Abstract
Background and Purpose
Diffusion-weighted MRI (DWI) of the brain is a promising technique to help predict functional outcome in comatose survivors of cardiac arrest. We aimed to evaluate prospectively the temporal-spatial profile of brain apparent diffusion coefficient (ADC) changes in comatose survivors during the first 8 days after cardiac arrest.
Methods
ADC values were measured by two independent and blinded investigators in predefined brain regions in 18 good and 15 poor outcome patients with 38 brain MRIs, and compared with 14 normal controls. The same brain regions were also assessed qualitatively by two other independent and blinded investigators.
Results
In poor outcome patients, cortical structures, in particular the occipital and temporal lobes, and the putamen exhibited the most profound ADC reductions, which were noted as early as 1.5 days and reached nadir between 3 to 5 days after the arrest. Conversely, when compared to normal controls, good outcome patients exhibited increased diffusivity, in particular in the hippocampus, temporal and occipital lobes, and corona radiata. By the qualitative MRI readings, one or more cortical gray matter structures were read as moderately-to-severely abnormal in all poor outcome patients imaged beyond 54 hours after the arrest, but not in the three patients imaged earlier.
Conclusions
Brain DWI changes in comatose post-cardiac arrest survivors in the first week after the arrest are region- and time-dependent and differ between good and poor outcome patients. With the increasing use of MRI in this context, it is important to be aware of these relationships.
Keywords: Coma, Cardiac Arrest, Magnetic Resonance Imaging, Diffusion Weighted Imaging
INTRODUCTION
Cardiopulmonary arrest is associated with high morbidity and mortality1-3 that are often caused by hypoxic-ischemic brain injury. Therefore, the ability to accurately assess the presence and severity of brain injury early after the arrest is a critical issue for healthcare providers and patients’ families.
Brain diffusion-weighted MRI (DWI) is exquisitely sensitive in the detection of early ischemic brain injury.4, 5 In the presence of acute ischemic infarction, the apparent diffusion coefficient (ADC) decreases by 25-40% in the affected territories within minutes to hours after symptom onset, reaching its nadir at about 24 hours, and remaining decreased for 7 to 14 days.6
Brain regions with reduced diffusion also occur in patients with severe global hypoxic-ischemic brain injury following cardiac arrest and correlate with outcome in preliminary reports.7-15 In a previous study, more than 10% of brain volume with an ADC value <650-700 ×10−6mm2/sec between two and four-and-a-half days after the arrest identified poor outcome patients with 100% specificity and 81% sensitivity.14 The timeline and the spatial profile of these DWI changes, however, have not been well defined. In this study, we aimed to define the temporal and spatial profile of brain ADC changes caused by global hypoxic-ischemic brain injury in specific and predefined brain regions during the first eight days after the cardiac arrest.
METHODS
Patients
Consecutive comatose post-cardiac arrest patients were prospectively enrolled. Details of the study methodology have been published previously.14 Briefly, adult patients who remained comatose after successful resuscitation for cardiac arrest were eligible. Patients were excluded if they had a pre-existing severe coexisting systemic disease or “do not resuscitate” status or a baseline modified Rankin Scale score of 3 or more. Patients underwent standardized neurological examinations at one hour and at one, two and three days after the arrest. Somatosensory evoked potentials (SSEP) were performed at three days after the arrest if patients remained comatose. The study was approved by the institutional review board and written consent was obtained from legally authorized representatives for each participant.
Patients were included in this study if they underwent a technically adequate brain DWI study within eight days of the arrest and if they survived for 6 months or if they died while meeting any of the following predefined clinical criteria shown to be highly specific for poor outcome: absent pupillary reflexes or motor response at three days, and/or bilateral absent cortical responses by SSEP after two days, and/or vegetative after one month. Patients who died due to withdrawal of life support or a cardiopulmonary complication without meeting any of these specific clinical criteria were not included in this report, because their outcomes were considered indeterminate. In patients treated with induced hypothermia, MRIs were obtained after passive rewarming to normal body temperature. Good vs. poor outcomes were defined as a 6-month Glasgow outcome scale (GOS) score of 3 or more vs. 1 or 2.
MRI Protocol and Image Processing
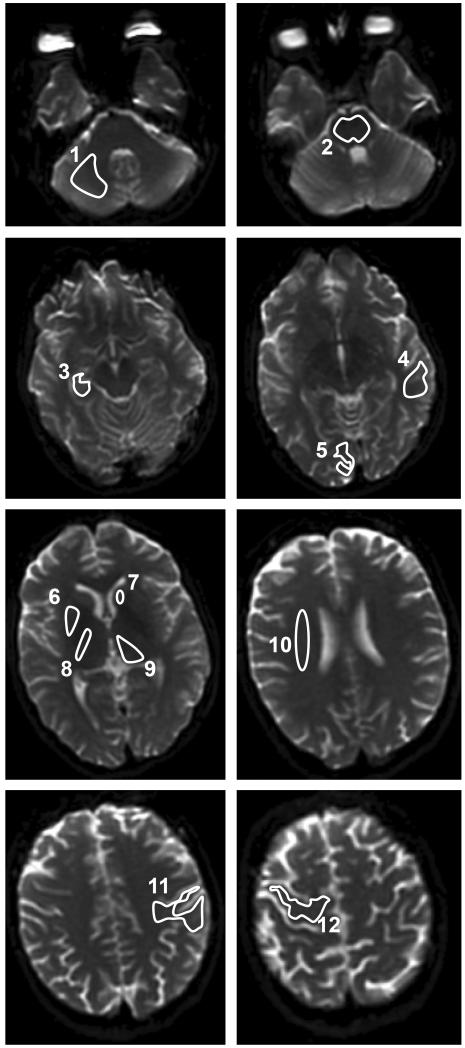
The MRI protocol for this study has been previously reported.14 Briefly, DWI images were acquired on a 1.5T GE Signa Horizon scanner (GE Medical Systems) with spin-echo echo-planar imaging, 128×128 acquisition matrix, 256×256 reconstructed matrix, field of view 240×240 mm, slice thickness/gap 5/1.5 or 5/2.5 mm, b=0 and 1000 sec/mm2, and diffusion-encoding along principal axes averaged to provide isotropically-weighted DWIs. Images were processed and analyzed using in-house developed Diffusion/Perfusion Analysis software (UCLA Stroke Center, Stanford Stroke Center). ADC maps were created. Predefined regions of interest (ROIs) were manually outlined bilaterally (except for midline structures) by two independent and blinded investigators in the frontal, parietal, occipital, and temporal lobes, hippocampus, corona radiata, internal capsule, caudate, putamen, thalamus, cerebellum, and pons (Figure 1). The program calculated the mean and standard deviation (SD) of the ADC value in the outlined ROIs.
