Abstract
This study aimed to elucidate the role of charge in mediating chondrocyte response to loading by employing synthetic 3D hydrogels. Specifically, neutral poly(ethylene glycol) (PEG) hydrogels were employed where negatively charged chondroitin sulfate (ChS), one of the main extracellular matrix components of cartilage, was systematically incorporated into the PEG network at 0%, 20% or 40% to control the fixed charge density. PEG hydrogels were employed as a control environment for extracellular events which occur as a result of loading, but which are not associated with a charged matrix (e.g., cell deformation and fluid flow). Freshly isolated bovine articular chondrocytes were embedded in the hydrogels and subject to dynamic mechanical stimulation (0.3 Hz, 15% amplitude strains, 6 hours) and assayed for nitric oxide production, cell proliferation, proteoglycan synthesis, and collagen deposition. In the absence of loading, incorporation of charge inhibited cell proliferation by ~75%, proteoglycan synthesis by ~22–50% depending on ChS content, but had no affect on collagen deposition. Dynamic loading had no effect on cellular responses in PEG hydrogels. However, dynamically loading 20% ChS gels inhibited nitrite production by 50%, cell proliferation by 40%, but stimulated proteoglycan and collagen deposition by 162% and 565%, respectively. Dynamic loading of 40% ChS hydrogels stimulated nitrite production by 62% and proteoglycan synthesis by 123%, but inhibited cell proliferation by 54% and collagen deposition by 52%. Upon removing the load and culturing under free swelling conditions for 36 hrs, the enhanced matrix synthesis observed in the 20% ChS gels was not maintained suggesting that loading is necessary to stimulate matrix production. In conclusion, extracellular events associated with a charged matrix has a dramatic affect on how chondrocytes respond to mechanical stimulation within these artificial 3D matrices suggesting that streaming potentials and/or dynamic changes in osmolarity may be important regulators of chondrocytes while cell deformation and fluid flow appear to have less of an effect.
Keywords: cartilage, chondrocyte, chondroitin sulfate, hydrogel, fixed charged density, dynamic load
1. Introduction
The extracellular matrix (ECM) of cartilage is composed of highly negatively charged aggrecan molecules intertwined among collagen type II fibrils. Aggrecan is made-up of predominantly sulfated glycosaminoglycans, namely chondroitin-4 sulfate, chondrotin-6 sulfate, and keratan sulfate, which contribute to the majority of the fixed negative density in the tissue (Maroudas et al., 1969; Haschall and Haschall, 1981; Frank and Grodzinsky, 1987). These negative charges are critical to the overall function of cartilage giving the tissue its ability to resist large compressive loads.
The biological effects of sulfated glycosaminoglycans have been widely studied for their potential therapeutic benefits. There is increasing evidence that chondroitin sulfate may act through chondro-protective and anti-inflammatory mechanisms to exert positive effects on chondrocytes. For example, exogenously delivering chondroitin sulfate to articular chondrocytes in vitro has been shown to lead to increased proteoglycan synthesis (Bassleer et al., 1998), inhibition of matrix degrading enzymes (Chan et al., 2005; Legendre et al., 2008) and decreased nitric oxide production (Legendre et al., 2008), a signaling molecule described as a mediator in osteoarthritis (Moncada et al., 1991; Sandell et al., 2001). Furthermore, several studies have described the benefits of chondroitin sulfate in applications for cartilage tissue engineering. For example, immobilizing chondroitin sulfate to synthetic scaffolds stimulated matrix synthesis by differentiated chondrocytes (Nishimoto et al., 2005; Hwang et al., 2007) and enhanced chondrogenesis of mesenchymal stem cells (Varghese et al., 2008).
The mechano-electrochemical properties of cartilage give rise to a whole host of extracellular events when the tissue is subject to dynamic mechanical loads, which ultimately influence chondrocyte function (Sah et al., 1989; Guilak et al., 1995; Kim et al., 1995; Mow et al., 1999; Hansen et al., 2001). The highly negatively charged matrix, at equilibrium, is shielded by positive ions, which are present in the interstitial fluid giving rise to osmotic swelling pressures. During dynamic loading, the mobile positive ions are forced in and out of the matrix creating an electric potential, commonly referred to as streaming potentials (Kim et al., 1995). Streaming potentials have been used as a non-destructive means for detecting proteoglycan loss associated with osteoarthritis (Kim et al., 1995; Légaré et al., 2002; Quenneville et al., 2004). Theoretical models have been developed to interpret streaming potentials and have suggested that streaming potentials play an important role in chondrocyte metabolism (Kim et al., 1995; Sun et al., 2004). Although, the exact mechanisms are not well understood. Recently, electrical stimulation was externally applied to chondrocyte-laden agarose gels to assess whether chondrocytes were sensitive to electrical stimuli in the absence of any mechanical signals (Akanji et al., 2008). Interestingly, matrix metabolism, cell proliferation and protein synthesis were not affected by electrical stimulation suggesting that perhaps the combined mechano-electrochemical signals are necessary.
