakraborty M, Kudo‐Saito C, Garnett CT, Schlom J. Multiple costimulatory modalities enhance CTL avidity. J Immunol. 2005; 174(10): 5994–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Aarts WM, Schlom J, Hodge JW. Vector‐based vaccine/cytokine combination therapy to enhance induction of immune responses to a self‐antigen and antitumor activity. Cancer Res. 2002; 62(20): 5770–5777. [PubMed] [Google Scholar]
- 30. Yang S, Hodge JW, Grosenbach DW, Schlom J. Vaccines with enhanced costimulation maintain high avidity memory CTL. J Immunol. 2005; 175(6): 3715–3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oh S, Hodge JW, Ahlers JD, Burke DS, Schlom J, Berzofsky JA. Selective induction of high avidity CTL by altering the balance of signals from APC. J Immunol. 2003; 170(5): 2523–2530. [DOI] [PubMed] [Google Scholar]
- 32. Yang S, Schlom J. Antigen‐presenting cells containing multiple costimulatory molecules promote activation and expansion of human antigen‐specific memory CD8+ T cells. Cancer Immunol Immunother. 2009; 58(4): 503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Grosenbach DW, Barrientos JC, Schlom J, Hodge JW. Synergy of vaccine strategies to amplify antigen‐specific immune responses and antitumor effects. Cancer Res. 2001; 61(11): 4497–4505. [PubMed] [Google Scholar]
- 34. Madan RA, Arlen PM, Mohebtash M, Hodge JW, Gulley JL. PROSTVAC‐VF: a vector‐based vaccine targeting PSA in prostate cancer. Expert Opin Investig Drugs. 2009; 18: 1001–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kantoff P, Glode L, Tannenbaum S, Bilhartz D, Pittman W, Schuetz T. Randomized, double‐blind, vector‐controlled study of targeted immunotherapy in patients (pts) with hormone‐refractory prostate cancer (HRPC) [abstract]. J Clin Oncol. 2006; 24(18S): 2501. [Google Scholar]
- 36. Gulley JL, Arlen PM, Madan RA, Tsang KY, Pazdur MP, Skarupa L, Jones JL, Poole DJ, Higgins JP, Hodge JW, Cereda V, Vergati M, Steinberg SM, Halabi S, Jones E, Chen C, Parnes H, Wright JJ, Dahut WL, Schlom J. Immunologic and prognostic factors associated with overall survival employing a poxviral‐based PSA vaccine in metastatic castrate‐resistant prostate cancer. Cancer Immunol Immunother. 2010; 59(5): 663–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halabi S, Small EJ, Kantoff PW, Kattan MW, Kaplan EB, Dawson NA, Levine EG, Blumenstein BA, Vogelzang NJ. Prognostic model for predicting survival in men with hormone‐refractory meta‐static prostate cancer. J Clin Oncol. 2003; 21(7): 1232–1237. [DOI] [PubMed] [Google Scholar]
- 38. Grunberg E, Eckert K, Maurer HR. Docetaxel treatment of HT‐29 colon carcinoma cells reinforces the adhesion and immunocytotoxicity of peripheral blood lymphocytes in vitro. Int J Oncol. 1998; 12(4): 957–963. [DOI] [PubMed] [Google Scholar]
- 39. Eckert K, Fuhrmann‐Selter T, Maurer HR. Docetaxel enhances the expression of E‐cadherin and carcinoembryonic antigen (CEA) on human colon cancer cell lines in vitro. Anticancer Res. 1997; 17(1A): 7–12. [PubMed] [Google Scholar]
- 40. Garnett CT, Schlom J, Hodge JW. Combination of docetaxel and recombinant vaccine enhances T‐cell responses and antitumor activity: effects of docetaxel on immune enhancement. Clin Cancer Res. 2008; 14(11): 3536–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sundelin K, Roberg K, Grenman R, Hakansson L. Effects of cisplatin, alpha‐interferon, and 13‐cis retinoic acid on the expression of Fas (CD95), intercellular adhesion molecule‐1 (ICAM‐1), and epidermal growth factor receptor (EGFR) in oral cancer cell lines. J Oral Pathol Med. 2007; 36(3): 177–183. [DOI] [PubMed] [Google Scholar]
- 42. Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low‐dose cyclophosphamide. Blood. 2005; 105(7): 2862–2868. [DOI] [PubMed] [Google Scholar]
- 43. Schellhammer P, Higano C, Berger E, Shore N, Small E, Penson D, Ferrari A, Sims R, Yuh L, Frohlich M, Kantoff P. A randomized, double‐blind, placebo‐controlled, multi‐center, phase III trial of sipuleucel‐T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC) [abstract]. American Urological Association Annual Meeting. 2009. [Google Scholar]
- 44. Petrylak DP. Defining the optimal role of immunotherapy and chemotherapy: advanced prostate cancer patients who receive sipuleucel‐T (PROVENGE) followed by docetaxel derive greatest survival benefit. The Chemotherapy Foundation Symposium . November, 2006; New York , NY . 9–13.