Figure 1.
Predefined brain regions of interest manually outlined for the apparent diffusion coefficient (ADC) value analyses: 1-cerebellum, 2-pons, 3-hippocampus, 4-temporal lobe, 5-occipital lobe, 6-putamen, 7-caudate, 8-internal capsule, 9-thalamus, 10-corona radiata, 11-parietal lobe, 12-frontal lobe.
All brain MRIs were also read systematically and qualitatively by a neuroradiologist and a neurologist with subspecialty certification in neurocritical care and vascular neurology. Evaluators were independent and blinded to patient identity and outcome. The FLAIR and the DWI sequences were scored systematically based on the severity of signal abnormality attributed to the cardiac arrest in 15 predefined regions corresponding to the regions included in the quantitative analyses: cortical gray and subcortical white matter in the frontal, parietal, temporal, occipital lobes, hippocampus, caudate nucleus, putamen, thalamus, cerebellum (cortex and white matter) and pons. All brain regions were scored according to the severity of signal abnormality on a 5-point scale with 0=no abnormality, 1=possibly abnormal, 2=mildly abnormal, 3=moderately abnormal and 4=severely abnormal. The average scores of the two raters were used.
Brain MRIs of 14 patients without evidence of acute infarction and without other acute parenchymal findings on their brain MRIs, and without a diagnosis of cerebrovascular disease were used as controls. The reason for obtaining MR imaging in these 14 patients were non-specific and transient neurologic symptoms including: headache, numbness, generalized weakness, altered mental status, and transient visual changes. The discharge diagnoses of these 14 patients were: migraine with aura or migraine equivalents (4), presyncope (2), sensory neuropathy (1), catatonia (1), polymyalgia rheumatica (1), perimesencephalic (non-aneurysmal) subarachnoid hemorrhage (1), systemic infection with altered mental status (1), and uncertain (3).
Data Analysis
MRIs were assigned to 48-hour time-intervals following the cardiac arrest. Each interval overlapped by 12-hours with the neighboring ones. Scans were assigned to two intervals if they were done during the overlapping time-window. Mean ADC values of the ROIs in the various brain structures of each patient within each time-interval in the good versus poor outcome groups were compared with the control group using the Mann-Whitney U test. All statistical tests were 2-tailed and significance was defined at α<0.05. To control for multiple comparisons, we adjusted the false discovery rate q*=0.05 using the step-up controlling procedure of Benjamini and Hochberg. Statistical analyses were performed using SPSS 17.0 (SPSS Inc.).
RESULTS
Twenty brain MRIs of 18 good outcome patients and 18 MRIs of 15 poor outcome patients were analyzed. The FLAIR sequence of one patient could not be interpreted qualitatively because of motion artifact. MRIs were obtained at a median (IQR) of 80 (55-117) hours after the arrest. Baseline characteristics of the good and poor outcome patients are presented in Table 1. Eleven patients in the good and ten in the poor outcome group underwent induced hypothermia. The 15 poor outcome patients met the following clinical criteria for unfavorable outcome: absent pupillary reflexes at 72 hours (N=5), absent motor response at 72 hours (N=12), absent SSEP after 48 hours (N=10), and inability to regain consciousness at 1 month (N=1). In the good outcome group, the 6-month GOS was 3 in six, 4 in five, and 5 in seven patients. The control group consisted of eight women and six men with a mean age 45±12 years. Baseline characteristics did not differ among the three groups.
Table 1.
Clinical characteristics of 33 comatose post-cardiac arrest patients and 14 controls
| Characteristic | Good Outcome Patients (N=18) |
Poor Outcome Patients (N=15) |
Controls (N=14) |
p-value |
|---|---|---|---|---|
| Age in years (mean ± SD) | 55 ± 20 | 56 ± 14 | 45±12 | 0.101 |
| Female gender # (%) | 4 (22) | 5 (33) | 8 (53) | 0.120 |
| History of diabetes mellitus # (%) | 3 (17) | 1 (7) | 4 (29) | 0.292 |
| History of hypertension # (%) | 5 (28) | 5 (33) | 8 (57) | 0.212 |
| History of coronary artery disease # (%) | 3 (17) | 2 (13) | 1 (7) | 0.723 |
| Arrest duration in minutes* (mean ± SD) | 19 ± 13 | 28±12 | 0.078 | |
| In-hospital cardiac arrest # (%) | 6 (33) | 3 (20) | 0.458 | |
| Induced hypothermia # (%) | 11 (61) | 10 (67) | 1 |
unknown in six patients
Qualitative MRI Readings (Table 2, Figure 2)
Table 2.
