Abstract
We tested the hypothesis that expression of presynaptic voltage-gated Na+ channel (Nav) subtypes coupled to neurotransmitter release differs between transmitter types and CNS regions in a nerve terminal-specific manner. Nav coupling to transmitter release was determined by measuring the sensitivity of 4-aminopyridine (4AP)-evoked [3H]glutamate and [14C]GABA release to the specific Nav blocker tetrodotoxin (TTX) for nerve terminals isolated from rat cerebral cortex, hippocampus, striatum and spinal cord. Expression of various Nav subtypes was measured by immunoblotting using subtype-specific antibodies. Potencies of TTX for inhibition of glutamate and GABA release were similar between CNS regions. However, the efficacies of TTX for inhibition of 4AP-evoked glutamate release were greater than for inhibition of GABA release in all regions except spinal cord. The relative nerve terminal expression of total Nav subtypes as well as of specific subtypes varied considerably between CNS regions. The region-specific potencies of TTX for inhibition of 4AP-evoked glutamate release correlated with greater relative expression of total nerve terminal Nav and Nav1.2. Nerve terminal-specific differences in the expression of specific Nav subtypes contribute to transmitter-specific and regional differences in pharmacological sensitivities of transmitter release.
Introduction
Calcium-triggered exocytosis of neurotransmitter-containing synaptic vesicles requires the coordinated activation of presynaptic voltage-gated ion channels including Na+ (Nav) and Ca2+ (Cav) channels (Meir et al. 1999, Vacher et. al. 2008). These members of the voltage-gated superfamily of ion channels are expressed as multiple subtypes with distinct functional roles, pharmacological properties, and tissue and developmental expression (Goldin 2001, Yu and Catterall 2003, Lai et al. 2004, Dib-Hajj and Priestly 2009). Compared to presynaptic Ca2+ channels coupled to neurotransmitter release (Catterall and Few 2008), relatively little is known about the expression and function of presynaptic Na+ channels. Nerve terminal Na+ channels are important in the amplification of presynaptic action potentials (Engel and Jonas, 2005). Inhibitors of Na+ and/or Ca2+ channels reduce neurotransmitter release from nerve terminals by reducing depolarization, Ca2+ entry, and Ca2+-dependent exocytosis (Wu and Saggau 1997). Transmitter-specific and regional differences in presynaptic Cav subtypes coupled to synaptic vesicle exocytosis lead to differences in the sensitivity of transmitter release to potent invertebrate neurotoxins (Bowman et al. 1993, Reid et al. 1997, Meir et al. 1999). A number of drugs including anticonvulsants, neuroprotective agents, and volatile anesthetics selectively inhibit glutamate release over other transmitters apparently by preferential inhibition of presynaptic Na+ channels relative to Ca2+ channels (Westphalen and Hemmings 2003, Sitges et al. 2007). The pharmacological selectivity of these Nav blockers supports differences in ion channel mechanisms between excitatory glutamatergic and inhibitory GABAergic synapses (Prakriya and Mennerick 2000).
Presynaptic specializations leading to distinct pharmacological sensitivities for the release of various neurotransmitters could result from differential expression of presynaptic ion channel subtypes (Bowman et al. 1993, Reid et al. 1997, Mechaly et al. 2005), ion channel accessory subunits (Wynne et al. 2009), ionotropic receptors (MacDermott et al. 1999, Long et al. 2009, Westphalen et al. 2009), post-translational ion channel modulation (Hemmings 1998, MacDermott et al. 1999), excitation-secretion coupling mechanisms (Prakriya and Mennerick 2000), and/or synaptic vesicle fusion machinery (Südhof 2004). We explored the hypothesis that nerve terminal-specific differences in presynaptic expression of Nav subtypes result in pharmacological differences in the Ca2+-dependent release of specific neurotransmitters. The effects of the specific subtype-selective Nav antagonists tetrodotoxin (Stuart and Sakmann 1994, Campos et al. 2004) and A-803467 (Jarvis et al. 2007) and of the subtype-selective Nav agonist veratridine (Farrag et al. 2008) on evoked glutamate and GABA release were determined for nerve terminals isolated from four functionally distinct regions of rat central nervous system (CNS). For comparison, the relative expression of the major CNS Nav subtypes was examined. There are 9 distinct Nav pore-forming α-subunit subtypes (Nav1.1–1.9) with distinct tissue distributions, electrophysiological properties, and toxin sensitivities (Goldin 2001, Strichartz 2008). Tetrodotoxin-sensitive (TTX-s) Nav subtypes (Nav1.1, 1.2, 1.3, 1.6, 1.7) are inhibited by TTX more potently than the TTX-resistant (TTX-r) subtypes (Nav1.4, 1.5, 1.8, 1.9) (Goldin 2001, Lai et al. 2004), and are selectively activated by the phytotoxin veratridine (VTD, Farrag et al. 2008). We utilized these pharmacological distinctions to probe for differences between CNS regions in the regulation of glutamate and GABA release by specific Nav subtypes. We hypothesized that differences in the presynaptic expression and/or function of Nav subtypes coupled to transmitter release result in differential sensitivities of release to subtype-selective drugs.
Materials and methods
Materials
Tetrodotoxin (TTX), 4-aminopyridine (4AP), veratridine (VTD), and A-803467 were from Sigma-Aldrich Chemical Co. (St. Louis, MO). L-[3H]Glutamate was from Amersham Radiochemical Centre (42 Ci/mmol; Buckinghamshire, UK). [14C]GABA was from PerkinElmer Inc. (28 mCi/mmol; Boston, MA) or American Radiolabel Chemicals Inc. (55 mCi/mmol; St. Louis, MO).
Nerve terminal preparation
Experiments were performed in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals as approved by the Weill Cornell Medical College Institutional Animal Care and Use Committee. Isolated nerve terminals (synaptosomes) from adult (200–300 g) male Sprague-Dawley rat (Charles River Laboratories, Troy, NY) cerebral cortex, hippocampus, striatum and spinal cord were prepared and demyelinated as described (Westphalen and Hemmings 2006a). Synaptosomes were incubated with 5–10 nM L-[3H]glutamate and 400–500 nM [14C]GABA for 15 min at 30°C in 10 ml Krebs-HEPES buffer (KHB, composition in mM: NaCl 140, KCl 5, HEPES 20, MgCl2 1, Na2HPO4 1.2, NaHCO3 5, EGTA 0.1, and D-glucose 10, pH 7.4 with NaOH). Synaptosomes were collected by centrifugation for 10 min at 20,000 × g at 4°C, resuspended in ice-cold 0.32 M sucrose, and loaded into release chambers.
