Abstract
Sepsis is a highly lethal disorder characterized by widespread apoptosis-induced depletion of immune cells and the development of a profound immunosuppressive state. IL-7 is a potent antiapoptotic cytokine that enhances immune effector cell function and is essential for lymphocyte survival. In this study, recombinant human IL-7 (rhIL-7) efficacy and potential mechanisms of action were tested in a murine peritonitis model. Studies at two independent laboratories showed that rhIL-7 markedly improved host survival, blocked apoptosis of CD4 and CD8 T cells, restored IFN-γ production, and improved immune effector cell recruitment to the infected site. Importantly, rhIL-7 also prevented a hallmark of sepsis (i.e., the loss of delayed-type hypersensitivity), which is an IFN-γ– and T cell-dependent response. Mechanistically, rhIL-7 significantly increased the expression of the leukocyte adhesion markers LFA-1 and VLA-4, consistent with its ability to improve leukocyte function and trafficking to the infectious focus. rhIL-7 also increased the expression of CD8. The potent antiapoptotic effect of rhIL-7 was due to increased Bcl-2, as well as to a dramatic decrease in sepsis-induced PUMA, a heretofore unreported effect of IL-7. If additional animal studies support its efficacy in sepsis and if current clinical trials continue to confirm its safety in diverse settings, rhIL-7 should be strongly considered for clinical trials in sepsis.
Sepsis, the systemic inflammatory response syndrome that occurs during severe infection, kills >210,000 people in the United States annually (1). Although patients with sepsis may die an early death due to a “cytokine storm” mediated by hyper-inflammation, the majority of patients survives the initial few days and develops a protracted hypoinflammatory, immunosuppressive state that is manifested by the inability of the patient to eradicate the primary infection and/or the development of new secondary infections (2, 3). The fact that many of the pathogens responsible for the fatal secondary nosocomial infections (e.g., Stenotrophomonas, Aceinetobacter, and Candida albicans) are not particularly virulent in patients with normal competent immune systems highlights the severe degree of immunosuppression in patients with sepsis. A defining feature of sepsis and an important mechanism of immunosuppression is the extensive apoptosis-induced depletion of immune effector cells (4–6). Three autopsy studies of patients who died of sepsis confirmed findings in animal models of sepsis and showed massive loss of Tand B lymphocytes and dendritic cells (7–9). Furthermore, observational clinical studies in patients with sepsis showed that the degree of apoptosis in circulating lymphocytes correlates with the severity of disease (10, 11).
There is compelling evidence in animal models that the profound apoptosis-induced loss of immune cells is a critical pathophysiologic factor in the immunosuppression and subsequent mortality of sepsis (12–15). The assertion that apoptosis is decisive in sepsis-induced mortality is supported by work showing that a variety of anti-apoptotic strategies improve overall animal survival in this lethal disorder (14–17). In particular, three independent investigative groups reported that overexpression of the antiapoptotic protein Bcl-2 in immune cells blocked sepsis-induced lymphocyte apoptosis and dramatically decreased mortality (17–19). These findings suggest that lymphocyte-protective antiapoptotic therapies may reduce morbidity and mortality in patients with sepsis.
IL-7 is a potent antiapoptotic cytokine that is essential for lymphocyte survival and expansion (20–25). In addition to its anti-apoptotic properties, IL-7 induces proliferation of naive CD4 and CD8 T cells (20, 25), potentially replenishing the loss of naive T cells that occurs during sepsis. The enormous potential of IL-7’s immune-boosting properties is best illustrated by the fact that it is currently being studied in four multinational clinical trials, including patients with HIV-1, cancer, and hepatitis C [(26–29) and http://clinicaltrials.gov]. In a recent dose-escalation, drug-safety study at the National Cancer Institute (Bethesda, MD), 16 patients with refractory cancer who received IL-7 had a >2-fold increase in circulating CD4 and CD8 T cells, a >60% increase in the size of spleen and lymph nodes, and a marked increase in lymphocyte intracellular Bcl-2 (26). In addition, a recent trial in HIV-1–infected patients who had persistently low CD4 T cell counts, despite effective viral suppression, showed that IL-7 was associated with robust and sustained increases in circulating CD4 and CD8 T cells (28).
Another important property of IL-7that may facilitate its entry into the clinical realm is its low incidence of side effects (26–30). Studies show that IL-7 is well tolerated in patients and, unlike IL-2, a closely related cytokine, it rarely induces fever, capillary leak syndrome, or other clinical abnormalities related to excessive release of proinflammatory cytokines (26–28). The fact that IL-7 rarely induces hyperinflammation may be partly related to a peculiar property of IL-7R. IL-7 transcriptionally represses expression of its own receptor (31, 32); therefore, there is less likelihood of hyperactivation of T cells by circulating IL-7.
The purpose of this investigation was to examine the efficacy of recombinant human IL-7 (rhIL-7) in a clinically relevant animal model of sepsis and to investigate potential mechanisms for its putative beneficial effects. This study demonstrates that rhIL-7 significantly increases intracellular Bcl-2, decreases the proapoptotic BH3 molecule PUMA, and blocks sepsis-induced death of CD4 and CD8 T cells. Furthermore, rhIL-7 had major beneficial effects on host immunity by increasing the recruitment of immune effector cells to the site of infection and preventing the loss of the host’s delayed-type hypersensitivity (DTH) response to antigenic challenge. These effects were likely due, in part, to rhIL-7’s ability to increase leukocyte adhesion molecules and to increase cellular expression of CD8. These effects were associated with a highly significant improvement in sepsis survival in mice receiving rhIL-7. In conclusion, rhIL-7 reverses fundamental immunologic defects in sepsis (i.e., the loss of critical immune effector cells) and the subsequent compromised host defenses. If the ongoing clinical trials on rhIL-7 verify its safety profile and if additional animal studies validate the efficacy of rhIL-7 in sepsis, rhIL-7 could move expeditiously into clinical trials in sepsis.
Materials and Methods
Mice
Animal studies were performed at Washington University School of Medicine (WUSM) and the University of Cincinnati College of Medicine (UCCM). Eight- to ten-week-old male C57BL6 mice purchased from The Jackson Laboratory (Bar Harbor, ME) or Charles River Laboratories (Wilmington, MA) were used for studies at WUSM and UCCM, respectively. CD1 male mice were purchased from Charles River Laboratories. All animal procedures were approved by the respective Animal Studies Committee at the two institutions.
Abs and reagents
The following Abs were purchased from BD Pharmingen (San Diego, CA): CD4-FITC (cat. #553729), CD4-PeCy5 (cat. #553050), CD8-PeCy5 (cat. #553034), B220-PeCy5 (cat. #553091), CD11c-FITC (cat. #553801), CD44-Pe (cat. #553134), CD69-Pe (cat. #01505B), CD25-Pe (cat. # 553075), Ki-67–Pe (cat. #556027), CD127-Pe (cat. #552734), and Bcl-2 (cat. #556536). CD62L-PeCy5 (cat. #15-0621-82) and DX5-FITC (cat. #11-5971-85) were purchased from eBioscience (San Diego, CA). PUMA (cat. #4976) and Bcl-XL (cat. #2764) were purchased from Cell Signaling Technology (Beverly, MA). TUNEL was performed using the APO-BRDU Kit (Phoenix Flow Systems, San Diego, CA).
rhIL-7 preparations
Two preparations of rhIL-7 were used for these studies; both demonstrated significant efficacy in sepsis (see Results). rhIL-7 (cat. #207-IL; R&D Systems, Minneapolis, MN) was stabilized with an anti–IL-7 Ab (M25; a kind gift from Dr. Phillipa Marrack, Denver, CO) to prolong the circulating t1/2 of the cytokine, exactly as described by Boyman et al. (22). Briefly, 2.5 μg rhIL-7 was mixed with 12.5 μg M25 per mouse and incubated for 15 min. Sterile saline was added to increase the total volume to 100 μl per mouse injected s.c. Studies in which rhIL-7 stabilized with anti-IL-7 Ab was used are shown in Figs. 1, 2, 4A, 4B, 6, 7C, and 8.
FIGURE 1.
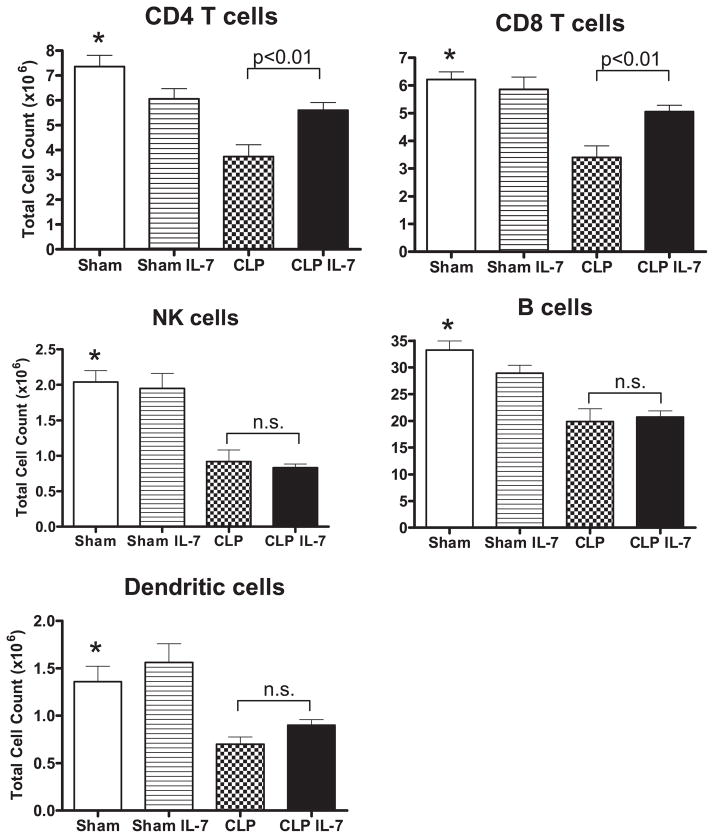
rhIL-7 prevents loss of CD4 and CD8 T cells in sepsis. Sham or septic mice were treated with 5 μg of rhIL-7 or diluent 90 min after surgery. Spleens were harvested for determination of absolute cell counts ~24 h later. Sepsis induced a loss in all classes of immune effector cells. rhIL-7 prevented the loss in CD4 and CD8 T cells but not in NK cells, B cells, or dendritic cells. *Sham versus CLP is significant at p < 0.01; CLP versus CLP + IL-7 is significant as indicated (n = 11 mice per group in each of the four groups; results from three combined studies).
FIGURE 2.