Qualitative scores in brain structures of 18 good and 15 poor outcome patients
| Structure | FLAIR qualitative score median (IQR, range) |
DWI qualitative score median (IQR, range) |
||||
|---|---|---|---|---|---|---|
| (maximum possible score) |
Good outcome (19 scans) |
Poor outcome (18 scans) |
p- value |
Good outcome (20 scans) |
Poor outcome (18 scans) |
p- value |
| Temporal lobe (8) | 0 (0-1, 0-2.5) | 3 (1-4, 0-4.5) | 0.001 | 0.25 (0-1, 0-2) | 3.5 (1.5-4, 0-5) | <0.001 |
| Gray Matter (4) | 0 (0-1, 0-2.5) | 3 (1-4, 0-4) | 0.001 | 0 (0-1, 0-2) | 3.5 (1.5-4, 0-4) | <0.001 |
| White Matter (4) | 0 (0-0, 0-0) | 0 (0-0, 0-0.5) | 0.578 | 0 (0-0, 0-0.5) | 0 (0-0, 0-1) | 0.965 |
| Occipital lobe (8) | 0 (0-1, 0-2.5) | 3.25 (2-4, 0-4.5) | <0.001 | 0 (0-1, 0-3) | 4 (3.5-4, 0-5) | <0.001 |
| Gray Matter (4) | 0 (0-1, 0-2.5) | 3.25 (2-4, 0-4) | <0.001 | 0 (0-1, 0-3) | 4 (3.5-4, 0-4) | <0.001 |
| White Matter (4) | 0 (0-0, 0-0) | 0 (0-0, 0-0) | 0.391 | 0 (0-0, 0-1.5) | 0 (0-0, 0-1) | 0.784 |
| Frontal lobe (8) | 0 (0-1, 0-2.5) | 3.25 (1-4.5, 0-5) | <0.001 | 0.5 (0-1.5, 0-2.5) | 3.75 (2-4, 0-5.5) | <0.001 |
| Gray Matter (4) | 0 (0-0.75, 0-2.5) | 3.25 (1-4.5, 0-4) | <0.001 | 0.5 (0-1.25, 0-2.5) | 3.75 (2-4, 0-4) | <0.001 |
| White Matter (4) | 0 (0-0, 0-0.5) | 0 (0-0.5, 0-1) | 0.142 | 0 (0-0, 0-0.5) | 0 (0-0, 0-1.5) | 0.965 |
| Parietal lobe (8) | 0 (0-1, 0-2.5) | 3.5 (2-4.5, 0-5) | <0.001 | 0.5 (0-1.75, 0-3) | 4 (2-4, 0-5.5) | <0.001 |
| Gray Matter (4) | 0 (0-0.75, 0-2.5) | 3.5 (1-4, 0-4) | <0.001 | 0.25 (0-1.5, 0-3) | 4 (2-4, 0-4) | <0.001 |
| White Matter (4) | 0 (0-0, 0-1) | 0 (0-0.5, 0-2) | 0.358 | 0 (0-0.5, 0-0.5) | 0 (0-0, 0-1.5) | 0.613 |
| Hippocampus (4) | 1 (0.5-2.25, 0-3.5) | 2.75 (2-4, 0-4) | 0.003 | 1.5 (0.5-2.5, 0-3.5) | 3 (1-4, 0-4) | 0.038 |
| Thalamus (4) | 1 (0-2, 0-3) | 3 (2.5-4, 0-4) | <0.001 | 1 (0.25-1.5, 0-3) | 2.25 (2-3, 0-4) | 0.002 |
| Caudate Nucleus (4) | 0.5 (0-1, 0-4) | 3.25 (2-4, 0-4) | <0.001 | 1 (0-1.75, 0-3.5) | 3.25 (2.5-3.5, 0-4) | <0.001 |
| Putamen (4) | 0 (0-1, 0-4) | 3.5 (1.5-4, 0-4) | <0.001 | 0.5 (0-1.25, 0-3.5 | 3 (2-3.5, 0-4) | 0.001 |
| Cerebellum (8) | 0 (0-0.25, 0-5) | 2.5 (0.5-4, 0-6.5) | <0.001 | 1.75 (0.5-2.25, 0-5) | 3.25 (2-4, 0-6) | 0.007 |
| Cortex (4) | 0 (0-0.25, 0-4) | 2.5 (0.5-3, 0-4) | <0.001 | 1 (0.5-2, 0-4) | 3 (2-4, 0-4) | 0.001 |
| White Matter (4) | 0 (0-0, 0-1) | 0 (0-0, 0-2.5) | 0.730 | 0 (0-0.5, 0-1.5) | 0 (0-0, 0-2) | 0.264 |
| Pons (4) | 0 (0-0, 0-1) | 0 (0-1.5, 0-3.5) | 0.070 | 0 (0-0, 0-0.5) | 0 (0-0.5, 0-3) | 0.276 |
Qualitative scores: 0=no abnormality, 1=possibly abnormal, 2=mildly abnormal, 3=moderately abnormal and 4=severely abnormal; lobar and cerebellar scores are sums of the component scores; all p-values <0.05 are significant after adjustment for multiple comparisons.
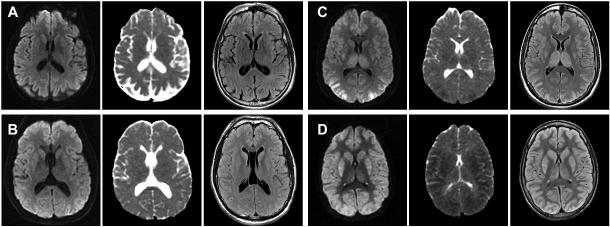
Figure 2.
Brain MRI (DWI, ADC, and FLAIR) of two patients in the good (A, B) and two patients in the poor (C, D) outcome groups between 55 and 99 hours after the arrest. The cortical and deep gray structures were qualitatively rated as normal and possibly abnormal in A, possibly and mildly abnormal in B, moderately and severely abnormal in C, and all severely abnormal in D.
Cortical structures
In the good outcome group, all but four patients were read to have normal cortical structures. Four patients were felt to have mild-to-moderate cortical gray matter abnormalities on the DWI sequence, the FLAIR sequence or both. Of these, one patient had a GOS=3, two a GOS=4, and one a GOS=5 at 6 months.