- 45. Arlen PM, Gulley JL, Parker C, Skarupa L, Pazdur M, Panicali D, Beetham P, Tsang KY, Grosen‐bach DW, Feldman J, Steinberg SM, Jones E, Chen C, Marte J, Schlom J, Dahut W. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen‐independent prostate cancer. Clin Cancer Res. 2006; 12(4): 1260–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Arlen PM, Skarupa L, Pazdur M, Seetharam M, Tsang KY, Grosenbach DW, Feldman J, Poole DJ, Litzinger M, Steinberg SM, Jones E, Chen C, Marte J, Parnes H, Wright J, Dahut W, Schlom J, Gulley JL. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007; 178(4 Pt 1): 1515–1520. [DOI] [PubMed] [Google Scholar]
- 47. Madan RA, Gulley JL, Dahut WL, Tsang KY, Steinberg SM, Schlom J, Arlen PM. Overall survival (OS) analysis of a phase II study using a pox viral‐based vaccine, PSA‐TRICOM, in the treatment of metastatic, castrate‐resistant prostate cancer (mCRPC). ASCO Annual Meeting, May 30–June 3, 2008: Chicago, IL. J Clin Oncol. 2008; 26(May 20 suppl): abstract 3005. [Google Scholar]
- 48. Zong ZW, Cheng TM, Su YP, Ran XZ, Li N, Ai GP, Xu H. Crucial role of SDF‐1/CXCR4 interaction in the recruitment of transplanted dermal multipotent cells to sublethally irradiated bone marrow. J Radiat Res (Tokyo). 2006; 47(3–4): 287–293. [DOI] [PubMed] [Google Scholar]
- 49. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel‐Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll‐like receptor 4‐dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007; 13(9): 1050–1059. [DOI] [PubMed] [Google Scholar]
- 50. Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine‐mediated T‐cell killing. Cancer Res. 2004; 64(12): 4328–4337. [DOI] [PubMed] [Google Scholar]
- 51. Hodge JW, Guha C, Neefjes J, Gulley JL. Synergizing radiation therapy and immunotherapy for curing incurable cancers. Opportunities and challenges. Oncology (Williston Park). 2008; 22(9): 1064–1084. [PMC free article] [PubMed] [Google Scholar]
- 52. Santin AD, Hermonat PL, Hiserodt JC, Chiriva‐Internati M, Woodliff J, Theus JW, Barclay D, Pecorelli S, Parham GP. Effects of irradiation on the expression of major histocompatibility complex class I antigen and adhesion costimulation molecules ICAM‐1 in human cervical cancer. Int J Radiat Oncol Biol Phys. 1997; 39(3): 737–742. [DOI] [PubMed] [Google Scholar]
- 53. Ishikawa E, Tsuboi K, Saijo K, Takano S, Ohno T. X‐irradiation to human malignant glioma cells enhances the cytotoxicity of autologous killer lymphocytes under specific conditions. Int J Radiat Oncol Biol Phys. 2004; 59(5): 1505–1512. [DOI] [PubMed] [Google Scholar]
- 54. Klein B, Loven D, Lurie H, Rakowsky E, Nyska A, Levin I, Klein T. The effect of irradiation on expression of HLA class I antigens in human brain tumors in culture. J Neurosurg. 1994; 80(6): 1074–1077. [DOI] [PubMed] [Google Scholar]
- 55. Hareyama M, Imai K, Kubo K, Takahashi H, Koshiba H, Hinoda Y, Shidou M, Oouchi A, Yachi A, Morita K. Effect of radiation on the expression of carcinoembryonic antigen of human gastric adenocarcinoma cells. Cancer. 1991; 67(9): 2269–2274. [DOI] [PubMed] [Google Scholar]