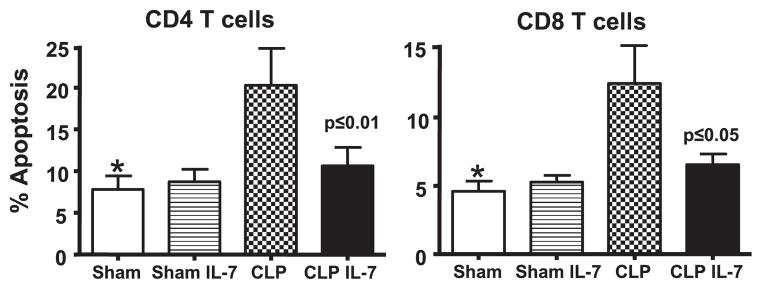
rhIL-7 prevents sepsis-induced apoptosis of T cells. Sham or septic mice were treated with 5 μg of rhIL-7 at 90 min after surgery. Spleens were harvested 24 h later, and apoptosis was determined by the TUNEL method. rhIL-7 prevented the marked increase in CD4 and CD8 T cell apoptosis occurring during sepsis. *Sham versus CLP is significantly different at p < 0.05; p value shown in the figure compares CLP versus CLP + IL-7. (n = 6–8 mice per group in the two sham groups, and n = 11 mice per group in the two CLP groups; results from three combined studies.)
FIGURE 4.
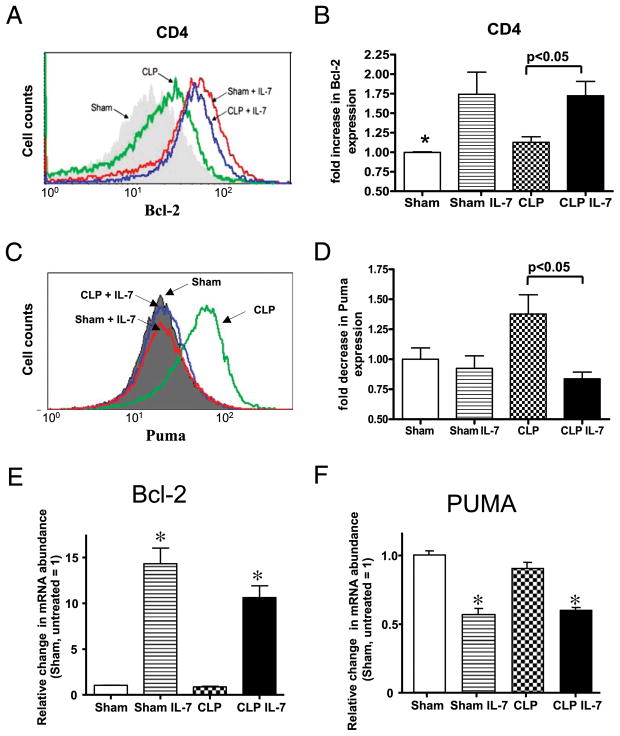
rhIL-7 increases antiapoptotic Bcl-2 and decreases proapoptotic PUMA protein and mRNA. A, Flow cytometry of intracellular staining for Bcl-2 in CD4 T cells. Sham or septic mice were treated with 5 μg of rhIL-7 at 90 min after surgery, spleens were harvested 24 h later, and lymphocytes were stained for intracellular Bcl-2. Flow cytometry was performed for relative quantitation of Bcl-2 in CD4 T cells. rhIL-7 increased Bcl-2 in sham and CLP mice. Each curve shows data from one representative mouse. B, The level of Bcl-2 in sham mice was normalized to a value of 1.0, and the Bcl-2 in other groups was expressed relative to this value. Note the increase in Bcl-2 in sham and septic mice treated with rhIL-7 (n = 6–8 mice in each group). *Sham versus sham + rhIL-7 is significantly different at p < 0.05. A highly similar effect of rhIL-7 also occurred on CD8 T cells (see Table I); two combined studies. C, Flow histogram of four individual representative mice. Note the increase in PUMA in CLP mice and the ability of rhIL-7 to prevent the sepsis-induced increase. D, Summary data for PUMA for all mice; one independent study (n = 4–5 mice per group). E, qRT-PCR for Bcl-2 and PUMA was performed on CD4 T cells isolated from sham- or CLP-operated mice 4 h after surgery and then incubated with or without rhIL-7 (5 ng/ml) for 5 h; one independent study. Note the dramatic increase in Bcl-2 gene expression in CD4 T cells from sham- and CLP-operated mice treated with rhIL-7. F, In addition, rhIL-7 caused a decrease in PUMA expression (n = 3 sham and n = 4 CLP mice in each group; one independent study). *p < 0.01.
FIGURE 6.
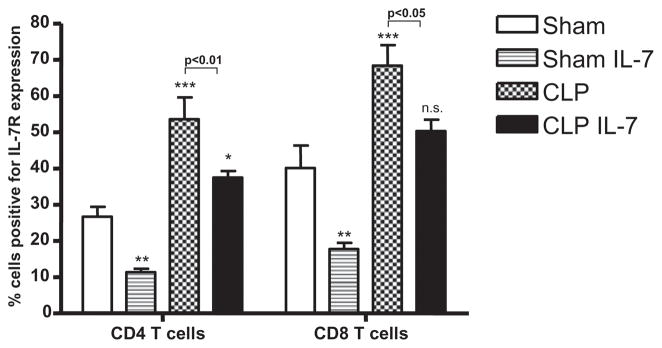
IL-7R expression is increased in sepsis. Sham or septic mice were treated with 5 μg of rhIL-7 for three consecutive days and killed on the third postoperative day. Sepsis induced a marked increase in IL-7R expression in CD4 and CD8 T cells. Treatment with rhIL-7 caused a decrease in IL-7R expression in sham and septic mice (n = 4 mice for each of the four groups; one independent study). *p < 0.05; **p < 0.01; ***p < 0.001 compared to sham mice.
FIGURE 7.
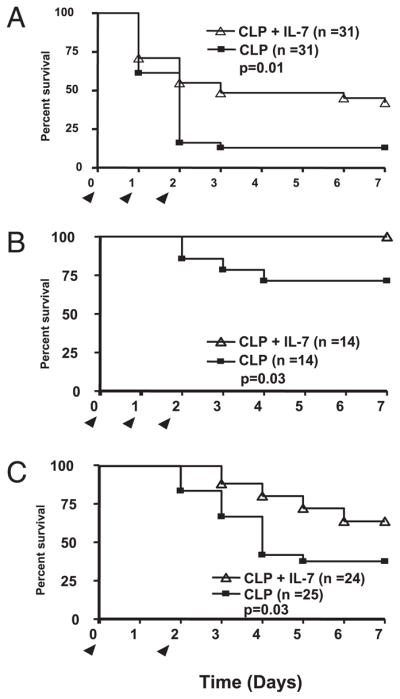
rhIL-7 improves survival in sepsis. A, CD-1 mice were injected with 5 μg of rhIL-7 90 min after CLP and again at 24 and 48 h. Septic control mice received the saline diluent. Survival was recorded for 7 d. Mice receiving rhIL-7 had a markedly improved survival. Results combined for two studies. B, Study design was identical to A, except that male C57BL6 mice were used. There was an increase in survival in mice treated with rhIL-7. Results combined for two studies. C, C57BL6 mice were treated with 5 μg/mouse IL-7 complexed with anti–IL-7 Ab (see Materials and Methods) administered immediately after CLP and again at 48 h postsurgery. Arrowheads indicate days of rhIL-7 injections.
FIGURE 8.
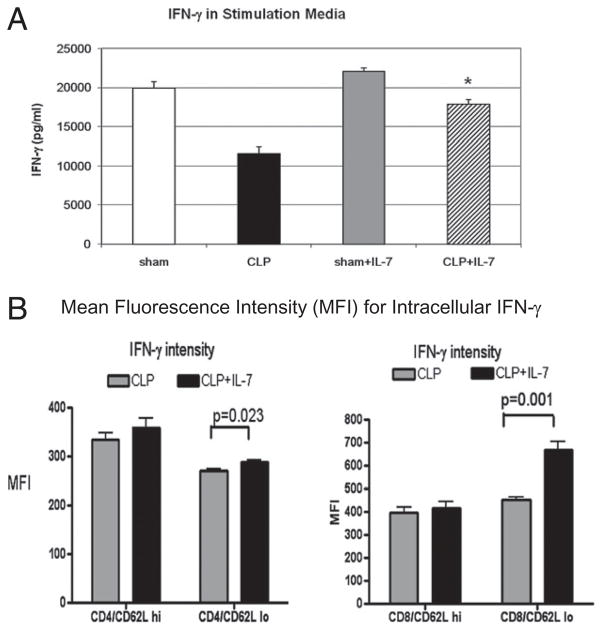
rhIL-7 reverses the sepsis-induced defect in IFN-γ production. A, Mice underwent sham or CLP surgery, and selected groups were treated with 5 μg of rhIL-7 90 min after surgery. Twenty-four hours later, splenocytes were harvested, and 1 × 107 cells were plated overnight in wells with 2 ml of RPMI 1640, as described previously. Anti-CD3 and anti-CD28 was added for lymphocyte stimulation. Supernatants were obtained, and IFN-γ was measured. Splenocytes from septic mice had a marked decrease in IFN-γ compared with sham-operated mice (p < 0.01), whereas splenocytes from septic mice that were treated with rhIL-7 did not have decreased IFN-γ production and were not different from sham-operated mice (*CLP versus CLP + IL-7; p < 0.05; n = 8 or 9 mice per group; results combined for two studies). B, CLP-operated mice were treated with 5 μg of rhIL-7 or with the saline diluent. Twenty-four hours later, splenocytes were harvested, and cells were stimulated for 24 h, followed by treatment with the Golgi inhibitor monesin for an additional 4 h. Cells had surface marker staining for CD4, CD8, and CD62L, followed by intracellular INF-γ staining. The MFI of INF-γ was evaluated by flow cytometry. CD4 and CD8 T cells that were CD62Llo (effector cells) had increased INF-γ MFI (n = 4 mice per group; one independent study).
A second preparation of rhIL-7 from Cytheris (Rockville, MD) was used for later studies because of a number of advantages. This preparation of rhIL-7 is fully humanized, glycosylated, has low immunogenicity, is manufactured under Good Manufacturing Practice specifications, and is currently being used for Food and Drug Administration-sponsored clinical trials in patients with HIV-1 and cancer. This preparation of IL-7 has a long circulating t1/2 (~12 h; Cytheris, unpublished data); therefore, it does not need stabilization with anti–IL-7 Ab. Studies in which clinical grade (non-Ab stabilized) rhIL-7 from Cytheris was used are shown in Figs. 3, 4C–F, 5, 7A, 7B, 9, and 10 and Supplemental Figs. 1–5.
FIGURE 3.