Glutamate and GABA release
Simultaneous release of glutamate and GABA was assayed as described (Westphalen and Hemmings 2006b). Briefly, dual-labeled synaptosomes (0.3–0.8 mg protein) confined by Whatman (Maidstone, UK) GF/B glass fiber filter disks were superfused at 0.5 ml/min with KHB at 37°C using a customized Brandel SF12 superfusion apparatus (Gaithersburg, MD) set to collect 1 min fractions. Synaptosomes were superfused with TTX (0.1–3000 nM) 12 min prior to, during and following a 2 min depolarizing pulse of either 1 mM 4AP or 10 μM VTD in the absence or presence of 1.9 mM free extracellular Ca2+ (obtained by adding 2 mM CaCl2 to KHB). In separate experiments, 4AP-evoked release was determined from synaptosomes exposed to 1 μM A-803467, a selective Nav1.8 blocker (Jarvis et al. 2007), in the presence of 1 μM TTX to block TTX-s Nav subtypes. Experiments were terminated by perfusion of 0.2 M perchloric acid to lyse synaptosomes. Radioactivity in each fraction was quantified by liquid scintillation spectrometry with dual isotope quench correction (Beckman Coulter LS 6000IC, Fullerton, CA) using BioSafe II scintillation cocktail (RPI, Mt. Prospect, IL).
Release of transmitter in each 1 min fraction of perfusate was expressed as a fraction of synaptosomal content of labeled transmitter prior to that fraction (fractional release [FR], Westphalen and Hemmings 2006b). The magnitude of evoked release was determined by subtracting baseline release (average of basal FR before and after stimulation) from cumulative FR values for each evoked release pulse to yield sum FR. Release data from each CNS region were generated from 2–5 (4AP-evoked) and 2–3 (VTD-evoked release) independent experiments. Each experiment included control (in duplicate), which was used to normalize data to the mean of all control values prior to curve fitting and/or statistical analysis. TTX concentration-effect data were fitted to a four-parameter logistic equation [Y=Imax+(I0−Imax)/(1+10^((X-LogIC50)*HillSlope))] by least-squares analysis to estimate IC50 (Prism 5.0; GraphPad Software, San Diego CA). The curve fit parameters I0 (upper plateau of the curve) and Imax (lower plateau of curve) were tested for significant differences from control or zero sum ΔFR, respectively. If no difference was found, I0 was constrained to control and/or Imax was constrained to zero sum ΔFR. Maximal release inhibition (I0 − Imax) was determined as percentage of control sum ΔFR. Significant differences between IC50 or Imax values were determined by F-test comparisons between best-fit values derived from separate curve fits to values derived from global fits with the tested parameter shared. The relative magnitude of control GABA vs. glutamate release was compared between secretegogues by subtracting sum ΔFR of glutamate from sum ΔFR of GABA and expressing the difference as percentage of evoked glutamate release. Mean sum ΔFR data were tested for differences between multiple groups by one-way analysis of variance (ANOVA) with Tukey post hoc test. Data compared between two groups used the Student t-test, with Welch correction if an F-test determined the group variances as not identical.
Quantitative immunoblotting
Immunoreactivity was analyzed in nerve terminal preparations using rabbit antibodies specific for the α-subunits of all Nav1 subtypes (pan-Nav1), or subtype-specific antibodies against rat amino acid residues 465–481 of Nav1.1, 467–485 of Nav1.2, 511–524 of Nav1.3 (Sigma-Aldrich), 1042–1061 of Nav1.6 (Millipore, Temecula, CA), 446–460 of Nav1.7 (Alomone Labs, Jerusalem, Israel), 1943–1956 or 1943–1957 of Nav1.8 (Millipore or Sigma-Aldrich), and 1748–1765 of Nav1.9 (Millipore). Rat cerebral cortex, hippocampus, striatum and spinal cord synaptosomes were prepared as described above with the addition of a protease inhibitor cocktail (4-(2-aminoethyl)benzenesulfonyl fluoride, aprotinin, bestatin, N-(trans-epoxysuccinyl)-L-leucine 4-guanidinobutylamide, leupeptin, and pepstatin A; Sigma-Aldrich). Protein concentrations were determined by the method of Bradford (Bradford 1976) using bovine serum albumin as a standard before solubilization in 1% (w/v) sodium dodecyl sulfate (SDS). Aliquots containing equal protein amounts were separated by SDS-polyacrylamide (7.5%) gel electrophoresis, and proteins were transferred onto polyvinylidine difluoride membrane (0.45 μm, Bio-Rad, Hercules, CA) as described (Hemmings et al. 1992). Protein amounts loaded were within the linear range of quantification for each antibody. Membranes were incubated in blocking buffer (5% dried milk powder (w/v) in PBST [10 mM Na-phosphate, 150 mM NaCl, 0.5% (v/v) Tween 20, pH 7.8]) for 1 h, probed with anti-pan Nav1 (1:1000 dilution; 10–20 μg protein load), anti-Nav1.1 (1:400 dilution; 20–30 μg protein load), anti-Nav1.2 (1:200 dilution; 10–20 μg protein load), anti-Nav1.3 (1:400 dilution; 100 μg protein load), anti-Nav1.6 (1:200 dilution; 50–120 μg protein load), anti-Nav1.7 (1:100 dilution; 80–100 μg protein load), anti-Nav1.8 (1:200 dilution; 90–100 μg protein load), anti-Nav1.9 (1:100 dilution; 100 μg protein load) for 1 h. Membranes were then washed 3 × 10 min with PBST and bound antibody was detected using horseradish peroxidase-conjugated goat-anti-rabbit antiserum and ECL (Amersham/GE Healthcare, Pittsburgh, PA).
