Abstract
Background
Pneumonia is common among patients with advanced dementia, especially toward the end of life. Whether antimicrobial treatment improves survival or comfort is not well understood. The objective of this study was to examine the effect of antimicrobial treatment for suspected pneumonia on survival and comfort in patients with advanced dementia.
Methods
From 2003 to 2009, data were prospectively collected from 323 nursing home residents with advanced dementia in 22 facilities in the area of Boston, Massachusetts. Each resident was followed up for as long as 18 months or until death. All suspected pneumonia episodes were ascertained, and antimicrobial treatment for each episode was categorized as none, oral only, intramuscular only, or intravenous (or hospitalization). Multivariable methods were used to adjust for differences among episodes in each treatment group. The main outcome measures were survival and comfort (scored according to the Symptom Management at End-of-Life in Dementia scale) after suspected pneumonia episodes.
Results
Residents experienced 225 suspected pneumonia episodes, which were treated with antimicrobial agents as follows: none, 8.9%; oral only, 55.1%, intramuscular, 15.6%, and intravenous (or hospitalization), 20.4%. After multivariable adjustment, all antimicrobial treatments improved survival after pneumonia compared with no treatment: oral (adjusted hazard ratio [AHR], 0.20; 95% confidence interval [CI], 0.10–0.37), intramuscular (AHR, 0.26; 95% CI, 0.12–0.57), and intravenous (or hospitalization) (AHR, 0.20; 95% CI, 0.09–0.42). After multivariable adjustment, residents receiving any form of antimicrobial treatment for pneumonia had lower scores on the Symptom Management at End-of-Life in Dementia scale (worse comfort) compared with untreated residents.
Conclusion
Antimicrobial treatment of suspected pneumonia episodes is associated with prolonged survival but not with improved comfort in nursing home residents with advanced dementia.
An estimated 5 million Americans have dementia, a number that is expected to increase to 13 million by 2030.1 Nursing homes (NHs) play an important role in their end-of-life care, as 70% will die in this setting.2 Infections, particularly pneumonia, are common in individuals with advanced dementia.3–7 Nursing home residents with end-stage dementia who develop pneumonia are often treated with antimicrobial agents, and many are hospitalized.3,6,8–11 However, the benefits of antimicrobial treatment are not well established in this population, and there is substantial variation in practice.10,11 Current literature provides limited and contrasting information on the ability of antimicrobial agents to affect 2 important treatment goals in advanced dementia: survival12,13 and comfort.14,15 Furthermore, prior work is limited by a lack of randomized designs, and some observational studies have not adjusted for factors that are associated with the likelihood of receiving treatment.12–15
Approximately 1 in 4 decisions facing families of NH patients with advanced dementia concern the treatment of infections.16 Ideally, these decisions are guided by the goals of care (eg, life prolongation, comfort). However, limited data regarding the outcomes of antimicrobial use hinder informed decision making. Although a randomized trial is the best method to assess the effectiveness of treatment options, conducting such a trial for pneumonia in advanced dementia would be ethically challenging and thus not likely to be undertaken. Therefore, the objective of this study, which was approved by the institutional review board of Hebrew SeniorLife Institute for Aging Research, Boston, Massachusetts, was to analyze data from a large, multisite, prospective cohort study of NH residents with advanced dementia to examine the effect of antimicrobial treatment on their survival and comfort.
METHODS
STUDY POPULATION
Participants were from the Choices, Attitudes, and Strategies for Care of Advanced Dementia at the End-of-Life (CASCADE) study; a prospective cohort study of NH residents with advanced dementia and their health care proxies (HCPs). Details of the CASCADE study methodology and cohort are described elsewhere.7,17 Residents were recruited from 22 NHs within 60 miles of Boston with at least 60 beds. Participant eligibility criteria were (1) age older than 60 years; (2) dementia (any type); (3) a Cognitive Performance Score18 of 5 or 6 (indicating severe or very severe cognitive impairment); and (4) a Global Deterioration Scale19 score of 7 (cannot recognize family, minimal verbal communication, total functional dependence, incontinence of urine and stool, and inability to ambulate independently). Residents had to have a designated HCP who could provide informed consent and communicate in English.
DATA COLLECTION
Resident data were obtained from baseline and quarterly medical record reviews, nurse interviews, and brief clinical examinations. The HCP data were obtained from baseline and quarterly telephone interviews. Residents and HCPs were followed up for 18 months or until death of the resident. If the resident died, a death assessment was conducted within 14 days.