In the poor outcome group, the cortical gray matter structures were the most severely affected of all regions of interest. One or more of these structures were read as moderate or severely abnormal on the DWI or FLAIR sequences in all but three patients. Two patients, who were scanned very early (at 2 and 7 hours after the arrest) were read to have normal cortex, and one patient, who was scanned at 14 hours, had only mild DWI signal abnormalities in his cortical gray matter structures. Generally, the DWI sequence was read as more abnormal than the FLAIR sequence. Further, the occipital cortex was the most severely and the temporal cortex the least severely affected cortical structure.
Hippocampus
Moderate-to-severe DWI and FLAIR signal abnormality was seen in four patients in the good outcome group and in more than half of the patients in the poor outcome group.
Deep gray nuclei
In the good outcome group, half of the patients were read as having normal deep gray nuclei. However, the other half was felt to have mild-to-moderate abnormalities on DWI and FLAIR in these structures.
In the poor outcome group, the caudate, putamen and thalamus were mildly-to-severely affected on the DWI and FLAIR sequences in all patients, except for the two patients who were scanned at 2 and 7 hours. Most patients had moderate-to-severe abnormalities in these structures.
Cerebellum and pons
Brainstem structures were felt to be normal in all good and poor outcome patients with the exception of three poor outcome patients who were felt to have moderate abnormalities on both the DWI and FLAIR sequences. Further, over half of the poor outcome patients had moderate-to-severe cerebellar abnormalities on DWI and FLAIR affecting the cerebellar cortex, but not the cerebellar white matter. Significant signal abnormalities in the brainstem and cerebellum did not occur in the good outcome group except for in one patient who had severe abnormalities of the cerebellar cortex both on the DWI and FLAIR sequences.
Quantitative MRI Measurements
The reproducibility of the quantitative ROI measurements was excellent (intraclass correlation coefficient of 0.923 and 0.935 for inter-rater and intra-rater reliability, respectively). The median of the mean ADC value for each ROI in each time-interval is presented in Table 3 for good and poor outcome patients and compared between the two groups as well as with normal controls.
Table 3.
ADC values in brain structures of good and poor outcome patients in 48-hour time-intervals following the cardiac arrest and in normal controls
| Controls (N=14) |
Good outcome (N=18) |
Poor outcome (N=15) |
||||
|---|---|---|---|---|---|---|
| Structure ADC ×10−6 mm2/sec median (IQR) |
Time-interval from the cardiac arrest to MRI in hours |
Structure ADC ×10−6 mm2/sec median (IQR) |
p-value compared to controls |
Structure ADC ×10−6 mm2/sec median (IQR) |
p-value compared to controls |
p-value between outcome groups |
| Temporal lobe: 788 (753-810) | 0-192 | 827 (777-860) | 0.047 | 683 (545-733) | 0.002* | <0.001* |
| 0-48 | 843 (787-863) | 0.432 | 788 (754-812) | 0.953 | 0.400 | |
| 37-84 | 843 (815-864) | 0.025 | 663 (513-687) | <0.001* | 0.001* | |
| 73-120 | 856 (820-862) | 0.024 | 604 (533-664) | 0.006* | 0.003* | |
| 109-156 | 813 (792-845) | 0.224 | 733 (710-771) | 0.300 | 0.183 | |
| 145-192 | 795 (766-820) | 0.574 | 657 (628-686) | 0.017* | 0.133 | |
| Occipital lobe: 754 (735-761) | 0-192 | 784 (760-821) | 0.004* | 619 (465-681) | <0.001* | <0.001* |
| 0-48 | 776 (762-797) | 0.244 | 681 (680-725) | 0.300 | 0.200 | |
| 37-84 | 819 (777-824) | 0.007* | 563 (434-614) | 0.002* | 0.001* | |
| 73-120 | 779 (752-827) | 0.070 | 514 (462-624) | <0.001* | 0.001* | |
| 109-156 | 776 (754-813) | 0.110 | 671 (648-678) | 0.003* | 0.017* | |
| 145-192 | 787 (767-823) | 0.018* | 620 (569-671) | 0.017* | 0.133 | |
| Frontal lobe: 702 (676-735) | 0-192 | 728 (686-745) | 0.323 | 635 (508-702) | 0.016* | 0.002* |
| 0-48 | 694 (688-716) | 0.953 | 682 (626-689) | 0.244 | 0.700 | |
| 37-84 | 735 (697-736) | 0.443 | 620 (450-702) | 0.039 | 0.042 | |
| 73-120 | 706 (662-745) | 0.868 | 535 (443-651) | 0.006* | 0.029 | |
| 109-156 | 734 (684-778) | 0.400 | 717 (627-734) | 1.000 | 0.517 | |
| 145-192 | 728 (714-763) | 0.192 | 523 (508-537) | 0.017* | 0.133 | |