Exposed films of immunoblots were scanned with a Typhoon Trio9410 variable mode imager (Amersham/GE Healthcare) using a 633 nm He-Ne laser and photomultiplier running at 600 V. Signal intensity was quantified using ImageQuant 5.2 software (Amersham/GE Healthcare) with local average background correction. Relative densities of immunoreactive bands were determined using 2–3 different amounts of protein per region to insure linearity. Immunoreactivity in each brain region was normalized to cerebral cortex on the same blot, and averaged from two exposure times. Relative expression in each CNS region were compared to cortex by repeated measures one-way ANOVA with Tukey post hoc test (Prism 5.0). Correlations of Nav immunoreactivity (relative to cortex) with potencies (1/IC50) of TTX for inhibition of 4AP-evoked transmitter release were tested for significance using the Pearson correlation (Prism 5.0).
Results
4AP-evoked release
The K+ channel blocker 4AP evokes transmitter release from isolated nerve terminals by a mechanism that requires sequential activation of Na+ and Ca2+ channels following transient membrane depolarizations (Tibbs et al. 1989). Stimulation of nerve terminals with a 2 min pulse of 1 mM 4AP in the presence of 1.9 mM free external Ca2+ evoked both L-[3H]glutamate and [14C]GABA release (Fig. 1). TTX inhibited 4AP-evoked glutamate and GABA release from all four CNS regions in a concentration-dependent manner (Fig. 1) with IC50 values of 5–30 μM (Fig. 2A). TTX inhibited 4AP-evoked glutamate release from hippocampal or striatal nerve terminals with greater potency than release from cortical nerve terminals. Inhibition of glutamate release from spinal cord nerve terminals was relatively low. TTX inhibited 4AP-evoked GABA release from cortical, striatal and spinal cord nerve terminals equipotently, and with less potency from hippocampal terminals. TTX inhibited glutamate and GABA release equipotently from cortical terminals, while potency for inhibition of glutamate release was greater than for GABA release from hippocampal and striatal terminals.
Figure 1.
Effects of tetrodotoxin on neurotransmitter release in various CNS regions. Data for inhibition of L-[3H]glutamate and [14C]GABA release evoked by 1 mM 4AP from rat cortical, hippocampal, striatal, and spinal cord synaptosomes were fitted to sigmoidal concentration-effect curves (mean±SEM; n=24–33). IC50 values and maximal inhibition values are shown in Fig 2. Control data are presented as mean±SD (n=10–18).
Figure 2.
Potency and efficacy for inhibition of glutamate and GABA release from isolated nerve terminals by tetrodotoxin. a: IC50 values (±SEM) derived by fitting data to sigmoidal concentration-effect curves (Fig. 1) for release from cerebral cortex (Cx), hippocampus (Hip), striatum (Str) and spinal cord (SC). Statistical comparisons between cerebral cortex and other CNS regions (*P < 0.05) and between glutamate and GABA release (†P < 0.05; †††P < 0.001) were analyzed by comparing logIC50 values between sigmoidal curve fits by F-tests. b: Maximal inhibition by TTX determined as the lower plateau of sigmoidal concentration-effect curve fits (Fig. 1) expressed as I0 − Imax/I0 × 100 (±SEM). Statistical comparisons between cerebral cortex and other CNS regions (***P < 0.001) and between glutamate and GABA release (††P < 0.01; †††P < 0.001) were analyzed by comparing lower plateaus of curves by F-tests.
Glutamate and GABA release evoked by 4AP was not completely inhibited by TTX: the lower plateaus of all curve fits (Imax) did not reach zero (cortex, hippocampus and striatum, P < 0.0001; spinal cord, P < 0.05). Maximal inhibition (efficacy) by TTX expressed as a percent of control release was significantly greater for glutamate release than for GABA release in cortex, hippocampus and striatum, but similar in spinal cord (Fig. 2B). Maximal inhibition by TTX of 4AP-evoked glutamate release from cortical, striatal, and spinal cord nerve terminals was similar, but less than that from hippocampal nerve terminals. Maximal inhibition by TTX of GABA release from cortex and spinal cord was less than that from hippocampal and striatal nerve terminals. Ca2+-independent 4AP-evoked release of both glutamate and GABA release from cortex was inhibited by TTX as previously reported (Westphalen and Hemmings 2003,Westphalen and Hemmings 2006b), but not from spinal cord (Fig. 3). In spinal cord, TTX inhibited GABA but not glutamate release.
Figure 3.
Effects of tetrodotoxin and A-803467 on 4AP-evoked glutamate and GABA release from cortex and spinal cord nerve terminals. Ca2+-independent 4AP-evoked glutamate release from cortex, but not spinal cord, was inhibited by 1 μM tetrodotoxin (TTX). TTX inhibited Ca2+-independent 4AP-evoked GABA release from both cortex and spinal cord. The selective Nav1.8 blocker A-803467 had no effect on TTX-insensitive glutamate or GABA release from cortical nerve terminals in the presence of Ca2+, but inhibited TTX-insensitive glutamate, but not GABA, release from spinal cord nerve terminals. *P < 0.05, **P < 0.01 by unpaired Student t-test with Welch correction (mean±SEM). Group sizes (n) for GABA are the same as for glutamate.
A role for selective Nav1.8 expression leading to relatively lower potency for TTX inhibition of 4AP-evoked glutamate release from spinal cord nerve terminals was tested using the selective Nav1.8 antagonist A-803467 (Jarvis et al. 2007). In the presence of TTX to inhibit TTX-s Nav subtypes, A-803467 further inhibited 4AP-evoked glutamate release from spinal cord but not from cortical nerve terminals, but had no effect on GABA release (Fig. 3). The additive inhibition by A-803467 and TTX approached residual Ca2+-independent glutamate release evoked by 4AP in spinal cord. A-803467 alone, at a high nonselective concentration (10 μM) that also inhibits TTX-s Nav channels (Jarvis et al. 2007), inhibited 4AP-evoked glutamate and GABA release from cortical nerve terminals by 30 ± 2% (n=3, data not shown).
Control sum ΔFR of GABA was greater than that of glutamate for all four rat CNS regions tested (by 1.6 – 2.3-fold; P < 0.001; Fig. 4A), as reported previously for rat cortex (Westphalen and Hemmings 2003). Control glutamate release from cortical nerve terminals (n=36) was equivalent to release from hippocampal (n=27) and striatal terminals (n=29), and greater than that from spinal cord terminals (n = 26; P < 0.001). Control GABA release from cortical nerve terminals was equivalent to release from hippocampal terminals, and greater than that from striatal (P < 0.001) and spinal cord terminals (P < 0.001).