SUSPECTED PNEUMONIA EPISODES
At each follow-up assessment, the resident’s chart was reviewed for the occurrence of all suspected pneumonia episodes, which were defined as documentation by a primary care provider (physician, nurse practitioner, or physician assistant) that the resident was suspected of having pneumonia. For each episode, the date of onset and the presence of unstable vital signs (respiratory rate, >30/min; temperature, >33°C; heart rate, >125/min; or systolic blood pressure, <90 mm Hg) were recorded. Additional documentation included whether aspiration was suspected and whether a chest radiograph was obtained and, if obtained, whether the radiograph confirmed a diagnosis of pneumonia.
TREATMENT OF PNEUMONIA
Treatment for each suspected pneumonia episode was categorized into a 4-part ordinal variable denoting increasing aggressiveness of care as follows: none, oral antimicrobial treatment, intramuscular antimicrobial treatment, and intravenous antimicrobial treatment or hospitalization. Intravenous antimicrobial treatment and hospitalization were grouped into a single category because these events often occur together. Episodes for which residents received antimicrobial agents by multiple routes were categorized under the more aggressive treatment.
OUTCOMES AFTER PNEUMONIA
Survival after each suspected pneumonia episode was defined as the number of days from episode onset until death (for residents who died during the study) or until the end of the follow-up period (for those who survived). Resident comfort was measured at baseline and quarterly using the Symptom Management at End-Of-Life in Dementia (SM-EOLD) scale, which was completed by a nurse caring for the resident.20 The scale has high internal consistency and reliability (α=.68–α=.78)20,21 and quantifies the frequency of the following symptoms during the previous 90 days: pain, dyspnea, depression, fear, anxiety, agitation, calm, skin breakdown, and resistance to care. Frequency is quantified as follows: never, once a month, 2 or 3 days per month, once a week, several days per week, or daily. Summary scores range from 0 to 45; higher scores indicate greater comfort. The SM-EOLD scores obtained at the same assessment as the suspected pneumonia episode were used as the outcome measure, and scores from the prior assessment were used as covariates to adjust for pre-episode comfort.
The SM-EOLD score was collected only from residents who were alive at the time of the assessment. Therefore, analyses examining this outcome excluded residents who died within 90 days of the pneumonia episode. For residents who died, a related scale, the Comfort Assessment in Dying with Dementia (CAD-EOLD) scale,20 was completed by the resident’s nurse within 2 weeks of death. This scale, which quantifies the frequency of symptoms during the last week of life, includes discomfort, pain, restlessness, shortness of breath, choking, gurgling, difficulty swallowing, fear, anxiety, crying, moaning, serenity, peace, and calm. Internal consistency and reliability is high (α=.85).20 Scores range from 14 to 42, with higher scores indicating greater comfort.
OTHER VARIABLES
Baseline resident characteristics included sociodemographics (age, race [white vs other], sex, and language [English vs other]); living on a special care dementia unit; HCP relationship to the resident; and comorbid illnesses (coronary artery disease, chronic obstructive pulmonary disease, active cancer of any type, or congestive heart failure). Other variables, which were obtained from the same assessment as the pneumonia episode, included cognitive status as measured by the Test for Severe Impairment22 (range, 0–24; lower scores indicate greater impairment, dichotomized as >0 vs 0); functional status as measured by the Bedford Alzheimer Nursing Severity Subscale23 (range, 7–28; higher scores indicate greater disability); do-not-hospitalize orders; hospice referral; the number of venipunctures over the past 90 days; the presence of a feeding tube; and the occurrence of an acute illness in the prior 90 days (eg, hip fracture, myocardial infarction, stroke, gastrointestinal bleed).
ANALYSIS
Means for continuous variables and proportions for categorical variables were calculated for resident characteristics and features of each suspected pneumonia episode. For SM-EOLD and CAD-EOLD scores, mean values were compared across the treatment groups using a nonparametric test for trend. Cox proportional hazards models were used to examine the association between the 4-level treatment variable and survival after each suspected pneumonia episode. The “no treatment” category was the referent group. Analyses were conducted at the level of the episode. Robust standard error estimates24,25 were used to adjust for clustering at the individual level because some residents had more than 1 pneumonia episode. Fixed effects were used to account for clustering at the facility level. Stable covariates were brought forward from the baseline assessment (eg, age), and dynamic variables (eg, Bedford Alzheimer Nursing Severity Subscale) were derived from the same assessment as the pneumonia episode. Bivariable associations between each independent variable with survival were determined using unadjusted Cox proportional hazards models, and variables associated with survival at the level of P<.10 were included in the multivariable model. The mutivariable model was also adjusted for age, sex, race, and the presence of unstable vital signs. After verification that the proportional hazards assumption had been met based on a test of the Schoenfeld residuals26 (P=.97), hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated.