| Parietal lobe: 750 (711-764) | 0-192 | 764 (727-799) | 0.377 | 607 (517-698) | 0.001* | <0.001* |
| 0-48 | 742 (720-757) | 0.859 | 743 (709-753) | 0.509 | 1.000 | |
| 37-84 | 773 (735-787) | 0.400 | 549 (485-663) | 0.001* | 0.008* | |
| 73-120 | 769 (661-804) | 0.815 | 555 (509-654) | 0.012* | 0.008* | |
| 109-156 | 793 (703-801) | 0.535 | 648 (582-718) | 0.300 | 0.117 | |
| 145-192 | 774 (739-806) | 0.277 | 539 (517-562) | 0.017* | 0.133 | |
| Hippocampus: 823 (792-849) | 0-192 | 906 (868-924) | <0.001* | 839 (780-902) | 0.694 | 0.015* |
| 0-48 | 896 (881-910) | 0.047* | 838 (813-882) | 0.676 | 0.700 | |
| 37-84 | 920 (874-924) | 0.038* | 840 (823-904) | 0.439 | 0.408 | |
| 73-120 | 887 (867-917) | 0.035* | 805 (701-873) | 0.494 | 0.059 | |
| 109-156 | 916 (872-947) | 0.002* | 780 (740-841) | 0.300 | 0.117 | |
| 145-192 | 970 (912-975) | 0.005* | 822 (780-864) | 0.817 | 0.267 | |
| Thalamus: 771 (742-790) | 0-192 | 794 (746-812) | 0.416 | 753 (665-788) | 0.235 | 0.087 |
| 0-48 | 808 (782-808) | 0.362 | 763 (738-769) | 0.362 | 0.400 | |
| 37-84 | 802 (761-808) | 0.443 | 720 (665-805) | 0.224 | 0.210 | |
| 73-120 | 778 (717-836) | 0.973 | 704 (623-788) | 0.207 | 0.108 | |
| 109-156 | 773 (736-805) | 0.913 | 788 (781-826) | 0.244 | 0.383 | |
| 145-192 | 806 (772-818) | 0.158 | 773 (773-773) | 1.000 | 0.533 | |
| Caudate: 759 (740-783) | 0-192 | 770 (738-810) | 0.457 | 754 (717-812) | 0.694 | 0.393 |
| 0-48 | 755 (735-776) | 0.953 | 731 (724-741) | 0.121 | 0.700 | |
| 37-84 | 797 (755-810) | 0.197 | 756 (568-812) | 0.829 | 0.536 | |
| 73-120 | 783 (751-858) | 0.238 | 785 (648-841) | 0.718 | 0.662 | |
| 109-156 | 747 (728-770) | 0.689 | 784 (761-813) | 0.509 | 0.517 | |
| 145-192 | 763 (738-822) | 0.798 | 816 (784-848) | 0.100 | 0.533 | |
| Putamen: 719 (699-744) | 0-192 | 746 (712-796) | 0.056 | 684 (571-730) | 0.037 | 0.001* |
| 0-48 | 744 (738-755) | 0.091 | 682 (661-712) | 0.244 | 0.200 | |
| 37-84 | 748 (746-776) | 0.038 | 615 (571-687) | 0.001* | 0.002* | |
| 73-120 | 759 (741-819) | 0.002* | 666 (502-699) | 0.051 | 0.013* | |
| 109-156 | 735 (712-787) | 0.224 | 800 (751-800) | 0.197 | 0.833 | |
| 145-192 | 698 (692-782) | 0.574 | 774 (746-801) | 0.100 | 0.533 | |
| Corona Radiata: 701 (680-720) | 0-192 | 732 (717-780) | 0.001* | 692 (664-760) | 0.985 | 0.026* |
| 0-48 | 688 (687-697) | 0.677 | 681 (672-688) | 0.300 | 0.400 | |
| 37-84 | 736 (721-782) | 0.020* | 751 (670-765) | 0.403 | 0.470 | |
| 73-120 | 759 (726-793) | <0.001* | 720 (673-760) | 0.602 | 0.142 | |
| 109-156 | 725 (720-729) | 0.025* | 760 (666-773) | 0.432 | 0.833 | |
| 145-192 | 726 (721-760) | 0.035* | 601 (571-630) | 0.017 | 0.133 | |
| Internal Capsule: 724 (685-746) | 0-192 | 727 (701-762) | 0.377 | 703 (650-749) | 0.561 | 0.158 |
| 0-48 | 718 (700-745) | 0.953 | 724 (716-726) | 0.768 | 1.000 | |
| 37-84 | 772 (717-774) | 0.149 | 699 (685-749) | 0.781 | 0.114 | |
| 73-120 | 739 (665-767) | 0.664 | 663 (624-761) | 0.353 | 0.282 | |
| 109-156 | 726 (670-739) | 0.971 | 761 (705-806) | 0.509 | 0.383 | |
| 145-192 | 727 (706-757) | 0.442 | 659 (650-669) | 0.033 | 0.133 | |
| Cerebellum: 683 (662-702) | 0-192 | 699 (673-746) | 0.129 | 667 (604-691) | 0.180 | 0.007* |
| 0-48 | 682 (623-758) | 0.953 | 706 (687-711) | 0.244 | 1.000 | |
| 37-84 | 732 (692-823) | 0.110 | 611 (554-681) | 0.072 | 0.042 | |
| 73-120 | 724 (699-782) | 0.002* | 637 (603-691) | 0.239 | 0.008* | |
| 109-156 | 696 (678-723) | 0.400 | 680 (659-702) | 0.859 | 0.517 | |
| 145-192 | 673 (667-718) | 0.959 | 648 (638-657) | 0.033 | 0.133 | |
| Pons: 665 (630-728) | 0-192 | 737 (661-772) | 0.056 | 706 (658-802) | 0.235 | 0.988 |
| 0-48 | 777 (744-867) | 0.091 | 690 (687-763) | 0.197 | 0.400 | |
| 37-84 | 763 (696-801) | 0.046 | 791 (658-802) | 0.305 | 0.837 | |
| 73-120 | 755 (660-803) | 0.127 | 691 (627-794) | 0.904 | 0.573 | |
| 109-156 | 672 (659-737) | 0.535 | 794 (754-818) | 0.091 | 0.117 | |
| 145-192 | 704 (644-751) | 0.645 | 646 (578-714) | 0.500 | 0.533 | |
significant after adjustment for multiple comparisons
Number of scans in the good and poor outcome groups: 0-48 hours – 3 vs. 3; 37-84 hours – 7 vs. 9; 73-120 hours – 8 vs. 6; 109-156 hours – 7 vs. 3; 145-192 hours – 4 vs. 2
Cortical structures
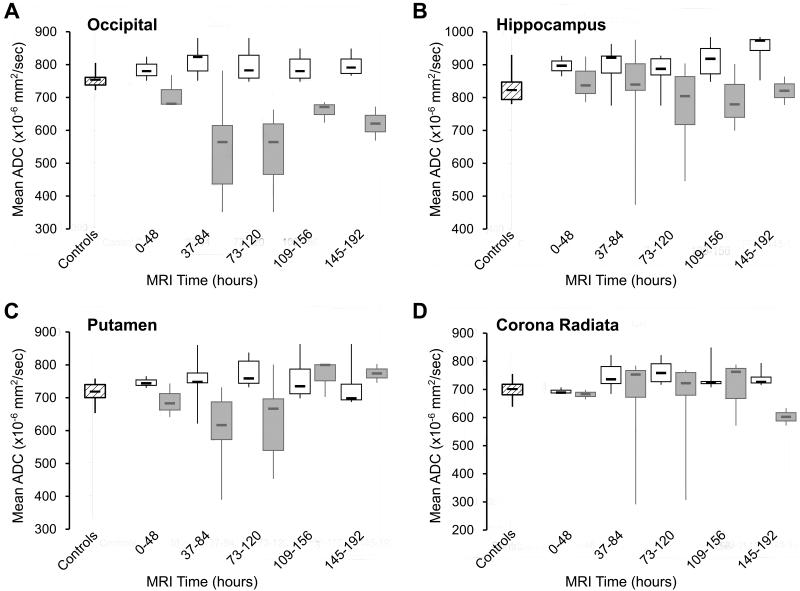