Figure 4.
Neurotransmitter release evoked by 4-aminopyridine or veratridine from various CNS regions. Release of L-[3H]glutamate and [14C]GABA from rat cortical (Cx), hippocampal (Hip), striatal (Str) and spinal cord (SC) nerve terminals evoked by 2 min pulses of 1 mM 4AP (a, n=14–22) or 10 μM VTD (b, n=10–31). Statistical comparisons between cortex and other CNS regions (***P < 0.001), and between glutamate and GABA (†P < 0.05; ††P < 0.01; †††P < 0.001) were analyzed by one-way ANOVA using Tukey’s post-hoc testing (mean±SD).
Veratridine-evoked release
Veratridine evokes transmitter release from isolated nerve terminals by effecting Nav activation through impaired channel inactivation leading to sustained membrane depolarization (Ulbricht 1998). 4AP-evoked GABA release was consistently greater than glutamate release in all four CNS regions (Fig. 4A). In contrast, VTD-evoked release of glutamate and GABA from cortical and striatal nerve terminals was similar, while GABA release was greater than glutamate release from hippocampal (1.5-fold; P < 0.01) and spinal cord (3-fold; P < 0.05) nerve terminals (Fig. 4B). The difference between GABA and glutamate release from spinal cord nerve terminals was greater for VTD-evoked release compared to 4AP-evoked release, primarily due to the lower efficacy of VTD-evoked glutamate release.
Although TTX inhibition of VTD-evoked release was qualitatively similar to inhibition of 4AP-evoked release (Fig. 1), TTX completely inhibited VTD-evoked release from all four regions tested (represented by cortex; Fig. 5A). This is consistent with an absolute requirement for activation of presynaptic Na+ channels for depolarization by VTD but not by 4AP. TTX inhibited VTD-evoked glutamate and GABA release from cortical, hippocampal, and striatal nerve terminals equipotently, but was far less potent in inhibiting glutamate release from spinal cord nerve terminals (Fig. 5B). The potency of TTX for inhibition of VTD-evoked GABA release did not differ between regions, and was greater compared to inhibition of spinal cord glutamate release (Fig. 5B).
Figure 5.
Effects of tetrodotoxin on neurotransmitter release evoked by veratridine. A: Inhibition by tetrodotoxin of L-[3H]glutamate and [14C]GABA release evoked by 10 μM VTD from rat cortical synaptosomes (n=20). B: IC50 values for inhibition by tetrodotoxin of transmitter release form cortical nerve terminals determined by fitting data to sigmoidal concentration-effect curves. Statistical comparisons between cortex and other CNS regions (***P < 0.001) and between glutamate and GABA (††P < 0.01) release curves were analyzed by comparing log IC50 values between sigmoidal curve fits by F-test (mean±SEM).
Na+ channel immunoreactivity
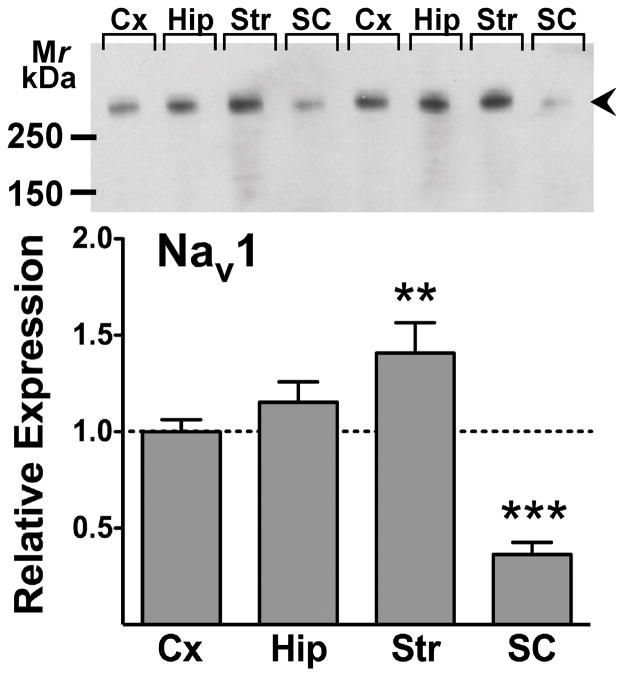
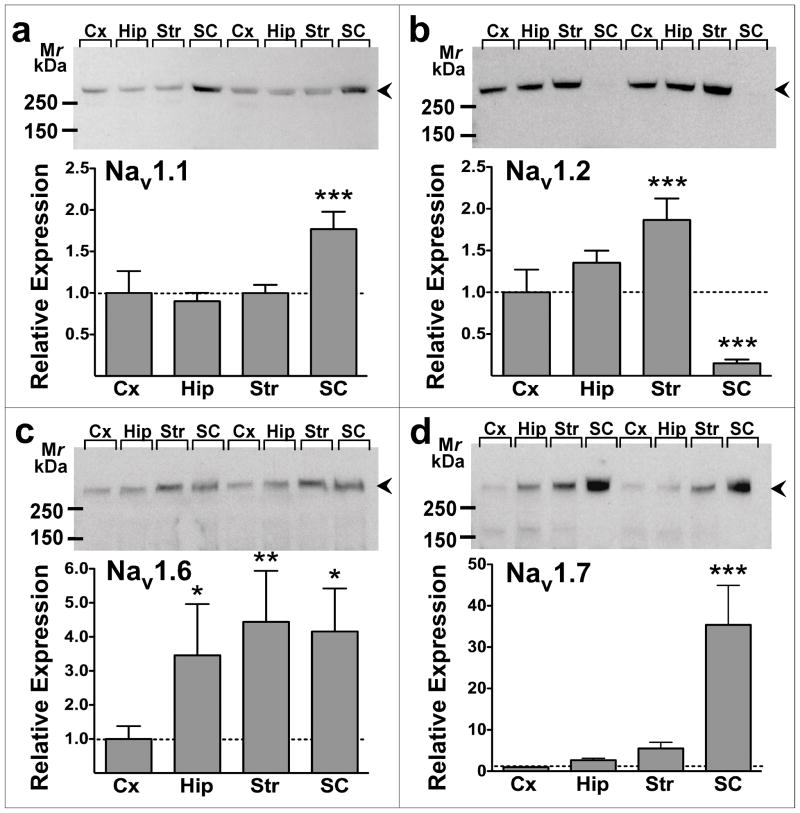
An antibody directed against a conserved epitope that recognizes all vertebrate Nav1 α-subunits (Toledo-Aral et al. 1997) was used to probe nerve terminal membrane preparations from rat cerebral cortex, hippocampus, striatum and spinal cord for total Nav expression (all subtypes) normalized to total protein (Fig. 6). Immunoblots revealed the presence of immunoreactivity at a relative molecular mass of 260–280 kDa corresponding to the glycosylated ~260 kDa Nav α-subunits present in adult rat brain (Messner and Catterall 1985). The relative expression of total Nav1 immunoreactivity in nerve terminal preparations was striatum (1.4-fold higher; P < 0.01) > hippocampus = cortex > spinal cord (0.4-fold lower; P < 0.001) (Fig. 6). Subtype-specific antibodies were used to probe relative expression of the major Na+ channel α-subunits expressed in rat CNS: Nav1.1, 1.2, 1.3, 1.6, 1.7, 1.8 and 1.9 (Goldin 2001). Cortical, hippocampal and striatal nerve terminals showed