Linear regression was used to examine the association between the 4-level treatment variable and comfort (SM-EOLD scale) after pneumonia episodes using a similar approach as described above for the survival analyses. The number of days between the suspected pneumonia episode and the SM-EOLD assessment was calculated and included as a covariate in the model. Analyses using the SM-EOLD scale as the outcome excluded episodes that occurred during the same assessment as resident death, because the SM-EOLD scale was not administered during these assessments. Therefore, among the subset of residents who died within 90 days of the suspected pneumonia episode, separate analyses, again using a similar approach to that described above, were conducted using the CAD-EOLD scale as the outcome measure. All statistical analyses were performed using Stata SE version 10.0 (StataCorp, College Station, Texas).
RESULTS
RESIDENT CHARACTERISTICS
Of the 323 residents recruited into the CASCADE study, 133 (41%) had at least 1 suspected pneumonia episode. These 133 residents experienced a total of 225 suspected pneumonia episodes over the follow-up period (ie, 92 residents experienced more than 1 episode). Table 1 presents the resident characteristics at the level of the pneumonia episode, given that this is the unit of analyses, but they are similar to those of the baseline CASCADE cohort.7 Of the residents with pneumonia episodes (mean age, 86 years), 81% were female, 92% were white, and 44% lived in a special care dementia unit.
Table 1.
Characteristics of Nursing Home Residents With Advanced Dementia Who Had Suspected Pneumonia Episodesa
| Characteristic | Pneumonia Episodes (n=225) |
|---|---|
| Age, mean (SD), y | 86.0 (7.0) |
| Female sex | 183 (81.3) |
| Race, white vs other | 208 (92.4) |
| BANS-S, mean (SD)b | 21.9 (2.3) |
| Test for Severe Impairment22 >0 | 25 (11.1) |
| Lives on special care dementia unit | 99 (44.0) |
| Comorbid illness | |
| Coronary artery disease | 34 (15.1) |
| Cancer, active | 1 (0.4) |
| Congestive heart failure | 69 (30.7) |
| Chronic obstructive pulmonary disease | 38 (16.9) |
| Other acute illness in past 90 d | 8 (3.6) |
| Presence of feeding tube | 35 (15.6) |
| Do-not-hospitalize order | 114 (50.7) |
Values are expressed as number (percentage) unless otherwise indicated.
Bedford Alzheimer Nursing Severity Subscale (range, 7–28; higher scores indicate greater disability).23
TREATMENT AND CHARACTERISTICS OF PNEUMONIA EPISODES
Among the 225 suspected pneumonia episodes, only 9% were not treated with antimicrobial agents (Table 2). The remaining episodes were treated with antimicrobial agents as follows: oral, 55%; intramuscular, 16%; and intravenous or hospitalization, 20%. Unstable vital signs were present in 38% of episodes. Aspiration was suspected in 56% of the suspected pneumonia episodes, and chest radiographs were obtained in 77% of cases. Among those residents who had a chest radiograph, pneumonia was radiographically confirmed in 84% of cases. For each treatment group, estimates of 90-day mortality after suspected pneumonia episodes, mean SM-EOLD scores, and percentage with suspected aspiration, unstable vital signs, and chest radiograph are presented in Table 2.
Table 2.
Characteristics of 225 Suspected Pneumonia Episodes Among Nursing Home Residents With Advanced Dementia
| Pneumonia Treatment | Pneumonia Episodes, No. (%) | % Alive 90 Days After Pneumonia Episode | SM-EOLD Score, Mean (SD) | No. (%) |
||
|---|---|---|---|---|---|---|
| Suspected Aspiration | Unstable Vital Signsa | Chest Radiograph Obtained | ||||
| No antimicrobial agent | 20/225 (8.9) | 32.8 | 39.4 (4.4) | 12 (60.0) | 8 (40.0) | 6 (30.0) |
| Oral antimicrobial agent | 124/225 (55.1) | 64.5 | 34.0 (8.1) | 61 (49.2) | 33 (26.6) | 105 (84.7) |
| Intramuscular antimicrobial agent | 35/225 (15.6) | 56.7 | 33.7 (7.2) | 18 (51.4) | 18 (51.4) | 26 (74.3) |
| Intravenous antimicrobial agent or hospitalization | 46/225 (20.4) | 60.6 | 30.5 (9.3) | 35 (76.1) | 26 (56.5) | 36 (78.3) |
Abbreviation: SM-EOLD, Symptom Management at End-of-Life in Dementia.