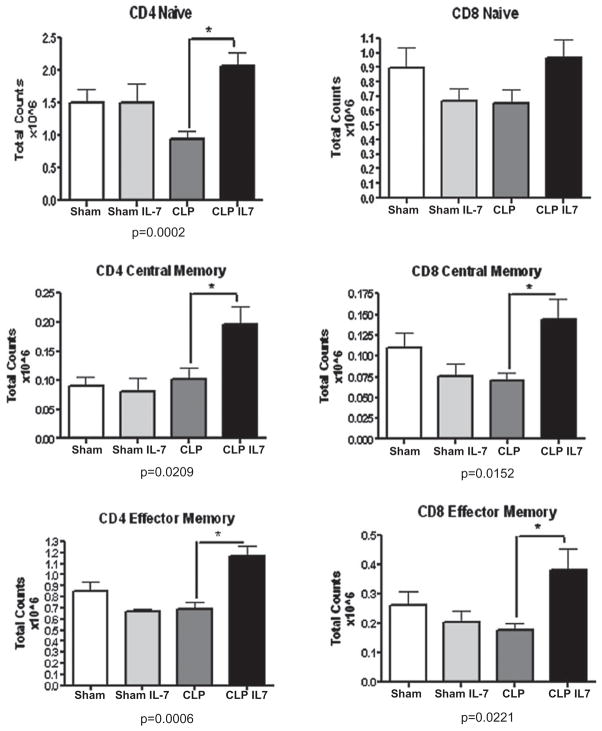
rhIL-7 increases CD4 and CD8 T cell subsets in lymph nodes. Sham or septic mice were treated with 5 μg of rhIL-7 at 90 min after surgery. Mesenteric lymph nodes were harvested 24 h later, and central memory, naive, and effector memory T cell subsets were assessed for CD4 and CD8 T cells via flow cytometry (see Materials and Methods). rhIL-7 had a significant ability to increase the absolute cell counts of CD4 central memory, naive, and effector memory T cells and CD8 central memory and effector memory T cells compared with CD4 and CD8 T cells from septic mice that did not receive rhIL-7 (n = 6 sham, n = 6 sham + rhIL-7, n = 9 CLP, and n = 10 CLP + rhIL-7; results from three combined studies). *p < 0.05.
FIGURE 5.
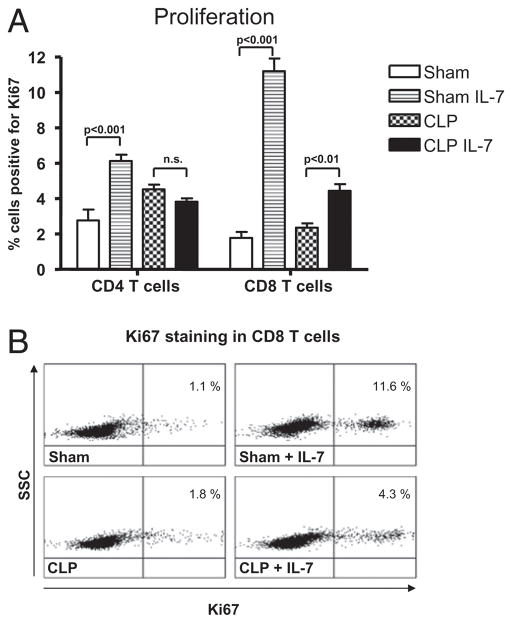
rhIL-7 increases cell proliferation. A, Sham or septic mice were treated with 5 μg of rhIL-7 at 90 min after surgery and again 24 and 48 h later. At 72 h after surgery, spleens were harvested, and lymphocytes were stained for the proliferation marker Ki67. rhIL-7 increased the percentage of CD4 and CD8 T cells that were positive for Ki67. rhIL-7 failed to increase proliferation in CD4 T cells in septic mice cells at 72 h but did increase proliferation in CD8 T cells. (n = 6–7 mice per group for sham and CLP; one independent study.) B, Flow cytometry dot plot showing the ability of rhIL-7 to increase proliferation, as indicated by the increase in CD8 T cells positive for Ki67. The numbers in the right upper quadrant represent the percentage of CD8 T cells positive for Ki67 (n = one representative animal per group).
FIGURE 9.
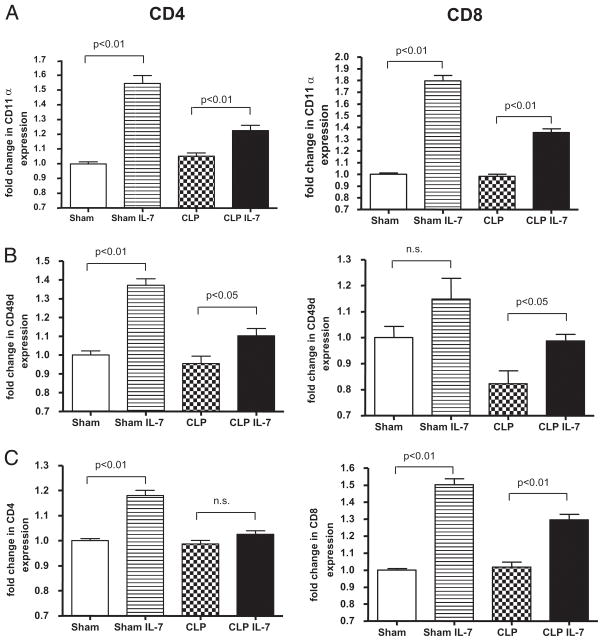
rhIL-7 increases LFA-1, VLA-4, CD4, and CD8 expression on T cells. Mice underwent sham or CLP surgery, and selected groups were treated with 5 μg of rhIL-7 90 min and 24 and 48 h after surgery. Splenocytes were harvested 72 h later and stained for (A) LFA-1 (CD11α), (B) VLA-4 (CD49d), (C) CD4, and CD8. The fold change in the MFI of the various Ags is expressed on the y-axis, with the splenocytes from the sham cells not treated with IL-7 arbitrarily assigned a value of 1.0. IL-7 caused increases in MFI in LFA-1 and VLA-4 in splenocytes from sham and septic mice, with a more prominent effect occurring in the sham mice (p < 0.05). IL-7 also caused an increase in the MFI of CD8 T cells from sham and septic mice and an increase in the MFI of CD4 T cells from sham, but not septic, mice (n = 10 sham mice, 9 sham + rhIL-7 mice, 13 CLP mice, and 12 CLP + rhIL-7 mice; results combined for two studies).
FIGURE 10.
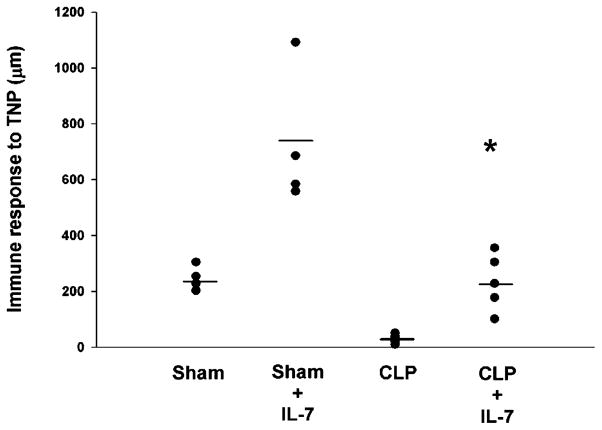
Sepsis induces a loss in the DTH response that is prevented by rhIL-7. Mice underwent sham or CLP surgery, and selected groups were treated with 2.5 μg of rhIL-7 90 min postsurgery and every 48 h for two additional doses. Four days postsurgery, mice were sensitized with trinitrophenyl (TNP). Four days after sensitization, mice had rechallenge with trinitrophenyl (footpad injection). The degree of footpad swelling was quantitated versus PBS injection (PBS internal control). rhIL-7 increased DTH in sham mice as expected. rhIL-7–treated septic mice sustained their DTH response, whereas untreated septic mice were incapable of mounting a response. Each filled circle represents one mouse (n = 5 mice per group). The horizontal line represents the mean value for each group. CLP versus CLP + rhIL-7 was statistically significant; p < 0.05. Results of one study but representative of three additional studies.
Sepsis model: cecal ligation and puncture
The cecal ligation and puncture (CLP) model was used to induce intra-abdominal peritonitis, as described previously (33, 34). Mice were anesthetized with isoflurane, and a midline abdominal incision was performed. The cecum was identified, ligated, and punctured with a needle. The needle gauge varied, depending upon the strain and source of the mice (see later discussion). The abdomen was closed in two layers, and 1 ml 0.9% saline was administered s.c. Sham-operated mice were treated identically, except that the cecum was not ligated or punctured.
Survival studies were performed by three investigators in two independent laboratories. Survival studies at WUSM were performed on two strains of mice, and the particular strain of mice used for each study is specified. CD1 mice were used in selected studies because of the expense of the C57BL6 mice and to confirm the findings regarding IL-7 seen in an inbred strain of mice (C57BL6) in an outbred strain of mice (CD1). A different gauge needle was used to puncture the cecum in the two different mouse strains because of previous studies demonstrating different sensitivities of the various mouse strains to survival following CLP (R.S. Hotchkiss and C.C. Caldwell, unpublished observations). In CD1 mice, CLP was performed using a 27-gauge needle with a double puncture. In studies involving C57BL6 mice, a 25-gauge needle was used for a single puncture. In both studies, rhIL-7 (Cytheris, 5 μg/mouse and injection) was administered 90 min after CLP and again at 24 and 48 h. Survival studies conducted at UCCM were performed on C57BL6 male mice, and rhIL-7 (5.0 μg rhIL-7 stabilized with the anti–IL-7 Ab M25) was administered immediately after CLP (cecum punctured once with a #23 needle) and again at 48 h postsurgery. Survival was followed for 7 d.
Quantification of absolute cell counts and apoptosis
Total cell counts per spleen were determined via the Vi-Cell counter (Beckman Coulter, Fullerton, CA). The percentages of individual cell phenotypes (e.g., CD4, CD8, and B) were determined via flow cytometric analysis. Thus, the absolute cell counts for each splenic subset population were calculated, as described previously (33, 34).
Apoptosis was quantified by flow cytometry using the TUNEL assay (33, 35). Flow cytometric analysis (50,000–100,000 events/sample) was performed on FACScan.
Determination of cell proliferation: Ki-67 staining
Sham- or CLP-operated mice were treated with 5 μg rhIL-7 or saline diluent at 90 min after surgery and 24 and 48 h later. At 72 h after surgery, spleen cells were harvested from sham and CLP mice and fixed, permeabilized, and stained with the cell proliferation marker Ki-67, as previously described (34).
Quantitative real-time PCR
In vivo quantitative real-time PCR studies
CD4 T cells were isolated by positive selection, as described previously (36). The purity of isolated resting CD4 T cells was generally >90%. Total RNA was isolated from spleen cells, as previously described (36). Quantitative real-time PCR (qRT-PCR) was performed in 96-well microtiter plates with an ABI Prism 7500 Sequence Detector System. Cell threshold values for each gene were determined, and fold-induction compared with GAPDH was calculated (36). qRT-PCR results were recorded for each gene as fold-change versus sham.
In vitro qRT-PCR determination
Mice underwent sham or CLP surgery; 4 h later, CD4 T cells were harvested by positive magnetic bead selection. One to two million cells were plated in incubation wells with 2 ml RPMI 1640 media. Selected wells were treated with 5 ng rhIL-7/ml for 5 h. Cells were obtained, and qRT-PCR was performed.