similar expression of Nav1.1, while expression in spinal cord terminals was 1.8-fold greater (P < 0.001, Fig. 7A). Cortical and hippocampal nerve terminals expressed comparable amounts of Nav1.2, while Nav1.2 expression was 2.0-fold greater (P < 0.001) in striatum and very low amounts (P < 0.001) are expressed in spinal cord (Fig. 7B). Expression of Nav1.6 was 3.5-fold (P < 0.05), 4.4-fold (P < 0.01) and 4.2-fold (P < 0.05) greater, respectively, in hippocampus, striatum and spinal cord relative to cortical nerve terminals (Fig. 7C). Cortical, hippocampal and striatal terminals expressed comparable amounts of Nav1.7, while Nav1.7 expression was 40-fold greater (P < 0.001) in spinal cord terminals (Fig. 7D). No Nav1.3, Nav1.8 or Nav1.9 immunoreactivity was detected in the regions tested with the antibodies used (data not shown).
Figure 6.
Relative expression of total voltage-gated Na+ channel immunoreactivity in rat CNS nerve terminal preparations. Representative immunoblot shown for pan-specific Nav1 antibody labeling of cerebral cortical (Cx), hippocampal (Hip), striatal (Str), and spinal cord (SC) nerve terminals (upper panel). Relative expression of immunoreactive bands (260–280 kDa; arrowheads) was compared to cortex by repeated measures one-way ANOVA. **P < 0.01, ***P < 0.001 vs. cortex (mean±SEM; n=14).
Figure 7.
Relative expression of voltage-gated Na+ channel subtype immunoreactivity in rat CNS nerve terminal preparations. Representative immunoblots (upper panels) shown for a: Nav1.1 (n=16), b: Nav1.2 (n=16), c: Nav1.6 (n=13), and d: Nav1.7 (n=7) labeling of cortical (Cx), hippocampal (Hip), striatal (Str), and spinal cord (SC) nerve terminals. Relative expression of immunoreactive bands (260–280 kDa; arrowheads) was compared to cortex by repeated measures one-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001 vs. cortex (mean±SEM).
Immunoreactive Nav1, Nav1.1, Nav1.2, and Nav1.6 were enriched in nerve terminal preparations compared to whole tissue homogenates in hippocampus (by 2.4-, 1.4-, 1.7-, and 3.1-fold, respectively), striatum (by 52-, 3.1-, 63-, and 9.5-fold, respectively) and spinal cord (by 5.1-, 46-, 22-, and 120-fold, respectively), but not in cerebral cortex (1.2-, 0.7-, 0.5-, and 0.9-fold, respectively). Immunoreactive Nav1.7 was enriched in striatal nerve terminal preparations compared to whole homogenates (1.5-fold), but not in cortex (1.1-fold), hippocampus (1.2-fold), or spinal cord (1.0-fold) preparations.
Relative expression of the TTX-s Nav subtypes Nav1.1, Nav1.6, or Nav1.7 correlated either negatively or did not correlate with potencies of TTX (1/IC50) for inhibition of 4AP-evoked glutamate release between CNS regions (Table 1). Expression of the TTX-s Nav1.2 and of total Nav1 immunoreactivity correlated positively with potency of TTX for inhibition of glutamate release. There were no correlations between Nav expression and potency of TTX for inhibition of GABA release.
Table 1. Relationship between Nav immunoreactivity and tetrodotoxin potency.
Correlation between regional expression of total Nav1 and specific Nav1 subtype immunoreactivity relative to cortex and the −log IC50 of tetrodotoxin for inhibition of 4AP-evoked glutamate or GABA release from nerve terminals prepared from cortex, hippocampus, striatum and spinal cord. Slope values were derived by linear regression and tested by Pearson’s correlation
| Immunoreactivity Nav | Tetrodotoxin potency (− log IC50) | |||
|---|---|---|---|---|
| Glutamate release | GABA release | |||
| Slope | Correlation (r2) | Slope | Correlation (r2) | |
| 1 | 1.01 | 0.99 ** | −0.11 | 0.08 |
| 1.1 | −0.96 | 0.89 | 0.20 | 0.26 |
| 1.2 | 0.55 | 0.95 * | −0.05 | 0.05 |
| 1.6 | 0.01 | <0.01 | 0.01 | 0.01 |
| 1.7 | −0.02 | 0.77 | 0.01 | 0.18 |
P < 0.05,
P < 0.01.