Unstable vital signs: respiratory rate greater than 30/min; temperature higher than 33°C; heart rate greater than 125/min; or systolic blood pressure lower than 90 mm Hg.
SURVIVAL AFTER PNEUMONIA EPISODES
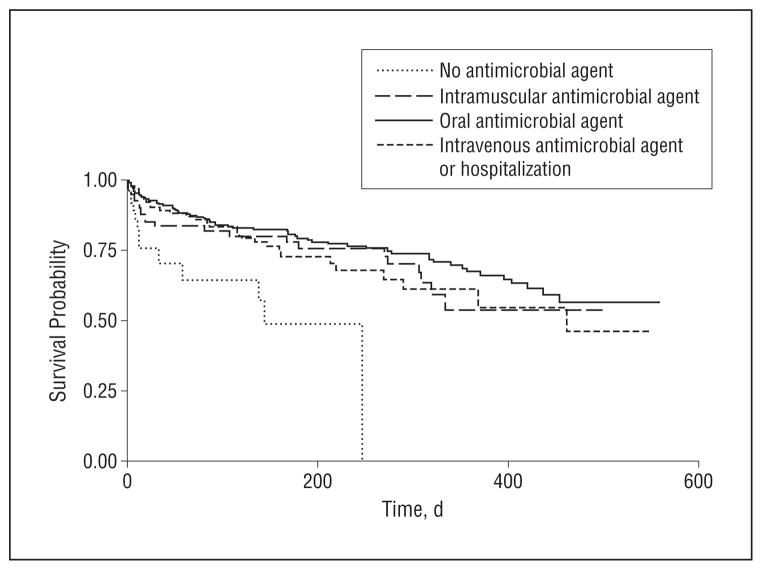
Antimicrobial treatment by any route was associated with lower mortality after suspected pneumonia episodes when compared with no treatment (Table 3 and Figure). The adjusted HRs (AHRs) and 95% CIs for each treatment route compared with no treatment were as follows: oral (AHR, 0.20; 95% CI, 0.10–0.37), intramuscular (AHR, 0.26; 95% CI, 0.12–0.57), and intravenous or hospitalization (AHR, 0.20; 95% CI, 0.09–0.42). There was no statistical difference in survival among the 3 antimicrobial treatment routes (P=.58). The average adjusted increase in survival time for suspected pneumonia episodes that were treated with antimicrobial agents was 273 days compared with untreated episodes (data not shown). Among other covariates, the presence of a do-not-hospitalize order was associated with greater mortality after pneumonia (AHR, 2.21; 95% CI, 1.34–3.66).
Table 3.
Factors Associated With Increased Mortality After Suspected Pneumonia
| Characteristic | Suspected Pneumonia Episodes (n=225) |
|
|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI)a | |
| Treatment | ||
| None | 1 [Reference] | 1 [Reference] |
| Oral antimicrobial agent | 0.30 (0.17–0.53) | 0.20 (0.10–0.37) |
| Intramuscular antimicrobial agent | 0.45 (0.24–0.85) | 0.26 (0.12–0.57) |
| Intravenous antimicrobial agent or hospitalization | 0.39 (0.21–0.73) | 0.20 (0.09–0.42) |
| Age, y | 1.04 (1.01–1.07) | 1.03 (0.99–1.08) |
| Female sex | 1.04 (0.67–1.63) | 0.85 (0.42–1.70) |
| Race, white vs other | 1.14 (0.60–2.17) | 1.94 (0.62–6.08) |
| BANS-Sb | 1.09 (1.01–1.17) | 1.04 (0.91–1.18) |
| Test for Severe Impairment22 >0 | 1.27 (0.76–2.11) | NA |
| Comorbid illness | ||
| Coronary artery disease | 1.10 (0.72–1.68) | NA |
| Active cancer | 1.39 (0.19–9.93) | NA |
| Congestive heart failure | 1.71 (1.22–2.39) | 1.53 (0.92–2.57) |
| Chronic obstructive pulmonary disease | 1.22 (0.82–1.83) | NA |
| Presence of feeding tube | 1.15 (0.75–1.76) | NA |
| Any unstable vital signc | 1.23 (0.88–1.71) | 1.17 (0.77–1.78) |
| Acute illness in prior 90 dd | 0.67 (0.25–1.81) | NA |
| Hospice referral | 2.38 (1.49–3.80) | 1.90 (0.83–4.38) |
| Suspected aspiration | 1.53 (1.09–2.14) | 1.39 (0.84–2.28) |
| Chest radiograph obtained | 0.62 (0.43–0.89) | 1.11 (0.69–1.79) |
| Do-not-hospitalize order | 2.06 (1.47–2.88) | 2.21 (1.34–3.66) |
Abbreviations: CI, confidence interval; HR, hazard ratio; NA, not applicable.