In the good outcome group, the ADC values of the temporal and occipital lobes were increased as compared to controls starting at 1.5 days and persisted throughout the first week after the arrest. In contrast, poor outcome patients exhibited early and marked reduced diffusion in the frontal, parietal, temporal and occipital lobes starting at 1.5 days and reaching a nadir between 3-5 days (Figure 3A). The ADC changes were most profound in the occipital cortex.
Figure 3.
Comparison of the time-averaged apparent diffusion coefficient (ADC) values of the occipital lobe (A), hippocampus (B), putamen (C) and corona radiata (D) in the poor  vs. the good
vs. the good  outcome groups and normal controls.
outcome groups and normal controls.
Hippocampus
In the good outcome patients, the hippocampus demonstrated increased diffusivity starting in the early time-intervals. In poor outcome patients, there was a trend toward a decline in ADC values in the 3-6.5 day time-interval, but the ADC values did not significantly differ from controls and good outcome patients in the individual time windows (Figure 3B; table 3).
Deep gray nuclei
The deep gray matter structures (thalamus, caudate and putamen) in the good outcome patients demonstrated an early trend toward increased diffusivity that reached significance only in the putamen between 3-5 days. Again, the opposite trend was noted in the poor outcome group with reduced diffusion in the first 5 days reaching significance in the putamen in the 1.5-3.5 day-interval (Figure 3C). Overall, reduced diffusivity in the poor outcome patients was more severe in the cortical structures than in the deep gray nuclei.
Corona radiata and internal capsule
In good outcome patients, the corona radiata, but not the internal capsule, showed mild increased diffusion in all time-intervals starting at 1.5 days with a peak between 3-5 days. In contrast, in the poor outcome group, marked reduced diffusion was noted in the corona radiata and in the internal capsule only between 6-8 days (Figure 3D).
Cerebellum and pons
In the good outcome group, the cerebellum exhibited increased diffusivity most markedly in the 3-5 day interval. Diffusivity in the cerebellum of the poor outcome patients tended to be decreased compared to controls starting at 1.5 days and was most pronounced between 6-8 days. The pons demonstrated a trend towards mild increased diffusion at most time-points in both groups, but more so in the good than the poor outcome patients.
Lastly, similar to the qualitative findings, poor outcome patients had significantly lower ADC values in each structure than good outcome patients when all time-windows were combined (i.e. 0 to 192 hours), except for the internal capsule, pons, caudate and thalamus (borderline).
DISCUSSION
DWI detects early cytotoxic edema by measuring the random motion of water protons, a process that is reduced by failure of the energy-requiring active water transport mechanism. DWI studies in animal models have demonstrated a decline in brain ADC values during cardiac arrest, which then reverse after successful resuscitation.16-18 In spite of successful reperfusion, however, secondary energy failure and ADC decrease follow after several hours.19
It has been shown in preliminary studies that quantitative MRI brain changes correlate with functional outcome in comatose post-cardiac arrest survivors.14, 20 The percentage of brain tissue below a threshold of 650-700 ×10−6mm2/sec was found to correlate with functional outcome at 3 months after the arrest.14 Based on whole brain quantitative DWI analyses the ideal time-window for prognostication appears to be between 49 and 108 hours after the arrest, when the ADC reductions are most apparent. None of the patients with more than ten percent of brain tissue with an ADC value below 650-700 ×10−6mm2/sec during this time-window regained consciousness.
In this prospective study, we analyzed in detail which brain structures at what time-point were most severely affected by ADC reduction, comparing good and poor outcome patients with normal controls and with each other. Our results show that ADC changes caused by global ischemic brain injury in humans are much delayed as compared to ADC changes caused by focal cerebral ischemia. In severe global ischemic brain injury associated with poor outcome, reduced diffusion may not be apparent in the initial hours after the arrest.