Discussion
Voltage-gated Na+ channels are critical upstream regulators of depolarization-evoked Ca2+-dependent exocytosis of amino acid neurotransmitters from small synaptic vesicles (Tibbs et al. 1989). We hypothesized that presynaptic differences in Nav subtype expression and/or function underlie neurotransmitter-specific and regional differences in the physiology and pharmacology of release. Although transmitter and regional differences are known for presynaptic Ca2+ channel subtypes (Bowman et al. 1993, Reid et al. 1997, Vacher et al. 2008), comparable differences have not been described for Na+ channels (Vacher et al. 2008, Dib-Hajj and Priestly 2009). Due to technical limitations in resolving presynaptic from postsynaptic ion channel localization using immunofluorescence microscopy, the localization of specific Nav subtypes to typical small CNS nerve terminals has been difficult. We used neurochemical approaches to analyze expression of the major CNS Nav subtype α–subunits in nerve terminals (synaptosomes) isolated from various CNS regions and correlations with transmitter release. Synaptosomes are a subcellular fraction highly enriched for functional nerve terminals capable of synthesizing, releasing, and re-accumulating various neurotransmitters depending on the region used for isolation (Nicholls 1993). We focused our analysis on the release of the abundant CNS neurotransmitters glutamate and GABA, which are released from the majority of CNS nerve terminals (Raiteri 2008).
We observed significant regional differences in both the sensitivities of depolarization-evoked glutamate and GABA release to inhibition by the Na+ channel blocker TTX as well as in the expression of Nav subtypes. The differences in TTX potency suggest nerve terminal-specific expression and/or function of specific Nav subtypes, which vary in sensitivity to TTX (Goldin 2001, Lai et al. 2004). Relative expression of specific Nav subtypes varied between CNS regions. Although isolated nerve terminal preparations can be contaminated by postsynaptic and glial membranes, they are highly enriched in presynaptic components (Dodd et al. 1981) and in Nav subtype expression, supporting presynaptic Nav expression. This is consistent with immunohistochemical studies of Nav1.2 (Gong et al. 1999) and Nav1.6 (Caldwell et al. 2000). Regional expression of Nav typically correlated better with TTX inhibition potency for glutamate vs. GABA release. Expression of total Nav1 and Nav1.2 immunoreactivity correlated with potency of TTX for inhibition of 4AP-evoked glutamate release, suggesting that expression of Nav1.2, and of overall nerve terminal Nav1 contribute to region-specific differences in sensitivity of glutamate release to inhibition by Nav blockers. Nav1.2 expression is therefore closely coupled to Na+ channel-dependent 4AP-evoked glutamate release compared to other Nav subtypes or to 4AP-evoked GABA release.
Fractional release of GABA evoked by 4AP was consistently greater than that of glutamate in all CNS regions. This demonstrates fundamental differences in release mechanisms that could lead to transmitter-selective pharmacological effects. TTX was generally less potent and consistently less efficacious in inhibiting 4AP-evoked GABA release compared to glutamate release, except in spinal cord. Spinal cord terminals exhibited distinct differences in potencies for TTX inhibition of transmitter release and in Nav subtype expression compared to forebrain-derived nerve terminals. Less total transmitter release was evoked from spinal cord nerve terminals by VTD, which selectively opens TTX-s channels (Farrag et al. 2008), compared to 4AP, consistent with greater involvement of TTX-r subtypes in spinal cord release. This interpretation is supported by the ability of the Nav1.8-selective inhibitor A-803467 to inhibit glutamate release in spinal cord but not in cortex, consistent with a component of Nav1.8-mediated glutamate release in spinal cord. Nav1.8 is highly resistant to TTX (Goldin 2001, Lai et al. 2004), so its expression could contribute to the lower potency of TTX inhibition of release. Nav1.8 immunoreactivity in spinal cord has been detected (Arroyo et al., 2002), but we were unable to detect it by immunoblotting. Together, our data indicate that quantitative and/or qualitative differences in expression of presynaptic Nav subtypes coupled to transmitter release lead to transmitter-specific and regional differences in sensitivities of release to the Nav subtype-selective inhibitors TTX and A-803467 and the agonist VTD.
Residual TTX-insensitive 4AP-evoked GABA release was consistently greater than residual glutamate release across regions except spinal cord. TTX-insensitive 4AP-evoked GABA release from cortical and spinal cord nerve terminals contains a Ca2+-dependent component, while 4AP-evoked glutamate release does not (Westphalen and Hemmings, 2006b). The presynaptic mechanisms contributing to these differences are unknown. Greater 4AP-evoked GABA vs. glutamate release was not evident in all CNS regions when release was evoked by selective activation of TTX-s Na+ channels using VTD (Farrag et al. 2008). This might be due to dissimilar contributions of Ca2+-dependent vs. Ca2+-independent glutamate and GABA release to release evoked by either 4AP or VTD. However, TTX completely inhibited VTD-evoked glutamate and GABA release with similar potencies across brain regions, and had greater efficacy for 4AP-evoked glutamate vs. GABA release inhibition across brain regions. These findings suggest that differences in the relative contributions of TTX-s Nav and of TTX-insensitive mechanisms to neurotransmitter release between GABAergic and glutamatergic terminals could determine their distinct pharmacological sensitivities.
The potency of TTX for inhibition of 4AP-evoked glutamate release varied between brain regions (IC50=5–14 nM) in the range of potencies reported for TTX inhibition of Na+ currents carried by TTX-s Nav subtypes (Goldin 2001, Lai et al. 2004). These Nav α-subunits exhibit distinct regional and developmental expression in mammalian CNS (Goldin 2001, Lai et al. 2004, Vacher et al. 2008): Nav1.1, Nav1.2 and Nav1.6 are all widely expressed, Nav1.3 is selectively expressed early in development, Nav1.7 has more limited expression, while Nav1.8 and Nav1.9 are reported to be exclusively expressed in dorsal root ganglion (DRG) neurons. Consistent with these reports, isolated nerve terminals from rat cortex, hippocampus and striatum expressed only Nav1.1, Nav1.2, Nav1.6, and Nav1.7 to varying degrees. The pharmacological identification of Nav1.8 in spinal cord terminals is consistent with afferent projections from Nav1.8 mRNA-rich DRG neurons to spinal cord (Ohshiro et al. 2007), the role of Nav1.8 in transmitter release from DRG neurons (Medvedeva et al. 2009), and the detection of Nav1.8 expression in rat spinal cord (Arroyo et al. 2002). Expression of Nav1.8 in rat spinal cord was not detectable by immunoblotting possibly due to a low level of expression, despite positive and negative controls confirming antibody specificity (data not shown).