Models also adjusted for clustering at facility and individual levels.
Bedford Alzheimer Nursing Severity Subscale (range, 7–28; higher scores indicate greater disability)23.
Unstable vital signs: respiratory rate greater than 30/min; temperature higher than 33°C; heart rate greater than 125/min; or systolic blood pressure lower than 90 mm Hg.
Acute illness (eg, hip fracture, myocardial infarction, stroke, gastrointestinal bleed).
Figure.
Survival after suspected pneumonia episode, by treatment: no antimicrobial agents, oral antimicrobial agents, intramuscular antimicrobial agents, and intravenous antimicrobial agents or hospitalization. Adjusted for age, sex, race, functional status (Bedford Alzheimer Nursing Severity Subscale),23 suspected aspiration, congestive heart failure, hospice referral, chest radiograph obtained, do-not-hospitalize order, and unstable vital signs.
COMFORT AFTER PNEUMONIA EPISODES
The SM-EOLD scale was used to evaluate comfort among residents who did not die within 90 days of the suspected pneumonia episode (n=159). The CAD-EOLD scale was used as the outcome measure for episodes that occurred in the 90-day interval before death.
The unadjusted mean SM-EOLD scores were highest (greater comfort) among episodes that were not treated with antimicrobial agents and were progressively lower (worse comfort) for increasing aggressiveness of care. The mean SM-EOLD scores for each antimicrobial treatment group, as shown in Table 2, were as follows: no treatment, 39.4; oral, 34.0; intramuscular, 33.7; and intravenous or hospitalization, 30.5 (P for trend, .008). Compared with no treatment, the adjusted linear regression coefficients for antimicrobial treatment were as follows (Table 4): oral (−7.63; P<.001), intramuscular (−7.39; P=.001), and intravenous or hospitalization (−12.26; P<.001). Greater functional impairment was also associated with decreased comfort for pneumonia episodes (regression coefficient, −0.87; P=.04). There was no association between treatment group and comfort in either unadjusted or adjusted analyses as measured by the CAD-EOLD scale.
Table 4.
Characteristics of Suspected Pneumonia Episodes Among Nursing Home Residents With Advanced Dementia and Their Association With Greater Comforta
| Characteristic | Suspected Pneumonia Episodes (n=159) |
||||
|---|---|---|---|---|---|
| No. (%) | Unadjusted Linear Regression |
Adjusted Linear Regressionb |
|||
| Coefficient | P Value | Coefficient (95% CI) | P Value | ||
| Treatment | |||||
| None | 9 (6) | 1 [Reference] | NA | 1 [Reference] | NA |
| Oral antimicrobial agent | 94 (59) | −5.49 (−11.09 to 0.12) | .06 | −7.63 (−11.30 to −3.95) | <.001 |
| Intramuscular antimicrobial agent | 22 (14) | −5.76 (−12.12 to 0.59) | .08 | −7.39 (−11.86 to −2.92) | .001 |
| Intravenous antimicrobial agent or hospitalization | 34 (21) | −8.97 (−14.99 to −2.96) | .004 | −12.26 (−17.10 to −7.43) | <.001 |
| Age, mean (SD), y | 85.2 (7) | −0.22 (−0.41 to −0.04) | .02 | −0.17 (−0.34 to 0.01) | .06 |
| Female sex | 131 (82) | 2.45 (−0.95 to 5.85) | .16 | 1.31 (−1.65 to 4.27) | .38 |
| Race, white vs other | 146 (92) | −4.25 (−8.96 to 0.47) | .08 | −1.43 (−6.19 to 3.33) | .55 |
| BANS-S, mean (SD)c | 21.9 (2) | −0.99 (−1.56 to −0.42) | .001 | −0.87 (−1.70 to −0.04) | .04 |
| Special care unit | 67 (42) | −2.26 (−4.87 to 0.36) | .09 | 1.86 (−1.53 to 5.25) | .28 |
| Chronic obstructive pulmonary disease | 27 (17) | −3.30 (−6.74 to 0.13) | .06 | −0.28 (−3.98 to 3.41) | .88 |
| Hospice referral | 12 (8) | −0.52 (−5.46 to 4.41) | .83 | NA | NA |
| Suspected aspiration | 80 (50) | −0.77 (−3.38 to 1.83) | .56 | NA | NA |
| Presence of feeding tube | 26 (16) | −3.94 (−7.41 to −0.46) | .03 | 0.99 (−3.22 to 5.20) | .64 |
| Venipunctures in last 90 d, mean (SD) | 4.4 (5) | 0.45 (−0.73 to −0.17) | .002 | −0.13 (−0.44 to 0.19) | .44 |
| Any unstable vital signd | 56 (35) | −0.80 (−3.53 to 1.93) | .56 | 1.15 (−1.61 to 3.90) | .41 |
| Acute illness in prior 90 de | 5 (3) | −1.74 (−9.21 to 5.73) | .65 | NA | NA |
| Do-not-hospitalize order | 63 (40) | 0.59 (−2.07 to 3.26) | .66 | NA | NA |
| Days between pneumonia episode and assessment of comfort, mean (SD) | 54.0 (27) | −0.04 (−0.09 to 0.01) | .12 | NA | NA |
Abbreviations: CI, confidence interval; NA, not applicable.