Since physicians are increasingly using brain MRI with DWI for prognostic purposes in post-cardiac arrest patients, it is important to be aware that ADC changes in these patients are both time- and region-dependent during the first week. We found that both the qualitative and quantitative MRI changes in poor outcome patients were most severe in the cortical regions and most apparent between 3-5 days after the arrest. Although ADC changes occur globally, they most profoundly affect the cortical gray matter structures in the poor outcome patients in the first week after the arrest. None of the patients with moderate-to-severe cortical abnormalities on their MRI awoke from their coma. Importantly, the three poor outcome patients who did not display substantial cortical abnormalities on their DWI MRI were imaged at 2, 7 and 14 hours after their arrests, suggesting that these changes are not apparent early after the arrest. In contrast to the gray matter structures, reduced diffusion in the white matter structures was not observed until the end of the first week. This DWI pattern likely reflects differences in tissue response to ischemic injury between various brain structures.21
Most patients who regained consciousness were qualitatively read as having normal cortical structures, with the exception of four patients who had mild-to-moderate abnormalities on the FLAIR and/or DWI sequence in one or more cortical gray matter structures. Furthermore, half of the good outcome patients had qualitative changes in the deep gray nuclei. Thus, mild-to-moderate cortical abnormalities and abnormalities in the deep gray nuclei did not exclude the possibility of regaining consciousness. Since qualitative interpretation of symmetrical and potentially subtle MRI changes is likely to vary between observers, we suspect that quantitative ADC changes may be more specific for prognostication.
Prior data on the temporal and spatial profile of brain ADC changes in the first week following cardiac arrest are scarce.20, 22 A recently published retrospective study confirms our observation that regional brain ADC values differ between good and poor outcome cardiac arrest patients and are time dependent.20 Comparisons with normal controls were not performed. To our surprise, when compared to normal controls, we observed increased or facilitated (rather than reduced or restricted) diffusion in good outcome patients who awoke from their coma. This change was only apparent on our quantitative ADC analyses and most pronounced in the cortical structures, the hippocampus, the putamen and the corona radiata. Increased diffusion has to our knowledge not been reported previously in humans in this context. We hypothesize that this increased diffusivity may represent mild global vasogenic edema caused by transient increased blood-brain barrier permeability.
While diffusivity in the hippocampus of the good outcome patients was consistently increased, no difference was observed in the poor outcome patients as compared to controls. One possible explanation is that severe hippocampal hypoxic-ischemic injury is associated with both vasogenic and cytotoxic edema counterbalancing each other’s effects on the hippocampal ADC values.
The differences in the DWI data of individual structures between good and poor outcome patients were very similar in the quantitative and qualitative analyses when all time-windows were combined. The only exception was the caudate nucleus, which was rated distinctly different in the two groups with the qualitative DWI readings, but not with the quantitative measurements. The most likely explanation for this observation is that there is increased T2 signal effect in the DWI maps observed qualitatively but not measured by the quantitative ADC maps.
An important limitation of this study is a limited number of MRI observations in each time-window, in particular in the very early time-window of 0-48 hours. The main reason why patients were not scanned during this time was because they were undergoing therapeutic hypothermia and we wanted to avoid any possible effect of hypothermia on the quantitative DWI MRI results. Additionally, patients who were taken off life support without meeting specific clinical criteria for poor outcome were excluded from this study. We decided not to include these patients because their outcomes were considered uncertain. Lastly, the results of this study only apply to patients who undergo brain MR imaging within 8 days following cardiac arrest. We are aware that some patients are too unstable from a critical care standpoint to undergo MR imaging during this early time period. MRI changes caused by severe hypoxic-ischemic brain injury are different after the first week.7
In summary, we found that moderate-to-severe reduced diffusion in cortical regions is strongly associated with the inability to regain consciousness and that this finding is most apparent on MRI between 3 and 5 days after the arrest. In contrast, patients who are able to wake up from their coma exhibit increased diffusion involving the temporal and occipital lobes, the corona radiata, and the hippocampus. As brain MRI is increasingly used for prognostic purposes in critically ill neurologic patients, physicians need to be aware that brain diffusion changes are both time and region dependent in the context of diffuse hypoxic-ischemic brain injury during the first week.
ACKNOWLEDGEMENTS
This study was funded by the National Institutes of Health 1R01HL089116 (Wijman), R01NS034866 (Moseley), 2R01EB002711 (Bammer), the American Heart Association National Scientist Development Award 0430275N (Wijman), and the Katherine McCormick Fund for Women (Hsia). The authors would like to thank Stephanie Kemp for administrative support of this study and Anna Finley Caulfield, Marion Buckwalter, Chitra Venkatasubramanian, Maarten Lansberg and Neil Schwartz for their assistance with patient enrollment.
Disclosures
This study was funded by the American Heart Association and the National Institutes of Health.
Dr. Mlynash reports no disclosures.
Dr. Campbell reports no disclosures.
Dr. Leproust is employed by Agilent Technologies, Inc.
Dr. Fischbein reports no disclosures.
Dr. Bammer was funded by NIH grant #2R01EB002711.
Dr. Eyngorn reports no disclosures.
Dr. Hsia received a stipend from the Katherine McCormick Fund for Women.
Dr. Moseley was funded by NIH grant #R01NS034866.