Rat brain Nav α-subunit mRNA expression varies between cell types (Vacher et al. 2008). Although not directly attributable to presynaptic distribution, mRNA expression detected by in situ hybridization indicates that multiple subtypes are expressed in specific CNS regions (Black et al. 1994, Felts et al. 1997). Multiple Nav α-subunit mRNA expression in individual neurons has been shown by in situ hybridization (Black et al. 1994) and single-cell RT-PCR (Mechaly et al. 2005). The specific Nav subtypes expressed in presynaptic terminals and coupled to action potential-evoked neurotransmitter release probably vary between axon terminal types given the considerable differences in regional, neuronal, and subcellular differences in Nav expression (Gong et al. 1999, Caldwell et al. 2000, Boiko et al. 2003, Engel and Jonas 2005). Our observations of differences between CNS regions in Nav subtype expression and in sensitivity of release to Nav-selective agents further supports such cellular and subcellular specialization in Nav expression and function.
The greater potency of TTX to inhibit 4AP-evoked glutamate release from hippocampal and striatal vs. cortical nerve terminals is consistent with the greater expression of the highly TTX-sensitive Nav1.6 subtype (IC50=1 nM; Lai et al. 2004) in hippocampal and striatal terminals. This is supported by evidence for presynaptic expression of Nav1.2 and Nav1.6 obtained by patch-clamp electrophysiological recordings of hippocampal mossy fiber boutons (Engel and Jonas 2005). Lower relative expression of the least TTX-sensitive subtype Nav1.2 (IC50=18 nM; Lai et al. 2004) was found in cortical vs. hippocampal and striatal terminals, suggesting that the balance between Nav subtype expression might determine presynaptic sensitivity to TTX. However, the TTX sensitivity of transmitter release depends not only on the TTX sensitivities of the expressed Nav subtypes, but also on their functional coupling to depolarization and downstream release mechanisms. Moreover, the methods used do not allow resolution of the relative expression of Nav subtypes within a particular nerve terminal type, but only of the average of the population of nerve terminals contained in each preparation.
In conclusion, our findings indicate that nerve terminal-specific differences in the expression of specific Nav subtypes contribute to transmitter-specific and regional differences in the pharmacological sensitivities of transmitter release.
Acknowledgments
This study was supported by National Institutes of Health Grant GM 58055 and the Department of Anesthesiology, Weill Cornell Medical College. We gratefully acknowledge the expert statistical advice of Dr. James Root, Departments of Psychiatry and Anesthesiology, Weill Cornell Medical College.
Abbreviations
- 4AP
4-aminopyridine
- ΔFR
sum fractional release
- FR
fractional release
- Nav
voltage-gated sodium channel
- VTD
veratridine
- TTX
tetrodotoxin
- TTX-s
tetrodotoxin-sensitive
- TTX-r
tetrodotoxin-resistant
References
- Arroyo EJ, Xu T, Grinspan J, Lambert S, Levinson SR, Brophy PJ, Peles E, Scherer SS. Genetic dysmyelination alters the molecular architecture of the nodal region. J Neurosci. 2002;22:1726–1737. doi: 10.1523/JNEUROSCI.22-05-01726.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black JA, Yokoyama S, Higashida H, Ransom BR, Waxman SG. Sodium channel mRNAs I, II and III in the CNS: cell-specific expression. Brain Res Mol Brain Res. 1994;22:275–289. doi: 10.1016/0169-328x(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Boiko T, Van Wart A, Caldwell JH, Levinson SR, Trimmer JS, Matthews G. Functional specialization of the axon initial segment by isoform-specific sodium channel targeting. J Neurosci. 2003;23:2306–2013. doi: 10.1523/JNEUROSCI.23-06-02306.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman D, Alexander S, Lodge D. Pharmacological characterisation of the calcium channels coupled to the plateau phase of KCl-induced intracellular free Ca2+ elevation in chicken and rat synaptosomes. Neuropharmacology. 1993;32:1195–1202. doi: 10.1016/0028-3908(93)90013-s. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Caldwell JH, Schaller KL, Lasher RS, Peles E, Levinson SR. Sodium channel Nav1.6 is localized at nodes of Ranvier, dendrites, and synapses. Proc Natl Acad Sci USA. 2000;97:5616–5620. doi: 10.1073/pnas.090034797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos FV, Moreira TH, Beirão PS, Cruz JS. Veratridine modifies the TTX-resistant Na+ channels in rat vagal afferent neurons. Toxicon. 2004;43:401–406. doi: 10.1016/j.toxicon.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Dib-Hajj S, Priestly T. Voltage-Gated Sodium Channels. In: Davies C, Kew JN, editors. Structure and Function of Ion Channels. Oxford University Press; London: 2009. pp. 131–171. [Google Scholar]
- Dodd PR, Hardy JA, Oakley AE, Edwardson JA, Perry EK, Delaunoy J-P. A rapid method for preparing synaptosomes: comparison, with alternative procedures. Brain Res. 1981;226:107–118. doi: 10.1016/0006-8993(81)91086-6. [DOI] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Farrag KJ, Bhattacharjee A, Docherty RJ. A comparison of the effects of veratridine on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in isolated rat dorsal root ganglion neurons. Pflügers Arch. 2008;455:929–938. doi: 10.1007/s00424-007-0365-5. [DOI] [PubMed] [Google Scholar]
- Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel alpha-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patterns in developing rat nervous system. Brain Res Mol Brain Res. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Resurgence of sodium channel research. Ann Rev Physiol. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na(+) channel alpha-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. J Comp Neurol. 1999;412:342–352. [PubMed] [Google Scholar]