Determination of circulating IL-7 concentration
Blood samples were obtained from sham or CLP mice ~24 h after surgery. Whole blood was collected by cardiac puncture in anesthetized mice in EDTA-coated vials, and plasma was obtained. Values for IL-7 were determined using a mouse IL-7 ELISA kit from R&D Systems, and the manufacturer’s protocol was followed exactly. The lower limit of detection for the assay was 6 pg/ml. Although human IL-7 is active in mice (i.e., human IL-7 does reproduce the effect of mouse IL-7), the mouse IL-7 kit from R&D Systems is specific for mouse IL-7 and does not detect human IL-7 (see www.rndsystems.com). In addition to quantitating circulating mouse IL-7, we measured human IL-7 in sham and septic mice treated with rhIL-7. Mice were injected with 5 μg rhIL-7 ~22 h prior to being killed, and blood was obtained. The values for human IL-7 were 324.0 ± 70.7 pg/ml in sham (n = 10) and 472.5 ± 48.1 pg/ml in septic mice (n = 13). The sham- and CLP-operated mice that did not receive rhIL-7 had no detectable levels of human IL-7.
Determination of circulating cytokine levels
Serum cytokines were quantified using the BD FACS Array and the Mouse Inflammation Kit, per the manufacturer’s recommendations, as previously described (34). The lower limits of detection were 17.5 pg/ml for IL-10, 5 pg/ml for IL-6, 22.7 pg/ml for monocyte chemoattractant protein-1, 7.3 pg/ml for TNF-α, 10.7 pg/ml for IL-12p70, and 2.5 pg/ml for IFN-γ.
Determination of DTH
Mice underwent CLP or sham surgery, as previously described (33). One group received 2.5 μg Ab-stabilized IL-7 s.c. 1 h postsurgery and another injection at 48 h. At 4 d postsurgery, all mice were immunized with injections of 100 μl 10 mM TNBS s.c. At day eight, all mice had antigenic challenge with 10 mM TNBS in the right footpad. PBS was injected in the left footpad as a control. Measurements (micrometers ± SE) were taken 24 h later and represent the difference between the right footpad (Ag challenge) versus the left footpad (PBS challenge).
Determination of IFN-γ in splenocyte incubations
Mice underwent sham or CLP surgery and were treated with 5 μg rhIL-7 90 min after surgery. Spleens were harvested 24 h later, and splenocyte suspensions were prepared, as previously described (36). Approximately 10 million splenocytes were plated in sterile wells with 2 ml RPMI 1640 media and incubated overnight, as described previously (35). An aliquot of the supernatant was obtained from the wells, and IFN-γ was quantitated using an ELISA kit from R&D Systems. The lower limit of detection of the kit was 6 pg/ml.
Determination of intracellular IFN-γ in splenocyte incubations
Mice underwent sham or CLP surgery and were treated with 5 μg rhIL-7 90 min after surgery. Spleens were harvested 24 h later, splenocyte suspensions were prepared, and cells were stimulated. Dissociated splenocytes (2 × 106) were stimulated overnight with anti-CD3 and anti-CD28; monesin was added for 4 h, followed by staining for CD4 and CD8 T cells and quantitation of intracellular IFN-γ by flow cytometry.
Statistical analysis
Data are reported as mean ± SEM and were analyzed using the statistical software program Prism (GraphPad, San Diego, CA). Except where specified, data were analyzed using one-way ANOVA with the Newman–Keuls or Dunnett posttest. Significance was accepted at p < 0.05.
Results
rhIL-7 prevents the depletion in absolute cell counts of CD4 and CD8 T cells in spleen
Mice underwent sham surgery or CLP and were treated 90 min later withrhIL-7orsalinediluent. A consistent finding in sepsisis depletion of innate and adaptive immune cells (13–15), and this is reflected in the current study. Sepsis induced major decreases in absolute cell counts in all five cell phenotypes examined, including CD4 T cells (49.3% ± 6.5% loss), CD8 T cells (45.2% ± 6.7% loss), B cells (40.2% ± 7.3% loss), NK cells (54.9% ± 7.9% loss), and dendritic cells (48.5% ± 5.7% loss) (Fig. 1). rhIL-7 effectively prevented the sepsis-induced loss in CD4 and CD8 T cells, such that absolute cell counts were not statistically different in the sham plus rhIL-7 group versus the CLP plus rhIL-7 group. rhIL-7 had no effect on the sepsis-induced loss of B cells, NK cells, or dendritic cells (Fig. 1).
To determine whether rhIL-7 prevented the loss of CD4 and CD8 T cells throughout the duration of sepsis, absolute cell counts for CD4 and CD8 T cells were quantified at 4 d postsham or CLP surgery. In sham animals, rhIL-7 caused an expected dramatic increase in total splenocytes, as well as the number of CD4 and CD8 T cells (Supplemental Fig. 1). Although the magnitude increase in absolute cell countsinsepticmicetreatedwithrhIL-7wasnotcomparabletothatof sham mice receiving rhL-7, it was markedly higher than in CLP mice that did not receive rhIL-7. The total splenocyte counts were 41.1 ± 3.0 × 106 and 58.8 ± 5.1 × 106 for CLP versus CLP plus rhIL-7, respectively; p < 0.05. The total CD4 T cell counts were 3.4 ± 0.3 × 106 and 5.5 ± 0.4 × 106 for CLP versus CLP plus rhIL-7, respectively; p < 0.01. Mice treated with rhIL-7 also had greater numbers of CD8 T cells (4.1 ± 0.4 × 106 cells and 9.5 ± 0.5 × 106 for CLP versus CLP plus rhIL-7, respectively; p < 0.01).
The protective effect of IL-7 to prevent depletion of tissue immune effector cells was also seen in the thymus. rhIL-7 decreased the sepsis-induced loss in double-positive, double-negative, and single-positive CD4/CD8 thymocytes (Supplemental Fig. 2).
Apoptotic death is an important mechanism for the depletion of splenic immune cells in sepsis (15–19). As depicted in Fig. 2, sepsis induced a >2-fold increase in apoptosis in splenic CD4 and CD8 T cells as determined by TUNEL assay. Importantly, rhIL-7 prevented the increased sepsis-induced apoptosis in these two lymphocyte subsets; this finding is consistent with the effects of rhIL-7 to preserve absolute CD4 and CD8 T cell counts in the spleen observed in Fig. 1. Thus, rhIL-7 significantly prevents the typical apoptotic demise of T cells and thymocytes during sepsis.
rhIL-7 preserves naive, central memory, and effector memory CD4 and CD8 T cells in the spleen and regional lymph nodes
The effects of rhIL-7 on naive (CD44lo/CD62Lhi), central memory (CD44hi/CD62Lhi), and effector memory (CD44hi/CD62Llo) CD4 and CD8 T cells in spleen and mesenteric lymph nodes were determined at 24 h after sham or sepsis surgery (Table I). rhIL-7 prevented the sepsis-induced decrease in splenic naive, central memory, and effector memory CD4 T cells; a similar protective effect was seen in naive and central memory (but not effector memory) CD8 T cells (Table I). Importantly, rhIL-7 had comparable protective effects in CD4 and CD8 T cell subsets located in the mesenteric lymph nodes, regional nodes in close proximity to the infected abdominal focus (Fig. 3).
Table I.
rhIL-7 treatment increases T cell absolute numbers, activated T cells and Bcl-2 expression in different T cell subsets
| Naive | Central Memory | Effector Memory | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CD4 T Cells | |||||||||
| × 106 | Bcl-2 MFI | CD69+ | × 106 | Bcl-2 MFI | CD69+ | × 106 | Bcl-2 MFI | CD69+ | |
| Sham | 5.6 ± 0.1 | 1398 ± 110 | 0.18 ± 0.01 | 0.74 ± 0.04 | 1233 ± 68 | 0.09 ± 0.01 | 0.89 ± 0.02 | 913 ± 59 | 0.16 ± 0.01 |
| Sham + IL7 | 6.6 ± 0.2 | 1729 ± 126 | 0.18 ± 0.01 | 0.91 ± 0.06 | 1607 ± 28 | 0.12 ± 0.01 | 1.0 ± 0.06 | 1147 ± 62 | 0.19 ± 0.02 |
| p Valuea | <0.01 | <0.01 | <0.05 | <0.05 | |||||
| CLP | 3.6 ± 0.3 | 1271 ± 13 | 0.32 ± 0.03 | 0.91 ± 0.1 | 1168 ± 38 | 0.26 ± 0.04 | 0.49 ± 0.03 | 812 ± 19 | 0.17 ± 0.02 |
| CLP + IL7 | 5.7 ± 0.5 | 1376 ± 11 | 0.42 ± 0.04 | 1.29 ± 0.2 | 1498 ± 38 | 0.30 ± 0.04 | 0.72 ± 0.04 | 1029 ± 30 | 0.26 ± 0.02 |
| p Valuea | <0.001 | <0.01 | <0.05 | <0.05 | <0.01 | <0.001 | <0.01 | <0.01 | |
| CD8 T Cells |
|||||||||
| Sham | 3.5 ± 0.1 | 1804 ± 115 | 0.11 ± 0.002 | 1.7 ± 0.22 | 2668 ± 216 | 0.07 ± 0.01 | 0.14 ± 0.01 | 1732 ± 116 | 0.01 ± 0.002 |
| Sh + IL7 | 4.6 ± 0.3 | 2435 ± 137 | 0.13 ± 0.009 | 2.0 ± 0.04 | 3411 ± 187 | 0.1 ± 0.01 | 0.12 ± 0.01 | 1966 ± 94 | 0.01 ± 0.003 |
| p Valuea | <0.05 | <0.05 | |||||||
| CLP | 3.3 ± 0.3 | 1498 ± 23 | 0.19 ± 0.02 | 1.23 ± 0.1 | 2340 ± 26 | 0.33 ± 0.06 | 0.21 ± 0.02 | 1634 ± 75 | 0.05 ± 0.01 |
| CLP+ IL7 | 5.0 ± 0.4 | 1824 ± 37 | 0.29 ± 0.03 | 2.00 ± 0.1 | 2573 ± 30 | 0.44 ± 0.06 | 0.25 ± 0.03 | 2121 ± 111 | 0.05 ± 0.01 |
| p Valuea | <0.01 | <0.001 | <0.05 | <0.001 | <0.001 | <0.05 | |||
Mice underwent sham or CLP surgery, and selected groups were treated with 5 μg of rhIL-7. Splenocytes were harvested 24 h later, enumerated, and analyzed for CD4, CD8, CD44, CD62L, CD69, and intracellular Bcl-2 by flow cytometry (n = 5 for sham and sham + rhIL-7; n = 8 for CLP and CLP + rhIL-7).
Determined by Student t test.