Outcome is the Symptom Management at End-Of-Life in Dementia (SM-EOLD) scale; higher scores indicate greater comfort.
Models also adjusted for prior SM-EOLD score and for clustering at facility and individual levels.
Bedford Alzheimer Nursing Severity Subscale (range, 7–28; higher scores indicate greater disability).23.
Unstable vital signs: respiratory rate greater than 30/min; temperature higher than 33°C; heart rate greater than 125/min; or systolic blood pressure lower than 90 mm Hg.
Acute illness (eg, hip fracture, myocardial infarction, stroke, gastrointestinal bleed).
COMMENT
To our knowledge, this prospective cohort study respresents the most rigorous effort to date to examine treatment outcomes of suspected pneumonia in advanced dementia. We found that antimicrobial agents are commonly prescribed (91%) for pneumonia episodes in this cohort. Survival was prolonged among residents who received antimicrobial treatment compared with those who were untreated. At the same time, our findings suggest that treatment with antimicrobial agents does not improve the comfort of residents with advanced dementia who have pneumonia, and more aggressive care may be associated with greater discomfort.
Short of a randomized trial, this study offers several advances over prior work that has examined the outcomes of antimicrobial treatment for suspected pneumonia in end-stage dementia.12–15 First, it is the largest prospective cohort study of NH residents rigorously defined as having advanced dementia conducted to date. Second, it extends earlier research by adjusting for clinical characteristics that are associated with the likelihood of receiving treatment. Finally, it examined residents who received no antimicrobial treatment and compared them with residents who received treatment by different routes. With these approaches, we report, for the first time, a strong association between antimicrobial treatment by any route and improved survival after pneumonia in persons with advanced dementia.
Comfort is often a main goal of care in advanced dementia,27 and it is an important concern when treatment options are being considered. Interventions such as parenteral therapy and hospital transfers can be burdensome for the frail elderly.28 Pneumonia is associated with discomfort in end-stage dementia,15 and some prior studies suggest that antimicrobial use may reduce that discomfort.14,15 Among residents who did not die in the 90 days after a suspected pneumonia episode, we found lower comfort levels in those who received antimicrobial treament compared with no treatment, as well as an association between greater discomfort and more aggressive routes of treatment. Although we cannot distinguish whether the residents’ discomfort was attributable to their pneumonia or to the treatment that they received, our analyses more fully adjusted for markers of episode severity as well as for other conditions and interventions that may cause discomfort compared with prior studies.14,15
High-quality medical decision making requires weighing the risks and benefits of treatment options against the primary goal of care. Taken together, our results suggest that when the most important goal for a resident with advanced dementia is to prolong survival, even if treatment may cause discomfort, then antimicrobial treatment may extend life by as much as 9 months after suspected pneumonia. However, for these residents, providers and family should consider limiting treatment to oral antimicrobial agents (or intramuscular if oral administration is not possible), which appear to achieve the same survival benefit, but with potentially less individual burden and health care costs, compared with more aggressive management approaches (eg, intravenous antimicrobial treatment or hospitalization). On the other hand, our results suggest that for residents with advanced dementia and suspected pneumonia for whom the primary goal of care is comfort, or for whom it is thought that an additional few months of life with advanced dementia will not outweigh the potential burdens of antimicrobial treatment, these agents should be withheld and palliation provided.