Dr. Wijman is funded by NIH grant #R01HL089116 and 2R01NS034866, and received the AHA National Scientist Development award #0430275N for this research.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eisenberg MS, Horwood BT, Cummins RO, Reynolds-Haertle R, Hearne TR. Cardiac arrest and resuscitation: A tale of 29 cities. Ann Emerg Med. 1990;19:179–186. doi: 10.1016/s0196-0644(05)81805-0. [DOI] [PubMed] [Google Scholar]
- 2.Becker LB, Ostrander MP, Barrett J, Kondos GT. Outcome of CPR in a large metropolitan area--where are the survivors? Ann Emerg Med. 1991;20:355–361. doi: 10.1016/s0196-0644(05)81654-3. [DOI] [PubMed] [Google Scholar]
- 3.Blackhall LJ, Ziogas A, Azen SP. Low survival rate after cardiopulmonary resuscitation in a county hospital. Arch Intern Med. 1992;152:2045–2048. [PubMed] [Google Scholar]
- 4.Moseley ME, Kucharczyk J, Mintorovitch J, Cohen Y, Kurhanewicz J, Derugin N, Asgari H, Norman D. Diffusion-weighted MR imaging of acute stroke: Correlation with t2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol. 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 5.Burdette JH, Ricci PE, Petitti N, Elster AD. Cerebral infarction: Time course of signal intensity changes on diffusion-weighted MR images. AJR Am J Roentgenol. 1998;171:791–795. doi: 10.2214/ajr.171.3.9725318. [DOI] [PubMed] [Google Scholar]
- 6.Provenzale JM, Sorensen AG. Diffusion-weighted MR imaging in acute stroke: Theoretic considerations and clinical applications. AJR Am J Roentgenol. 1999;173:1459–1467. doi: 10.2214/ajr.173.6.10584783. [DOI] [PubMed] [Google Scholar]
- 7.Arbelaez A, Castillo M, Mukherji SK. Diffusion-weighted MR imaging of global cerebral anoxia. AJNR Am J Neuroradiol. 1999;20:999–1007. [PMC free article] [PubMed] [Google Scholar]
- 8.Wijdicks EFM, Campeau NG, Miller GM. MR imaging in comatose survivors of cardiac resuscitation. AJNR Am J Neuroradiol. 2001;22:1561–1565. [PMC free article] [PubMed] [Google Scholar]
- 9.McKinney AM, Teksam M, Felice R, Casey SO, Cranford R, Truwit CL, Kieffer S. Diffusion-weighted imaging in the setting of diffuse cortical laminar necrosis and hypoxic-ischemic encephalopathy. AJNR Am J Neuroradiol. 2004;25:1659–1665. [PMC free article] [PubMed] [Google Scholar]
- 10.Singhal AB, Topcuoglu MA, Koroshetz WJ. Diffusion MRI in three types of anoxic encephalopathy. Journal of the Neurological Sciences. 2002;196:37–40. doi: 10.1016/s0022-510x(02)00019-9. [DOI] [PubMed] [Google Scholar]
- 11.Barrett KM, Freeman WD, Weindling SM, Brott TG, Broderick DF, Heckman MG, Crook JE, Divertie GD, Meschia JF. Brain injury after cardiopulmonary arrest and its assessment with diffusion-weighted magnetic resonance imaging. Mayo Clin Proc. 2007;82:828–835. doi: 10.4065/82.7.828. [DOI] [PubMed] [Google Scholar]
- 12.Hald JK, Brunberg JA, Dublin AB, Wootton-Gorges SL. Delayed diffusion-weighted MR abnormality in a patient with an extensive acute cerebral hypoxic injury. Acta Radiol. 2003;44:343–346. doi: 10.1080/j.1600-0455.2003.00050.x. [DOI] [PubMed] [Google Scholar]
- 13.Greer DM. MRI in anoxic brain injury. Neurocrit Care. 2004;1:213–215. doi: 10.1385/NCC:1:2:213. [DOI] [PubMed] [Google Scholar]
- 14.Wijman CA, Mlynash M, Caulfield AF, Hsia AW, Eyngorn I, Bammer R, Fischbein N, Albers GW, Moseley M. Prognostic value of brain diffusion-weighted imaging after cardiac arrest. Ann Neurol. 2009;65:394–402. doi: 10.1002/ana.21632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Els T, Kassubek J, Kubalek R, Klisch J. Diffusion-weighted MRI during early global cerebral hypoxia: A predictor for clinical outcome? Acta Neurol Scand. 2004;110:361–367. doi: 10.1111/j.1600-0404.2004.00342.x. [DOI] [PubMed] [Google Scholar]
- 16.de Crespigny AJ, Rother J, Beaulieu C, Moseley ME, Hoehn M. Rapid monitoring of diffusion, DC potential, and blood oxygenation changes during global ischemia. Effects of hypoglycemia, hyperglycemia, and TTX. Stroke. 1999;30:2212–2222. doi: 10.1161/01.str.30.10.2212. [DOI] [PubMed] [Google Scholar]
- 17.Fischer M, Bockhorst K, Hoehn-Berlage M, Schmitz B, Hossmann K-A. Imaging of the apparent diffusion coefficient for the evaluation of cerebral metabolic recovery after cardiac arrest. Magnetic Resonance Imaging. 1995;13:781–790. doi: 10.1016/0730-725x(95)00030-k. [DOI] [PubMed] [Google Scholar]
- 18.Krep H, Bottiger BW, Bock C, Kerskens CM, Radermacher B, Fischer M, Hoehn M, Hossmann KA. Time course of circulatory and metabolic recovery of cat brain after cardiac arrest assessed by perfusion- and diffusion-weighted imaging and MR-spectroscopy. Resuscitation. 2003;58:337–348. doi: 10.1016/s0300-9572(03)00151-5. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Silva MD, Liu KF, Helmer KG, Omae T, Fenstermacher JD, Sotak CH, Fisher M. Secondary decline in apparent diffusion coefficient and neurological outcomes after a short period of focal brain ischemia in rats. Ann Neurol. 2000;48:236–244. [PubMed] [Google Scholar]
- 20.Wu O, Sorensen AG, Benner T, Singhal AB, Furie KL, Greer DM. Comatose patients with cardiac arrest: Predicting clinical outcome with diffusion-weighted MR imaging. Radiology. 2009;252:173–181. doi: 10.1148/radiol.2521081232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierpaoli C, Alger JR, Righini A, Mattiello J, Dickerson R, Des Pres D, Barnett A, Di Chiro G. High temporal resolution diffusion MRI of global cerebral ischemia and reperfusion. J Cereb Blood Flow Metab. 1996;16:892–905. doi: 10.1097/00004647-199609000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi S, Higano S, Ishii K, Matsumoto K, Sakamoto K, Iwasaki Y, Suzuki M. Hypoxic brain damage: Cortical laminar necrosis and delayed changes in white matter at sequential MR imaging. Radiology. 1993;189:449–456. doi: 10.1148/radiology.189.2.8210374. [DOI] [PubMed] [Google Scholar]