The ability of rhIL-7 to prevent the depletion of T cells expressing the early inducible activation marker CD69 on the selected CD4 and CD8 T cell subsets was also quantitated (Table I). rhIL-7 treatment resulted in increased numbers of CD69-expressing naive CD4 and CD8 T cells and effector memory CD4 T cells (Table I).
rhIL-7 increases intracellular Bcl-2 in CD4 and CD8 T cells
At 24 h after administration, rhIL-7 induced a rapid increase in intracellular Bcl-2 determined by mean fluorescence intensity (MFI) in CD4and CD8T cells in sham and septic animals. The MFI of the sham mice that did not receive rhIL-7 was assigned a value of 1.0, and the MFI values of the other groups were compared with this value. In sham-treated mice, rhIL-7 caused a 1.7 ± 0.3- and a 1.5 ± 0.2-fold increase in the MFI of CD4 and CD8 T cells, respectively; p < 0.05 (Fig. 4, Table I.). The increase in MFI in CD4 T cells for Bcl-2 is demonstrated via flow cytometry for individual mice treated with rhIL-7 (Fig. 4A). Compared with sham mice, rhIL-7 caused an ~1.7 ± 0.2-fold increase in the MFI of CD4 and CD8 T cells in CLP mice; p < 0.05. The effects of rhIL-7 on Bcl-2 MFI in naive, central memory, and effector memory CD4 and CD8 T cells are presented in Table I; rhIL-7 increased Bcl-2 in all three CD4 and CD8 T cell subsets in septic mice.
rhIL-7 prevents increased proapoptotic BH3 mRNA in sepsis and decreases PUMA protein
Previous work from the laboratory (R.S. Hotchkiss) showed that sepsis induces a significant increase in proapoptotic BH3 family members Bim and PUMA in CD4 T cells (36). Increases in Bim and PUMA contribute to apoptosis in sepsis, based on studies showing that mice deficient in Bim or Puma have dramatically decreased sepsis-induced apoptosis (35). To investigate the potential effect of rhIL-7 on proapoptotic BH3 family members, qRT-PCR was performed on splenic CD4 T cells isolated at 8 and 18 h from sham- or CLP-operated mice. Five micrograms of rhIL-7 was administered 90 min after sham or CLP surgery. At 8 h after surgery, splenic CD4 T cells were harvested. Sepsis induced a 2-to 3-fold increase in mRNA for Bim, Bmf, and Puma that was prevented by treatment with rhIL-7 (Supplemental Fig. 3A). By 18 h after sepsis onset, mRNA for Puma remained elevated, and treatment with rhIL-7 remained effective in reducing PUMA mRNA (Supplemental Fig. 3B).
In addition to in vivo studies, we performed in vitro studies on isolated splenic CD4T cells to examine the effect of rhIL-7 on mRNA forBcl-2 and PUMA. Mice underwent sham or CLP surgery;4h later, splenic CD4 T cells were harvested using magnetic bead selection. Approximately 1–2 million cells were incubated in wells, and selected wells were treated with 5 ng of rhIL-7/ml. Five hours later, cells were harvested, and qRT-PCR was performed for Bcl-2 and PUMA. There was a dramatic increase in mRNA for Bcl-2 in cells from sham- and CLP-operated mice that were treated with rhIL-7 (Fig. 4E). The decreased mRNA for PUMA caused by rhIL-7 that was observed in the in vivo studies was reproduced in the in vitro studies (Fig. 4F).
Given the in vivo and in vitro results showing a potent effect of rhIL-7 in decreasing mRNA for PUMA in sepsis, we examined the effect of rhIL-7 on intracellular PUMA protein levels determined by MFI using flow cytometry. Mice were treated with rhIL-7 or saline diluent, as described above. Splenocytes were harvested ~24 h after surgery, and intracellular PUMA staining was performed in CD4 T cells. PUMA was markedly increased in septic mice; however, mice treated with rhIL-7 had no such increase (Fig. 4C, 4D). Compared with septic mice that did not receive rhIL-7, septic mice treated with rhIL-7 had a 39% lower MFI for PUMA (p < 0.05).
rhIL-7 increased cell proliferation in CD8 T cells at 72 h after CLP
We examined the ability of rhIL-7 to induce proliferation of T cells in sepsis, because the known potent proliferative effect of IL-7 could contribute to the elevated T cell numbers observed in the current study. Previous work from our laboratory (R.S. Hotchkiss) showed that CD4 and CD8 T cells do not undergo proliferation in the first 48 h after sepsis onset (33, 34). Although the exact mechanism is unknown, studies indicate that sepsis can inhibit cell proliferation (33, 34, 37). To determine the effect of rhIL-7 on T cell proliferation, splenocytes were harvested and stained for Ki67, an indicator of cell proliferation. Although rhIL-7 had not increased cell proliferation at 24 h after sham or CLP surgery (data not shown), by 72 h after surgery it caused a >2-fold increase in CD4 T cell proliferation in sham mice but failed to increase proliferation in CD4 T cells from mice with sepsis (Fig. 5A). rhIL-7 was effective in increasing proliferation in CD8 T cells in sham and septic mice (Fig. 5). In sham mice, there was a >6-fold increase in Ki67 staining, whereas CD8 T cells from septic mice had an ~2-fold increase in Ki67+ staining.
IL-7R expression is increased in sepsis and is downregulated by treatment with rhIL-7
Cellular expression of IL-7R is labile and adjusts rapidly depending upon a number of factors, including the size of the lymphocyte pool and the concentration of circulating IL-7 (see later discussion) (31, 32). In the current study, the percentage of cells expressing IL-7R was significantly increased in the CD4 and CD8 T cells 3 d after sepsis onset (Fig. 6). Administration of rhIL-7 ~90 min after surgery caused a marked decrease in the percentage of CD4 and CD8 T cells expressing IL-7R in sham- and CLP-operated mice. There was a 57.4% ± 3.6% and a 55.7% ± 4.2% decrease in IL-7R expression in CD4 and CD8 T cells, respectively, in sham mice treated with rhIL-7 (Fig. 6). The percentage decrease in IL-7R expression in CD4 and CD8 T cells in septic mice receiving rhIL-7 was of a smaller magnitude (30.1% ± 3.4% and 26.5% ± 4.7%, respectively). Additionally, IL-7R expression was determined on sham-operated mice and on septic C57BL6 mice at a different time point postsurgery. IL-7R expression was increased as soon as 1 h after CLP compared with sham-operated mice. Forty-eight hours after CLP, IL-7R expression levels on naive CD4 and CD8 T cells were increased 130% and 159%, respectively, whereas they were increased 181% and 194% on CD4 and CD8 effector memory cells, respectively (data not shown).
Circulating murine IL-7 is increased in sepsis
Plasma was obtained from sham- or CLP-operated mice ~24 h after surgery, and circulating IL-7 was quantitated using an ELISA kit from R&D Systems. Circulating IL-7 was 9.8 ± 5.0 pg/ml (n = 15) and 95.6 ± 20.0 pg/ml (n = 15) for sham and CLP, respectively.
rhIL-7 improves survival in sepsis
Having demonstrated that rhIL-7 had potent effects on the preservation of CD4 and CD8 T cells, we sought to determine whether these protective effects translated into improved survival. The survival studies were conducted by two independent investigative groups: WUSM and UCCM. At WUSM, rhIL-7 was tested in two different strains of male mice: C57BL6 and CD1. rhIL-7 was administered 90 min after CLP, with additional doses administered 24 and 48 h after CLP. rhIL-7 improved survival in C57BL6 (p = 0.01; Fig. 7A) and CD1 mice (p = 0.03; Fig. 7B). There was a difference in survival in the untreated group of C57BL6 and CD1 mice that is indicative of the differences in sensitivity to the CLP model in these two mouse strains (R.S. Hotchkiss, unpublished data). In survival studies at UCCM, rhIL-7 complexed with anti–IL-7 Ab was administered to C57BL6 male mice immediately after CLP and again 48 h later. rhIL-7 therapy resulted in a markedly improved survival at 7 d (p = 0.03; Fig. 7C).
rhIL-7 reverses the sepsis-induced defect in IFN-γ production
A series of mechanistic studies was performed to determine potential means by which rhIL-7 improves survival. IFN-γ plays a critical role in macrophage activation, mediation of Th1 immunity, and promotion of an effective DTH response. Sepsis induces decreased IFN-γ production, and this defect was reported to be a central feature in the morbidity and mortality of sepsis in animal models of sepsis and in clinical studies (38–41). We determined the effect of rhIL-7 on the ability of stimulated splenocytes to secrete IFN-γ. Mice underwent sham or CLP surgery and were treated with 5 μg of rhIL-7 or saline diluent 90 min following surgery. Twenty-four hours later, spleens were harvested, and splenocytes were prepared. Approximately 1 × 107 splenocytes were placed in wells and stimulated with anti-CD3 and anti-CD28 overnight, as described previously (33, 34). Splenocytes from mice that underwent CLP had a marked decrease (~42%) in IFN-γ production compared with sham-operated mice; p < 0.001 (Fig. 8A). In contrast, IFN-γ production in splenocytes from septic mice treated with rhIL-7 was not statistically different from sham mice. Furthermore, IFN-γ production in sham mice treated with rhIL-7 was not statistically different from sham mice not treated with rhIL-7.
In a related set of studies, we determined the effect of rhIL-7 on IFN-γ production in CD4 and CD8 T cells after in vitro stimulation. Dissociated splenocytes (2 × 106) were stimulated overnight with anti-CD3 and anti-CD28, monesin was added for 4 h, followed by staining for CD4 and CD8 T cells and quantitation of intracellular IFN-γ by flow cytometry (Fig. 8B). rhIL-7 caused a slight, but statistically significant, increase in the MFI of IFN-γ in CD4 T effector cells and a greater and more prominent increase in CD8 T effector cells (Fig. 8B). Considered together, these results are consistent with the ability of rhIL-7 to reverse the sepsis-induced defect in IFN-γ production, without causing excessive production of this potent cytokine. Thus, rhIL-7 reverses a fundamental defect in immunity that occurs in sepsis.
In addition to IFN-γ, we quantitated circulating pro- and anti-inflammatory cytokines 24 h after surgery. As expected, sepsis caused a significant increase in proinflammatory (TNF-α, IL-6, and monocyte chemoattractant protein-1) and anti-inflammatory (IL-10) cytokines compared with sham mice (Supplemental Fig. 4). rhIL-7 did not impact circulating pro- or anti-inflammatory cytokines in sham or septic mice at this time point.