This study should be considered in light of certain limitations. First, it is an observational study and not a randomized trial. Therefore, although we used multivariable techniques, it is possible that unmeasured sources of bias or confounding limit the validity of our findings. However, we evaluated potential confounders, including markers of disease severity, resident functional status, measures of comfort care, and advance care planning. Second, suspected pneumonia episodes were identified using NH records. Although strict confirmation of infections was not obtained, our approach represents the real-world practices of treating frail NH residents for whom treatment decisions for infections are often made empirically, without extensive testing to firmly establish diagnostic criteria. However, we note that pneumonia was confirmed in 84% of cases in which a chest radiograph was obtained. While the absolute number of residents who did not receive antimicrobial treatment was relatively small, detectable differences were still found in both the survival and the comfort outcomes among treatment groups. Furthermore, this study did not analyze outcomes related to the use of specific antimicrobial agents; it analyzed only their route of administration. Lastly, the CASCADE study was limited to the Boston area and mostly white residents, and the generalizability of our findings to other geographic areas or racial groups is uncertain. However, the facility and resident characteristics are comparable to similar cohorts nationwide.17
This study’s findings have important implications for practice. The management of infections, especially pneumonia, is one of the common decisions confronting the families and practitioners who are providing care to the growing number of Americans with advanced dementia residing in NHs.16 For these patients, our results indicate that antimicrobial treatment for suspected pneumonia may be a double-edged sword, as it was associated with both survival and discomfort. These observations may help families and providers of residents with advanced dementia align the potential advantages and disadvantages of antimicrobial treatment with their goals of care.
Acknowledgments
Funding/Support: This study was supported in part by grants R01 AG024091 and K24AG033640 (Dr Mitchell) from the National Institute on Aging and the National Institutes of Health and by a Hartford Geriatrics Health Outcomes Research Scholars Award (Dr Givens).
Role of the Sponsors: The funding sources had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; or in the preparation of the manuscript.
Footnotes
Financial Disclosure: None reported.
Author Contributions: Dr Givens had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Givens and Mitchell. Acquisition of data: Mitchell. Analysis and interpretation of data: Givens, Jones, Shaffer, Kiely, and Mitchell. Drafting of the manuscript: Givens. Critical revision of the manuscript for important intellectual content: Jones, Shaffer, Kiely, and Mitchell. Statistical analysis: Givens, Jones, and Shaffer. Obtained funding: Mitchell. Administrative, technical, and material support: Kiely and Mitchell. Study supervision: Mitchell.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60(8):1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell SL, Teno JM, Miller SC, Mor V. A national study of the location of death for older persons with dementia. J Am Geriatr Soc. 2005;53(2):299–305. doi: 10.1111/j.1532-5415.2005.53118.x. [DOI] [PubMed] [Google Scholar]
- 3.Chen JH, Lamberg JL, Chen YC, et al. Occurrence and treatment of suspected pneumonia in long-term care residents dying with advanced dementia. J Am Geriatr Soc. 2006;54(2):290–295. doi: 10.1111/j.1532-5415.2005.00524.x. [DOI] [PubMed] [Google Scholar]
- 4.Beard CM, Kokmen E, Sigler C, Smith GE, Petterson T, O’Brien PC. Cause of death in Alzheimer’s disease. Ann Epidemiol. 1996;6(3):195–200. doi: 10.1016/1047-2797(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 5.Thomas BM, Starr JM, Whalley LJ. Death certification in treated cases of pre-senile Alzheimer’s disease and vascular dementia in Scotland. Age Ageing. 1997;26(5):401–406. doi: 10.1093/ageing/26.5.401. [DOI] [PubMed] [Google Scholar]
- 6.D’Agata E, Mitchell SL. Patterns of antimicrobial use among nursing home residents with advanced dementia. Arch Intern Med. 2008;168(4):357–362. doi: 10.1001/archinternmed.2007.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med. 2009;361(16):1529–1538. doi: 10.1056/NEJMoa0902234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Steen JT, Ooms ME, van der Wal G, Ribbe MW. Withholding or starting antibiotic treatment in patients with dementia and pneumonia: prediction of mortality with physicians’ judgment of illness severity and with specific prognostic models. Med Decis Making. 2005;25(2):210–221. doi: 10.1177/0272989X05275400. [DOI] [PubMed] [Google Scholar]