- 56. Tseng CW, Trimble C, Zeng Q, Monie A, Alvarez RD, Huh WK, Hoory T, Wang MC, Hung CF, Wu TC. Low‐dose radiation enhances therapeutic HPV DNA vaccination in tumor‐bearing hosts. Cancer Immunol Immunother. 2009; 58(5): 737–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ye GW, Park JB, Park YJ, Choi YS, Sin JI. Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine‐driven CTL‐mediated killing induces synergistic anti‐tumor activity. Mol Ther. 2007; 15(8): 1564–1570. [DOI] [PubMed] [Google Scholar]
- 58. Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up‐regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol. 2003; 170(12): 6338–6347. [DOI] [PubMed] [Google Scholar]
- 59. Chamoto K, Takeshima T, Wakita D, Ohkuri T, Ashino S, Omatsu T, Shirato H, Kitamura H, Togashi Y, Nishimura T. Combination immunotherapy with radiation and CpG‐based tumor vaccination for the eradication of radio‐ and immuno‐resistant lung carcinoma cells. Cancer Sci. 2009; 100(5): 934–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Garnett CT, Palena C, Chakraborty M, Tsang KY, Schlom J, Hodge JW. Sublethal irradiation of human tumor cells modulates phenotype resulting in enhanced killing by cytotoxic T lymphocytes. Cancer Res. 2004; 64(21): 7985–7994. [DOI] [PubMed] [Google Scholar]
- 61. Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, Tsang KY, Yokokawa J, Hodge JW, Menard C, Camphausen K, Coleman CN, Sullivan F, Steinberg SM, Schlom J, Dahut W. Combining a recombinant cancer vaccine with standard defi nitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005; 11(9): 3353–3362. [DOI] [PubMed] [Google Scholar]
- 62. Lechleider RJ, Arlen PM, Tsang KY, Steinberg SM, Yokokawa J, Cereda V, Camphausen K, Schlom J, Dahut WL, Gulley JL. Safety and immunologic response of a viral vaccine to prostate‐specific antigen in combination with radiation therapy when metronomic‐dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008; 14(16): 5284–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eary JF, Collins C, Stabin M, Vernon C, Petersdorf S, Baker M, Hartnett S, Ferency S, Addison SJ, Appelbaum F, Gordon EE. Samarium‐153‐EDTMP biodistribution and dosimetry estimation. J Nucl Med. 1993; 34(7): 1031–1036. [PubMed] [Google Scholar]
- 64. Chakraborty M, Wansley EK, Carrasquillo JA, Yu S, Paik CH, Camphausen K, Becker MD, Goeckeler WF, Schlom J, Hodge JW. The use of chelated radionuclide (samarium‐153‐ethyle‐nediaminetetramethylenephosphonate) to modulate phenotype of tumor cells and enhance T cell‐mediated killing. Clin Cancer Res. 2008; 14(13): 4241–4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Dalgleish A, Quatan N, Michael A, Wushishi F, Pandha H. Increased time to progression and sustained PSA velocity responses in a phase II trial in advanced metastatic prostate cancer following treatment with ONY‐P1, an allogeneic whole cell vaccine [abstract]. J Clin Oncol. 2005; 23(16S): 4726. 16034048 [Google Scholar]
- 66. Murphy GP, Tjoa BA, Simmons SJ, Ragde H, Rogers M, Elgamal A, Kenny GM, Troychak MJ, Salgaller ML, Boynton AL. Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate. 1999; 39(1): 54–59. [DOI] [PubMed] [Google Scholar]
- 67. Tjoa BA, Simmons SJ, Elgamal A, Rogers M, Ragde H, Kenny GM, Troychak MJ, Boynton AL, Murphy GP. Follow‐up evaluation of a phase II prostate cancer vaccine trial. Prostate. 1999; 40(2): 125–129. [DOI] [PubMed] [Google Scholar]
- 68. North SA, Graham K, Bodnar D, Venner P. A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J Urol. 2006; 176(1): 91–95. [DOI] [PubMed] [Google Scholar]