IL-7 increases expression of leukocyte adhesion markers in sepsis
Integrins are essential for cell trafficking and for optimal activation of CD4 and CD8 T cells. Integrins are important in the host response to sepsis because mice deficient in LFA-1 are more susceptible to the lethal effects of Streptococcus pneumoniae. Consequently, the effect of rhIL-7 on leukocyte adhesion markers LFA-1 (CD11α) and VLA-4 (CD49d) was evaluated by quantitating MFI at 72 h after sham or CLP treatment (Fig. 9). Individual cell expression of CD4 and CD8 was also determined. The MFI for sham mice was assigned a value of 1.0, and the data for CLP- and rhIL-7–treated mice were referenced to this value. rhIL-7 caused a significant increase in the expression of LFA-1 in CD4 and CD8 T cells in sham and septic mice, although the magnitude of the increase was greater in sham mice (Fig. 9). rhIL-7 also increased VLA-4 in CD4 T cells in sham and septic mice but only in CD8 T cells from septic mice. rhIL-7 also significantly increased CD4 expression in sham mice and increased CD8 expression in sham and septic mice. Finally, rhIL-7 increased the expression of CD62L in CD8 T cells but not CD4 T cells in sham and septic mice (Supplemental Fig. 5).
rhIL-7 preserves the DTH response in septic mice
The DTH response requires a coordinated interaction of cells of the innate and adaptive immune system and is known to require T cells and IFN-γ. As noted by other investigators, IL-7 enhances the response to Ag (42). We observed this phenomenon in the marked increase in the DTH response in sham mice treated with rhIL-7 compared with sham mice not treated with rhIL-7 (Fig. 10). Mice with sepsis failed to mount a DTH response to antigenic challenge, which is consistent with a profound defect in immunity during sepsis. Importantly, septic mice that received rhIL-7 had a significant DTH response that was indistinguishable from sham mice that did not receive rhIL-7, although it was of a lesser magnitude than the response of sham mice treated with rhIL-7; p < 0.05 (Fig. 10). Thus, rhIL-7 preserved the integrated immunologic function that is normally ablated in sepsis.
Discussion
The immunologic and inflammatory response to sepsis is extremely complex and typified by an early net proinflammatory response followed by immunosuppression (43–47). There is increasing recognition that the immunosuppressive phase of sepsis, which has been termed immunoparalysis, is a major contributing factor to its lethality (2–4, 46). Patients with sepsis may die of an inability to eradicate their initial infection or by developing secondary nosocomial infections. One of the most important factors in sepsis immunosuppression is the apoptosis-induced depletion of immune effector cells (13–16). In addition to numerous animal studies documenting widespread loss of immune cells in sepsis, three autopsy studies of patients who died of sepsis documented massive loss of CD4 T cells, B cells, dendritic cells, and other immune elements (7–9). In addition to the loss in critical immune cells, apoptosis is detrimental to host immunity through a second independent mechanism. Phagocytic cells that consume apoptotic cells become anergic and/or induce T cells to acquire a Th2 anti-inflammatory phenotype that can inhibit the ability of the host to eradicate the pathogens (48–51). A number of investigative groups, including our own, have demonstrated that prevention of apoptosis improves survival in sepsis, thereby supporting the hypothesis that apoptosis of immune cells is a central pathophysiologic event in the disease (13–16, 18). Therefore, development of a clinically applicable therapy that could prevent sepsis-induced apoptosis of immune cells and maintain or enhance immune effector cell function could have a major impact on morbidity and mortality in this disorder that kills >210,000 patients annually.
The present work is significant because it demonstrates that rhIL-7, a potent antiapoptotic cytokine, was able to prevent the loss of CD4 and CD8 T cells as reflected by its effects on absolute cell counts and apoptosis. These protective effects occurred in spleen as well as in mesenteric lymph nodes that are inclose proximity to the septic focus. rhIL-7’s protection extended broadly to most classes of CD4 and CD8 T cells (i.e., naive, central memory, and effector memory subsets) and was persistent throughout the duration of sepsis (Supplemental Fig. 1).
Although IL-7 inhibits apoptosis via several mechanisms, one of its most important antiapoptotic actions is due to its ability to increase Bcl-2 (52). In the current study, rhIL-7 acted rapidly within 24 h to significantly increase the abundance of intracellular Bcl-2 in CD4 and CD8 T cells. The in vitro qRT-PCR data showed a >10-fold increase in mRNA for Bcl-2 in CD4 T cells by 5 h after treatment with rhIL-7 (Fig. 4E). These findings are consistent with work by Sportés et al. (26), who showed that cancer patients treated with rhIL-7 had a dramatic increase in MFI of Bcl-2 in circulating CD4 and CD8 T cells. The Bcl-2–inducing property of IL-7 is particularly important considering the studies that examined the protective effect of transgenic overexpression of Bcl-2 in sepsis. Three independent groups demonstrated that mice that overexpress Bcl-2 are resistant to sepsis-induced apoptosis and have improved survival (13, 18, 19).
A surprising finding was the ability of rhIL-7 to prevent the - sepsis-induced increase in mRNA for proapoptotic BH3 molecules, including Bim, PUMA, and BMF. Previous work from our group 7 showed that Bim-null mice and PUMA-null mice have significant decreases in sepsis-induced lymphocyte apoptosis (35). The ability of rhIL-7 to decrease PUMA in sham- and CLP-operated mice occurred at 8 and 18 h following in vivo administration, and the effect was observed in isolated CD4 T cells using in vitro studies (Fig. 4F). Significantly, this rhIL-7–mediated decrease in mRNA for PUMA was associated with an ~39% decrease in intracellular PUMA protein in CD4 T cells (Fig. 4C, 4D). An additional antiapoptotic effect of rhIL-7 was its ability to increase mRNA for Bcl-2 at 5 h (Fig. 4E); however, this effect was not seen at later time points (Supplemental Fig. 3). Given the increase in Bcl-2 protein (Fig. 4A, 4B), we speculate that the effect of rhIL-7 on mRNA for Bcl-2 may be time dependent.
The most important finding of the current study was the ability of IL-7 to improve survival in sepsis. The beneficial effects of IL-7 on survival were demonstrated for two different preparations of IL-7 in two different institutions (see Materials and Methods). rhIL-7 complexed with anti–IL-7 Ab and rhIL-7 from Cytheris (which has a long circulating half-life and does not require Ab stabilization) had salutary effects in sepsis. Recent work showed that complexing rhIL-7 to an anti–IL-7 mAb increases its efficacy by 50- to 100-fold (22, 53). Exceedingly small amounts of anti–IL-7 Ab dramatically prolong the circulating half-life of IL-7 (22, 53). Similar effects were shown for IL-2 and -15. The rhIL-7 preparation from Cytheris is the formulation being used in the multiple clinical trials previously discussed; it possesses low immunogenicity and has an excellent safety record in patients (26–29).
A key goal of the current study was to determine potential mechanisms of action for the beneficial effect of rhIL-7 in addition to its ability to block apoptosis. One potential mechanism is the ability of rhIL-7 to reverse the decrease in IFN-γ production that is a characteristic finding in animal and clinical studies in sepsis (39–41). Studies examining the amount of IFN-γ produced by CD4 and CD8 splenocytes (i.e., intracellular staining for IFN-γ) showed that CD4 and CD8 T cells from septic mice treated with rhIL-7 had an increase in MFI for IFN-γ compared with septic mice that did not receive rhIL-7 (Fig. 10B). These results are consistent with work by Levy et al. (28), who documented that rhIL-7 increased the median frequency of CD4 T cells producing IFN-γ in HIV-1–infected adults. Animal studies showed that treatments that reverse the defect in IFN-γ production can improve survival in sepsis (39–41). Furthermore, Döcke et al. (39) reported that administration of IFN-γ restored monocyte TNF-α production and improved survival in a group of patients with sepsis. In the current study, splenocytes from septic mice treated with rhIL-7 had a reversal of the sepsis-induced defective production of IFN-γ (Fig. 10). IFN-γ production in sham mice treated with rhIL-7 was not different from sham mice not treated with rhIL-7. This latter finding suggests that rhIL-7 did not prime cells for excessive production, but rather reversed the sepsis-induced defect. One possible explanation for the increase in IFN-γ production in the splenocytes from septic mice treated with rhIL-7 is that splenocytes from rhIL-7–treated mice had improved survival during the overnight incubation and, therefore, produced more IFN-γ. It is also possible that rhIL-7 improved the ability of the individual splenocytes to produce more IFN-γ.
A second potential mechanism for the salutary effect of IL-7 in sepsis is its action to increase expression of the leukocyte adhesion molecules LFA-1 and VLA-4. The effect of IL-7 on integrin expression has been examined to a very limited degree; the present report is the first study to show that IL-7 increases the expression of multiple integrins in an in vivo setting and during a pathologic state. A successful immune response depends on the ability of the immune effector cells to migrate from one location in the body to another (54). The increase in leukocyte adhesion markers results in improved leukocyte migration and tissue penetration to the site of infection or to other areas in which critical cell cross-talk will occur. Integrins, especially LFA-1, are essential for most adhesion-dependent lymphocyte functions, including Ag- and APC-induced Th cell stimulation, cytotoxic lymphocyte-mediated killing of target cells, and adhesion of lymphocytes to vascular endothelium (53–56). Although the role of integrins in sepsis is not well defined, Prince et al. noted that mice genetically deficient in LFA-1 that were challenged with i.p. Streptococcus pneumoniae had increased bacterial colony counts in spleen and liver, increased incidence of otitis media and meningitis/encephalitis, and increased mortality compared with wild type mice (57). The increase in LFA-1 and VLA-4 in the current study is probably related to a direct ability of rhIL-7 to increase integrin expression as well as a potential ability of rhIL-7 to prevent apoptosis in cells that are expressing these integrins. A key finding is that rhIL-7 increased LFA-1 and VLA-4 in CD4 and CD8 T cells from sham-operated mice to an even greater extent than in the CLP-operated mice (Fig. 9). Because there will be no increased apoptosis in T cells from sham-operated mice, the ability of rhIL-7 to increase LFA-1 and VLA-4 in sham mice and, therefore, likely in the setting of sepsis as well, is probably a direct effect.
Importantly, rhIL-7 increased central and effector memory CD4 and CD8 T cells in mesenteric lymph nodes of septic mice. Multiple mechanisms likely contribute to this increase in cell number. Certainly, increases in Bcl-2 levels likely provide protection from apoptosis to these cells. In addition, increased Ki-67 staining (Fig. 5) indicates that rhIL-7 promotes cellular proliferation. Further, rhIL-7–driven increases in integrin expression could enhance T cell numbers by facilitating lymphocyte trafficking to and from sites of infection. Finally, rhIL-7 promotes the expression of chemokine receptors that promote lymphocyte migration (30). Beq et al. (30) recently showed that Rhesus macaques that received rhIL-7 had increased T cell expression of homing chemokine receptors, including CXCR4, CCR6, and CCR9, as well as increased tissue expression of numerous chemokines. This effect of rhIL-7 was associated with massive and rapid T cell migration from the blood into various organs, including lymph nodes and parts of the intestine. One intriguing possibility is that rhIL-7 promotes lymphocyte viability and, in doing so, allows them to respond to signals (i.e., chemokines) that let them increase the expression of integrins, migrate to sites of infection, and promote pathogen elimination.