- 9.van der Steen JT, Ooms ME, Ader HJ, Ribbe MW, van der Wal G. Withholding antibiotic treatment in pneumonia patients with dementia: a quantitative observational study. Arch Intern Med. 2002;162(15):1753–1760. doi: 10.1001/archinte.162.15.1753. [DOI] [PubMed] [Google Scholar]
- 10.Mehr DR, van der Steen JT, Kruse RL, Ooms ME, Rantz M, Ribbe MW. Lower respiratory infections in nursing home residents with dementia: a tale of two countries. Gerontologist. 2003;43(special issue 2):85–93. doi: 10.1093/geront/43.suppl_2.85. [DOI] [PubMed] [Google Scholar]
- 11.van der Steen JT, Kruse RL, Ooms ME, et al. Treatment of nursing home residents with dementia and lower respiratory tract infection in the United States and the Netherlands: an ocean apart. J Am Geriatr Soc. 2004;52(5):691–699. doi: 10.1111/j.1532-5415.2004.52204.x. [DOI] [PubMed] [Google Scholar]
- 12.Kruse RL, Mehr DR, van der Steen JT, et al. Antibiotic treatment and survival of nursing home patients with lower respiratory tract infection: a cross-national analysis. Ann Fam Med. 2005;3(5):422–429. doi: 10.1370/afm.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Steen JT, Mehr DR, Kruse RL, Ribbe MW, van der Wal G. Treatment strategy and risk of functional decline and mortality after nursing-home acquired lower respiratory tract infection: two prospective studies in residents with dementia. Int J Geriatr Psychiatry. 2007;22(10):1013–1019. doi: 10.1002/gps.1782. [DOI] [PubMed] [Google Scholar]
- 14.Van Der Steen JT, Pasman HR, Ribbe MW, Van Der Wal G, Onwuteaka-Philipsen BD. Discomfort in dementia patients dying from pneumonia and its relief by antibiotics. Scand J Infect Dis. 2009;41(2):143–151. doi: 10.1080/00365540802616726. [DOI] [PubMed] [Google Scholar]
- 15.van der Steen JT, Ooms ME, van der Wal G, Ribbe MW. Pneumonia: the demented patient’s best friend? discomfort after starting or withholding antibiotic treatment. J Am Geriatr Soc. 2002;50(10):1681–1688. doi: 10.1046/j.1532-5415.2002.50460.x. [DOI] [PubMed] [Google Scholar]
- 16.Givens JL, Kiely DK, Carey K, Mitchell SL. Healthcare proxies of nursing home residents with advanced dementia: decisions they confront and their satisfaction with decision-making. J Am Geriatr Soc. 2009;57(7):1149–1155. doi: 10.1111/j.1532-5415.2009.02304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell SL, Kiely DK, Jones RN, Prigerson H, Volicer L, Teno JM. Advanced dementia research in the nursing home: the CASCADE study. Alzheimer Dis Assoc Disord. 2006;20(3):166–175. doi: 10.1097/00002093-200607000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morris JN, Fries BE, Mehr DR, et al. MDS Cognitive Performance Scale. J Gerontol. 1994;49(4):M174–M182. doi: 10.1093/geronj/49.4.m174. [DOI] [PubMed] [Google Scholar]
- 19.Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139 (9):1136–1139. doi: 10.1176/ajp.139.9.1136. [DOI] [PubMed] [Google Scholar]
- 20.Volicer L, Hurley AC, Blasi ZV. Scales for evaluation of end-of-life care in dementia. Alzheimer Dis Assoc Disord. 2001;15(4):194–200. doi: 10.1097/00002093-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Kiely DK, Volicer L, Teno J, Jones RN, Prigerson HG, Mitchell SL. The validity and reliability of scales for the evaluation of end-of-life care in advanced dementia. Alzheimer Dis Assoc Disord. 2006;20(3):176–181. doi: 10.1097/00002093-200607000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Albert M, Cohen C. The Test for Severe Impairment: an instrument for the assessment of patients with severe cognitive dysfunction. J Am Geriatr Soc. 1992;40(5):449–453. doi: 10.1111/j.1532-5415.1992.tb02009.x. [DOI] [PubMed] [Google Scholar]
- 23.Volicer L, Hurley AC, Lathi DC, Kowall NW. Measurement of severity in advanced Alzheimer’s disease. J Gerontol. 1994;49(5):M223–M226. doi: 10.1093/geronj/49.5.m223. [DOI] [PubMed] [Google Scholar]
- 24.Huber P. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability. Vol. 1. Berkeley: University of California Press; 1967. The behavior of maximum likelihood estimates under nonstandard conditions; pp. 221–233. [Google Scholar]
- 25.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–838. [Google Scholar]
- 26.Grambsch P, Therneau T. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81(3):515–526. [Google Scholar]
- 27.Luchins DJ, Hanrahan P. What is appropriate health care for end-stage dementia? J Am Geriatr Soc. 1993;41(1):25–30. doi: 10.1111/j.1532-5415.1993.tb05943.x. [DOI] [PubMed] [Google Scholar]
- 28.Morrison RS, Ahronheim JC, Morrison GR, et al. Pain and discomfort associated with common hospital procedures and experiences. J Pain Symptom Manage. 1998;15(2):91–101. [PubMed] [Google Scholar]