- Hemmings HC, Jr, Girault JA, Nairn AC, Bertuzzi G, Greengard P. Distribution of protein phosphatase inhibitor-1 in brain and peripheral tissues of various species: comparison with DARPP-32. J Neurochem. 1992;59:1053–1061. doi: 10.1111/j.1471-4159.1992.tb08347.x. [DOI] [PubMed] [Google Scholar]
- Hemmings HC., Jr General anesthetic effects on protein kinase C. Toxicol Lett. 1998;100–101:89–95. doi: 10.1016/s0378-4274(98)00170-2. [DOI] [PubMed] [Google Scholar]
- Jarvis MF, Honore P, Shieh CC, et al. A-803467, a potent and selective Nav1.8 sodium channel blocker, attenuates neuropathic and inflammatory pain in the rat. Proc Natl Acad Sci USA. 2007;104:8520–8525. doi: 10.1073/pnas.0611364104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J, Porreca F, Hunter JC, Gold MS. Voltage-gated sodium channels and hyperalgesia. Annu Rev Pharmacol Toxicol. 2004;44:371–397. doi: 10.1146/annurev.pharmtox.44.101802.121627. [DOI] [PubMed] [Google Scholar]
- Long P, Mercer A, Begum R, Stephens GJ, Sihra TS, Jovanovic JN. Nerve terminal GABAA receptors activate Ca2+/calmodulin-dependent signaling to inhibit voltage-gated Ca2+ influx and glutamate release. J Biol Chem. 2009;284:8726–37. doi: 10.1074/jbc.M805322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Mechaly I, Scamps F, Chabbert C, Sans A, Valmier J. Molecular diversity of voltage-gated sodium channel alpha subunits expressed in neuronal and non-neuronal excitable cells. Neuroscience. 2005;130:389–396. doi: 10.1016/j.neuroscience.2004.09.034. [DOI] [PubMed] [Google Scholar]
- Medvedeva YV, Kim M-S, Schnizler K, Usachev YM. Functional tetrodotoxin-resistent Na+ channels are expressed presynaptically in rat dorsal root ganglia neurons. Neuroscience. 2009;159:559–569. doi: 10.1016/j.neuroscience.2008.12.029. [DOI] [PubMed] [Google Scholar]
- Meir A, Ginsburg S, Butkevich A, Kachalsky SG, Kaiserman I, Ahdut R, Demirgoren S, Rahamimoff R. Ion channels in presynaptic nerve terminals and control of transmitter release. Physiol Rev. 1999;79:1019–1088. doi: 10.1152/physrev.1999.79.3.1019. [DOI] [PubMed] [Google Scholar]
- Messner DJ, Catterall WA. The sodium channel from rat brain. Separation and characterization of subunits. J Biol Chem. 1985;260:10597–10604. [PubMed] [Google Scholar]
- Nicholls DG. The glutamatergic nerve terminal. Eur J Biochem. 1993;212:613–631. doi: 10.1111/j.1432-1033.1993.tb17700.x. [DOI] [PubMed] [Google Scholar]
- Ohshiro H, Ogawa S, Shinjo K. Visualizing sensory transmission between dorsal root ganglion and dorsal horn neurons in co-culture with calcium imaging. J Neurosci Methods. 2007;165:49–54. doi: 10.1016/j.jneumeth.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Mennerick S. Selective depression of low-release probability excitatory synapses by sodium channel blockers. Neuron. 2000;26:671–682. doi: 10.1016/s0896-6273(00)81203-9. [DOI] [PubMed] [Google Scholar]
- Raiteri M. Presynaptic metabotropic glutamate and GABAB receptors. Handb Exp Pharmacol. 2008;184:373–407. doi: 10.1007/978-3-540-74805-2_12. [DOI] [PubMed] [Google Scholar]
- Reid CA, Clements JD, Bekkers JM. Nonuniform distribution of Ca2+ channel subtypes on presynaptic terminals of excitatory synapses in hippocampal cultures. J Neurosci. 1997;17:2738–2745. doi: 10.1523/JNEUROSCI.17-08-02738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitges M, Guarneros A, Nekrassov V. Effects of carbamazepine, phenytoin, valproic acid, oxcarbazepine, lamotrigine, topiramate and vinpocetine on the presynaptic Ca2+ channel-mediated release of [3H]glutamate: comparison with the Na+ channel-mediated release. Neuropharmacology. 2007;53:854–862. doi: 10.1016/j.neuropharm.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Strichartz GR. Novel ideas of local anaesthetic actions on various ion channels to ameliorate postoperative pain. Br J Anaesth. 2008;101:45–47. doi: 10.1093/bja/aen101. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature. 1994;367:69–72. doi: 10.1038/367069a0. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tibbs GR, Barrie AP, Van Mieghem FJE, McMahon HT, Nicholls DG. Repetitive action potentials in isolated nerve terminals in the presence of 4-aminopyridine: Effects on cytosolic free Ca2+ and glutamate release. J Neurochem. 1989;53:1693–1699. doi: 10.1111/j.1471-4159.1989.tb09232.x. [DOI] [PubMed] [Google Scholar]
- Toledo-Aral JJ, Moss BL, He ZJ, et al. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proc Natl Acad Sci USA. 1997;94:1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulbricht W. Effects of veratridine on sodium currents and fluxes. Rev Physiol Biochem Pharmacol. 1998;133:1–54. doi: 10.1007/BFb0000612. [DOI] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Gomez RS, Hemmings HJ. Nicotinic receptor-evoked hippocampal norepinephrine release is highly sensitive to inhibition by isoflurane. Br J Anaesth. 2009;102:355–360. doi: 10.1093/bja/aen387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Selective depression by general anesthetics of glutamate versus GABA release from isolated cortical nerve terminals. J Pharmacol Exp Ther. 2003;304:1188–1196. doi: 10.1124/jpet.102.044685. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: basal release. J Pharmacol Exp Ther. 2006a;316:208–215. doi: 10.1124/jpet.105.090647. [DOI] [PubMed] [Google Scholar]
- Westphalen RI, Hemmings HC., Jr Volatile anesthetic effects on glutamate versus GABA release from isolated rat cortical nerve terminals: 4-aminopyridine-evoked release. J Pharmacol Exp Ther. 2006b;316:216–223. doi: 10.1124/jpet.105.090662. [DOI] [PubMed] [Google Scholar]
- Wu L-G, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]
- Wynne PM, Puig SI, Martin GE, Treistman SN. Compartmentalized beta subunit distribution determines characteristics and ethanol sensitivity of somatic, dendritic, and terminal large-conductance calcium-activated potassium channels in the rat central nervous system. J Pharmacol Exp Ther. 2009;329:978–986. doi: 10.1124/jpet.108.146175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biol. 2003;4:207. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]