In addition to IL-7’s ability to increase integrin expression, the present work demonstrates that it also increased the MFI of CD8 by ~50.3% ± 3.5% and 29.3% ± 3.2% on individual cytotoxic T cells in sham and CLP mice, respectively (Fig. 9). CD8 is a coreceptor for MHC class I molecules and binds Lck on the cytoplasmic face of the plasma membrane. The increased expression of CD8 and integrins induced by IL-7 likely leads to more sustained interactions at the immunologic synapse between the CD8 TCR and MHC class I and, thereby, improves cytotoxic T cell activation. The present findings represent the first report that IL-7 increases CD8 expression in a clinical disorder and are consistent with the one prior study on this topic by Park et al., who observed that signals from IL-7 and other common γ-chain cytokines transcriptionally increase CD8 coreceptor expression in individual CD8 T cells (58).
In addition to improving survival, the most significant effect of rhIL-7isitsabilitytorestore the DTH response insepsis. Patients with sepsis lose their DTH response to recall Ags, a finding that is emblematic of their severely impaired immune function (59). In addition to the preservation of functional T cells, a potential mechanism for the improved DTH response in septic mice treated with rhIL-7 may be IL-7’s reported ability to enhance T cell–APC interactions (20, 22, 23). The present work is consistent with studies by Fry et al. (42), who determined that IL-7 restored the ability of athymic T cell-depleted hosts that underwent adoptive transfer of lymph node cells to appropriately reject nonmatched skin grafts. They stated that the restoration of immune competence seemed to be mediated by a combination of inhibition of apoptosis, enhanced costimulation, and modulation of APC function.
In addition to its antiapoptotic effects, rhIL-7 acts to restore CD8 T cells that are depleted during sepsis by its ability to induce proliferation (25). Geiselhart et al. (25) showed that a 2-d administration of IL-7 in vivo increased basal proliferation of CD4 and CD8 T cells by 4- and 18-fold, respectively. In a recent National Cancer Institute study of 16 patients with refractory cancer, patients who received the highest doses of rhIL-7 had a >60% increase in spleen size and an ~2- and 4-fold increase in circulating CD4 T and CD8 T cells, respectively (26). These effects of rhIL-7 occurred within 14–28 d of therapy. Additional studies showed that >40% of CD4 T cells and >50% of CD8 T cells were positive for Ki67, a marker of cell proliferation. In animal studies, the ability of IL-7 to induce proliferation of T cells starts to occur within 48–72 h. In the current study, rhIL-7 caused an approximate doubling in CD8 T cell proliferation in septic mice at 72 h after administration of rhIL-7 (Fig. 5).
A not unexpected discovery was the increase in IL-7R expression on CD4 and CD8 T cells from septic mice compared with sham-operated mice (Fig 8). As noted in numerous studies (14, 60, 61), as well as documented in the current study (Figs. 1, 4), sepsis induces massive loss of immune effector cells, including CD4 and CD8 T cells. This loss in immune cells triggers homeostatic proliferative mechanisms that ultimately result in replenishment of tissue stores of CD4 and CD8 T cells in surviving animals. IL-7 is critical for a proper homeostatic proliferative response, and the increased IL-7R expression in CD4 and CD8 T cells likely serves to enhance IL-7’s effect (20, 24, 26). T cells from septic mice had markedly upregulated IL-7Rs (CD127), and septic mice had an increase in circulating IL-7 compared with sham mice. Research indicates that the amount of IL-7R expression on a cell determines how vigorously the cell responds to IL-7 (31). Previous work from our group showed that sepsis inhibits homeostatic proliferation in CD4 T cells (33, 34); the ability of rhIL-7 to increase proliferation in CD8 T cells from septic mice (Fig. 5) may be advantageous in overcoming this inhibition.
A theoretical advantage of the use of rhIL-7 in sepsis is that it is unlikely to exacerbate the initial hyperinflammatory phase that usually precedes the more sustained secondary hypoinflammatory stage of sepsis (26–29). The early phase of sepsis is often characterized by a proinflammatory cytokine-mediated syndrome of fever, hypotension or shock, and acute lung injury (2, 4, 12). The administration of agents that accentuate the inflammation may worsen early mortality in sepsis (62). Unlike IL-2, a closely related cytokine, IL-7 rarely induces fever, capillary leak syndrome, or other clinical abnormalities related to the release of excessive proinflammatory cytokines (26–29). Studies show that IL-7 can induce proliferation in the absence of cytokine production and without increases in most lymphocyte-activation markers (22). One potential explanation for the fact that IL-7 does not exacerbate inflammation is related to the fact that as T cells become activated, there is downregulation of IL-7R expression (31). This down-regulation of the receptor likely helps to prevent hyperactivation of the T cells. We speculate that rhIL-7 may preserve the potential for effector function of T cells without causing outright activation. This speculation is supported by the results of the splenocyte-incubation studies in which rhIL-7 reversed the defect in sepsis-inducted IFN-γ production but did not increase IFN-γ production in the sham treated-mice.
In conclusion, although there are a number of potentially promising therapies in sepsis, we speculate that rhIL-7 is particularly attractive because it ameliorates many of the key pathophysiologic processes that are believed to be central to the lethality of sepsis. Specifically, rhIL-7 blocks sepsis-induced depletion of CD4 and CD8 T cells, enhances lymphocyte recruitment, prevents the sepsis-induced loss in immunity as evidenced by preserved DTH response, does not exacerbate the proinflammatory response in sepsis, and improves survival. If current clinical trials of rhIL-7 continue to demonstrate its safety in multiple patient populations, rhIL-7 could move rapidly into clinical trials in sepsis.
Acknowledgments
This work was supported in part by National Institutes of Health Grants GM44118 and GM55194 (to R.S.H.), GM72760 (to C.C.C.), AI057753 (to D.A.H.), and EY015570 and EY06765 (to T.A.F.); the Alan A. and Edith L. Wolff Foundation; and the Shriners of North America Project #8560.
Abbreviations used in this paper
- CLP
cecal ligation and puncture
- DTH
delayed-type hypersensitivity
- MFI
mean fluorescence intensity
- qRT-PCR
quantitative real-time PCR
- rhIL-7
recombinant human IL-7
- UCCM
University of Cincinnati College of Medicine
- WUSM
Washington University School of Medicine
Footnotes
The online version of this article contains supplemental material.
Disclosures
Dr. Michel Morre is the CEO of Cytheris Corporation, a company that makes IL-7 for clinical trials. The other authors have no financial conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Monneret G. How to identify systemic sepsis-induced immunoparalysis. Advances in Sepsis. 2005;4:42–49. [Google Scholar]
- 3.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 4.Hotchkiss RS, Coopersmith CM, McDunn JE, Ferguson TA. The sepsis seesaw: tilting toward immunosuppression. Nat Med. 2009;15:496–497. doi: 10.1038/nm0509-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiramatsu M, Hotchkiss RS, Karl IE, Buchman TG. Cecal ligation and puncture (CLP) induces apoptosis in thymus, spleen, lung, and gut by an endotoxin and TNF-independent pathway. Shock. 1997;7:247–253. doi: 10.1097/00024382-199704000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Chung CS, Xu YX, Wang W, Chaudry IH, Ayala A. Is Fas ligand or endotoxin responsible for mucosal lymphocyte apoptosis in sepsis? Arch Surg. 1998;133:1213–1220. doi: 10.1001/archsurg.133.11.1213. [DOI] [PubMed] [Google Scholar]
- 7.Hotchkiss RS, Swanson PE, Freeman BD, Tinsley KW, Cobb JP, Matuschak GM, Buchman TG, Karl IE. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit Care Med. 1999;27:1230–1251. doi: 10.1097/00003246-199907000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Felmet KA, Hall MW, Clark RS, Jaffe R, Carcillo JA. Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J Immunol. 2005;174:3765–3772. doi: 10.4049/jimmunol.174.6.3765. [DOI] [PubMed] [Google Scholar]
- 9.Toti P, De Felice C, Occhini R, Schuerfeld K, Stumpo M, Epistolato MC, Vatti R, Buonocore G. Spleen depletion in neonatal sepsis and chorioamnionitis. Am J Clin Pathol. 2004;122:765–771. doi: 10.1309/RV6E-9BMC-9954-A2WU. [DOI] [PubMed] [Google Scholar]
- 10.Le Tulzo Y, Pangault C, Gacouin A, Guilloux V, Tribut O, Amiot L, Tattevin P, Thomas R, Fauchet R, Drénou B. Early circulating lymphocyte apoptosis in human septic shock is associated with poor outcome. Shock. 2002;18:487–494. doi: 10.1097/00024382-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hotchkiss RS, Osmon SB, Chang KC, Wagner TH, Coopersmith CM, Karl IE. Accelerated lymphocyte death in sepsis occurs by both the death receptor and mitochondrial pathways. J Immunol. 2005;174:5110–5118. doi: 10.4049/jimmunol.174.8.5110. [DOI] [PubMed] [Google Scholar]
- 12.Riedemann NC, Guo RF, Ward PA. The enigma of sepsis. J Clin Invest. 2003;112:460–467. doi: 10.1172/JCI19523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotchkiss RS, Swanson PE, Knudson CM, Chang KC, Cobb JP, Osborne DF, Zollner KM, Buchman TG, Korsmeyer SJ, Karl IE. Overexpression of Bcl-2 in transgenic mice decreases apoptosis and improves survival in sepsis. J Immunol. 1999;162:4148–4156. [PubMed] [Google Scholar]
- 14.Chung CS, Song GY, Lomas J, Simms HH, Chaudry IH, Ayala A. Inhibition of Fas/Fas ligand signaling improves septic survival: differential effects on macrophage apoptotic and functional capacity. J Leukoc Biol. 2003;74:344–351. doi: 10.1189/jlb.0102006. [DOI] [PubMed] [Google Scholar]
- 15.Oberholzer C, Oberholzer A, Clare-Salzler M, Moldawer LL. Apoptosis in sepsis: a new target for therapeutic exploration. FASEB J. 2001;15:879–892. doi: 10.1096/fj.00-058rev. [DOI] [PubMed] [Google Scholar]
- 16.Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18:1185–1191. doi: 10.1096/fj.03-1230com. [DOI] [PubMed] [Google Scholar]
- 17.Hotchkiss RS, Tinsley KW, Swanson PE, Chang KC, Cobb JP, Buchman TG, Korsmeyer SJ, Karl IE. Prevention of lymphocyte cell death in sepsis improves survival in mice. Proc Natl Acad Sci USA. 1999;96:14541–14546. doi: 10.1073/pnas.96.25.14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwata A, V, Stevenson M, Minard A, Tasch M, Tupper J, Lagasse E, Weissman I, Harlan JM, Winn RK. Over-expression of Bcl-2 provides protection in septic mice by a trans effect. J Immunol. 2003;171:3136–3141. doi: 10.4049/jimmunol.171.6.3136. [DOI] [PubMed] [Google Scholar]
- 19.Oberholzer C, Oberholzer A, Bahjat FR, Minter RM, Tannahill CL, Abouhamze A, LaFace D, Hutchins B, Clare-Salzler MJ, Moldawer LL. Targeted adenovirus-induced expression of IL-10 decreases thymic apoptosis and improves survival in murine sepsis. Proc Natl Acad Sci USA. 2001;98:11503–11508. doi: 10.1073/pnas.181338198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 21.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–679. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 22.Boyman O, Ramsey C, Kim DM, Sprent J, Surh CD. IL-7/anti-IL-7 mAb complexes restore T cell development and induce homeostatic T Cell expansion without lymphopenia. J Immunol. 2008;180:7265–7275. doi: 10.4049/jimmunol.180.11.7265. [DOI] [PubMed] [Google Scholar]
- 23.Alpdogan O, van den Brink MR. IL-7 and IL-15: therapeutic cytokines for immunodeficiency. Trends Immunol. 2005;26:56–64. doi: 10.1016/j.it.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 25.Geiselhart LA, Humphries CA, Gregorio TA, Mou S, Subleski J, Komschlies KL. IL-7 administration alters the CD4:CD8 ratio, increases T cell numbers, and increases T cell function in the absence of activation. J Immunol. 2001;166:3019–3027. doi: 10.4049/jimmunol.166.5.3019. [DOI] [PubMed] [Google Scholar]
- 26.Sportès C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Fleisher TA, Krumlauf MC, Babb RR, Chow CK, et al. Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med. 2008;205:1701–1714. doi: 10.1084/jem.20071681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg SA, Sportès C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwarz SL, Stetler-Stevenson M, Morton KE, Mavroukakis SA, Morre M, et al. IL-7 administration to humans leads to expansion of CD8+ and CD4+ cells but a relative decrease of CD4+ T-regulatory cells. J Immunother. 2006;29:313–319. doi: 10.1097/01.cji.0000210386.55951.c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy Y, Lacabaratz C, Weiss L, Viard JP, Goujard C, Lelièvre JD, Boué F, Molina JM, Rouzioux C, Avettand-Fénoêl V, et al. Enhanced T cell recovery in HIV-1-infected adults through IL-7 treatment. J Clin Invest. 2009;119:997–1007. doi: 10.1172/JCI38052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sereti I, Dunham RM, Spritzler J, Aga E, Proschan MA, Medvik K, Battaglia CA, Landay AL, Pahwa S, Fischl MA, et al. ACTG 5214 Study Team. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. 2009;113:6304–6314. doi: 10.1182/blood-2008-10-186601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beq S, Rozlan S, Gautier D, Parker R, Mersseman V, Schilte C, Assouline B, Rancé I, Lavedan P, Morre M, Cheynier R. Injection of glycosylated recombinant simian IL-7 provokes rapid and massive T-cell homing in rhesus macaques. Blood. 2009;114:816–825. doi: 10.1182/blood-2008-11-191288. [DOI] [PubMed] [Google Scholar]
- 31.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 32.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 33.Unsinger J, Herndon JM, Davis CG, Muenzer JT, Hotchkiss RS, Ferguson TA. The role of TCR engagement and activation-induced cell death in sepsis-induced T cell apoptosis. J Immunol. 2006;177:7968–7973. doi: 10.4049/jimmunol.177.11.7968. [DOI] [PubMed] [Google Scholar]
- 34.Unsinger J, Kazama H, McDonough JS, Hotchkiss RS, Ferguson TA. Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury. J Leukoc Biol. 2009;85:382–390. doi: 10.1189/jlb.0808491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang KC, Unsinger J, Davis CG, Schwulst SJ, Muenzer JT, Strasser A, Hotchkiss RS. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. 2007;21:708–719. doi: 10.1096/fj.06-6805com. [DOI] [PubMed] [Google Scholar]
- 36.Brahmamdam P, Watanabe E, Unsinger J, Chang KC, Schierding W, Hoekzema AS, Zhou TT, McDonough JS, Holemon H, Heidel JD, et al. Targeted delivery of siRNA to cell death proteins in sepsis. Shock. 2009;32:131–139. doi: 10.1097/SHK.0b013e318194bcee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coopersmith CM, Stromberg PE, Davis CG, Dunne WM, Amiot DM, 2nd, Karl IE, Hotchkiss RS, Buchman TG. Sepsis from Pseudomonas aeruginosa pneumonia decreases intestinal proliferation and induces gut epithelial cell cycle arrest. Crit Care Med. 2003;31:1630–1637. doi: 10.1097/01.CCM.0000055385.29232.11. [DOI] [PubMed] [Google Scholar]
- 38.Zantl N, Uebe A, Neumann B, Wagner H, Siewert JR, Holzmann B, Heidecke CD, Pfeffer K. Essential role of gamma interferon in survival of colon ascendens stent peritonitis, a novel murine model of abdominal sepsis. Infect Immun. 1998;66:2300–2309. doi: 10.1128/iai.66.5.2300-2309.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Döcke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, Volk HD, Kox W. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. 1997;3:678–681. doi: 10.1038/nm0697-678. [DOI] [PubMed] [Google Scholar]
- 40.Hotchkiss RS, Chang KC, Grayson MH, Tinsley KW, Dunne BS, Davis CG, Osborne DF, Karl IE. Adoptive transfer of apoptotic splenocytes worsens survival, whereas adoptive transfer of necrotic splenocytes improves survival in sepsis. Proc Natl Acad Sci USA. 2003;100:6724–6729. doi: 10.1073/pnas.1031788100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prass K, Meisel C, Höflich C, Braun J, Halle E, Wolf T, Ruscher K, Victorov IV, Priller J, Dirnagl U, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J Exp Med. 2003;198:725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fry TJ, Christensen BL, Komschlies KL, Gress RE, Mackall CL. Interleukin-7 restores immunity in athymic T-cell-depleted hosts. Blood. 2001;97:1525–1533. doi: 10.1182/blood.v97.6.1525. [DOI] [PubMed] [Google Scholar]
- 43.Riedemann NC, Guo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–524. doi: 10.1038/nm0503-517. [DOI] [PubMed] [Google Scholar]
- 44.Oberholzer A, Oberholzer C, Moldawer LL. Sepsis syndromes: understanding the role of innate and acquired immunity. Shock. 2001;16:83–96. doi: 10.1097/00024382-200116020-00001. [DOI] [PubMed] [Google Scholar]
- 45.Hotchkiss RS, I, Karl E. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 46.Murphy T, Paterson H, Rogers S, Mannick JA, Lederer JA. Use of intracellular cytokine staining and bacterial superantigen to document suppression of the adaptive immune system in injured patients. Ann Surg. 2003;238:401–410. doi: 10.1097/01.sla.0000086661.45300.14. discussion 410–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang X, Venet F, Wang YL, Lepape A, Yuan Z, Chen Y, Swan R, Kherouf H, Monneret G, Chung CS, Ayala A. PD-1 expression by macrophages plays a pathologic role in altering microbial clearance and the innate inflammatory response to sepsis. Proc Natl Acad Sci USA. 2009;106:6303–6308. doi: 10.1073/pnas.0809422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao Y, Herndon JM, Zhang H, Griffith TS, Ferguson TA. Antiinflammatory effects of CD95 ligand (FasL)-induced apoptosis. J Exp Med. 1998;188:887–896. doi: 10.1084/jem.188.5.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ferguson TA, Herndon J, Elzey B, Griffith TS, Schoenberger S, Green DR. Uptake of apoptotic antigen-coupled cells by lymphoid dendritic cells and cross-priming of CD8(+) T cells produce active immune unresponsiveness. J Immunol. 2002;168:5589–5595. doi: 10.4049/jimmunol.168.11.5589. [DOI] [PubMed] [Google Scholar]
- 50.Green DR, Beere HM. Apoptosis. Gone but not forgotten. Nature. 2000;405:28–29. doi: 10.1038/35011175. [DOI] [PubMed] [Google Scholar]
- 51.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wojciechowski S, Tripathi P, Bourdeau T, Acero L, Grimes HL, Katz JD, Finkelman FD, Hildeman DA. Bim/Bcl-2 balance is critical for maintaining naive and memory T cell homeostasis. J Exp Med. 2007;204:1665–1675. doi: 10.1084/jem.20070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finkelman FD, Madden KB, Morris SC, Holmes JM, Boiani N, Katona IM, Maliszewski CR. Anti-cytokine antibodies as carrier proteins. Prolongation of in vivo effects of exogenous cytokines by injection of cytokine-anti-cytokine antibody complexes. J Immunol. 1993;151:1235–1244. [PubMed] [Google Scholar]
- 54.Evans R, Patzak I, Svensson L, De Filippo K, Jones K, McDowall A, Hogg N. Integrins in immunity. J Cell Sci. 2009;122:215–225. doi: 10.1242/jcs.019117. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Li D, Nurieva R, Yang J, Sen M, Carreño R, Lu S, McIntyre BW, Molldrem JJ, Legge GB, Ma Q. LFA-1 affinity regulation is necessary for the activation and proliferation of naive T cells. J Biol Chem. 2009;284:12645–12653. doi: 10.1074/jbc.M807207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wohler J, Bullard D, Schoeb T, Barnum S. LFA-1 is critical for regulatory T cell homeostasis and function. Mol Immunol. 2009;46:2424–2428. doi: 10.1016/j.molimm.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prince JE, Brayton CF, Fossett MC, Durand JA, Kaplan SL, Smith CW, Ballantyne CM. The differential roles of LFA-1 and Mac-1 in host defense against systemic infection with Streptococcus pneumoniae. J Immunol. 2001;166:7362–7369. doi: 10.4049/jimmunol.166.12.7362. [DOI] [PubMed] [Google Scholar]
- 58.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;10:1027–1028. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 59.Meakins JL, Pietsch JB, Bubenick O, Kelly R, Rode H, Gordon J, MacLean LD. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann Surg. 1977;186:241–250. doi: 10.1097/00000658-197709000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hotchkiss RS, Tinsley KW, Swanson PE, Grayson MH, Osborne DF, Wagner TH, Cobb JP, Coopersmith C, Karl IE. Depletion of dendritic cells, but not macrophages, in patients with sepsis. J Immunol. 2002;168:2493–2500. doi: 10.4049/jimmunol.168.5.2493. [DOI] [PubMed] [Google Scholar]
- 61.Hotchkiss RS, Tinsley KW, Swanson PE, Schmieg RE, Jr, Hui JJ, Chang KC, Osborne DF, Freeman BD, Cobb JP, Buchman TG, Karl IE. Sepsis-induced apoptosis causes progressive profound depletion of B and CD4+ T lymphocytes in humans. J Immunol. 2001;166:6952–6963. doi: 10.4049/jimmunol.166.11.6952. [DOI] [PubMed] [Google Scholar]
- 62.Zeni F, Freeman B, Natanson C. Anti-inflammatory therapies to treat sepsis and septic shock: a reassessment. Crit Care Med. 1997;25:1095–1100. doi: 10.1097/00003246-199707000-00001. [DOI] [PubMed] [Google Scholar]