Synthetic hydrogels offer a unique 3D culture environment where the macroscopic properties of the hydrogel can be readily controlled while multiple chemistries can be systematically incorporated into the hydrogel network to create multifunctional environments (Nicodemus et al., 2008). For example, the macroscopic properties of poly(ethylene glycol) (PEG) hydrogels have been tailored to match the mechanical properties of cartilage. When chondrocytes were encapsulated in PEG hydrogels exhibiting compressive moduli up to 900 kPa and cultured under free swelling or dynamically loaded conditions, these high moduli environments supported chondrocyte viability and matrix synthesis (Bryant and Anseth, 2002; Villanueva et al., 2008). To create 3D environments that better mimic the native ECM, several groups have modified glycosaminoglycans with crosslinkable groups (Smeds and Grinstaff, 2000; Bryant et al., 2004b; Li et al., 2004). By co-polymerizing multi-functional ECM analogs with synthetic chemistries, environments can be created with tightly controlled biological functionalities and macroscopic properties (Bryant et al., 2004b). These environments may serve to help isolate the role of ECM components in mediating cellular functions. For example, a recent study demonstrated that incorporating chondroitin sulfate into PEG hydrogels not only enhanced chondrogenesis of goat derived mesenchymal stem cells, but also appeared to inhibit the hypertrophic phenotype, suggesting a beneficial role for chondroitin sulfate in mediating chondrogenesis (Varghese et al., 2008).
Previous studies from our group have demonstrated that cell proliferation and matrix synthesis are either not affected or inhibited by dynamic loading during early culture times when freshly isolated bovine chondrocytes are encapsulated in pure PEG hydrogels (Villanueva et al., 2008). We hypothesized that incorporation of cartilage-specific matrix molecules may be important to initiate mechanotransduction events leading to enhanced tissue deposition. A number of studies have reported enhanced matrix synthesis in cartilage explants subject to physiological dynamic loading regimes suggesting an important role of the ECM in mediating mechanotransduction events in chondrocytes (Maroudas et al., 1969; Sah et al., 1989; Kim et al., 1995). Our goal for this study was to employ synthetic hydrogels based on bioinert PEG where chondroitin sulfate is systematically incorporated into the neutral PEG network to probe the role of fixed negative charges in mediating chondrocyte response under dynamic mechanical loading. Because dynamic loading induces several extracellular events independent of a charged matrix including cell deformation and fluid flow, we initially assessed the hydrogel macroscopic properties as well as cell deformation as a function of hydrogel formulation and fixed charge density (FCD). Neutral PEG hydrogels served as a control environment for extracellular events which occur as a result of loading, but which are not associated with a charged matrix. To assess the role of FCD on chondrocytes in the absence and presence of dynamic mechanical loading, chondrocyte response was measured by nitric oxide production, as a marker for intracellular signaling events, cell proliferation, which has been previously shown to be stimulated in freshly isolated bovine chondrocytes by dynamic loading in agarose constructs (Chowdhury et al., 2003; Lee and Bader, 1997), and extracellular matrix synthesis of newly deposited sulfated proteoglycans and collagen.
2. Results
2.1 Properties of the PEG and PEG-ChS gels
Synthetic 3D hydrogels were fabricated by co-polymerizing PEG divinyl macromers with chondroitin sulfate multivinyl macromers where the concentration of the two macromers in solution prior to polymerization was varied to control the chondroitin sulfate content and the fixed negative density in the resulting hydrogel. Three hydrogels were fabricated where the total macromer concentration was held constant for all gel formulations, but the PEG:ChS macromer ratio was varied by 100:0, 80:20, and 60:40 and are referred to as 0, 20 or 40% (w/w) ChS, respectively. The actual amount of chondroitin sulfate that was covalently attached and incorporated into the hydrogel network was determined by the 1,9-dimethylmethylene blue dye method. Based on the actual concentration of chondroitin sulfate in the hydrogels, the FCD was determined and the values are given in Table 1. In addition, literature values for fixed charged density of bovine articular cartilage are provided (Wang et al., 2002). PEG-ChS gels containing 20% ChS were estimated to have FCDs close to the range reported for bovine articular cartilage. PEG-ChS gels containing 40% ChS had estimated FCDs which were higher than native cartilage.
Table 1.
Estimated fixed charged density in PEG-ChS hydrogels
| Fixed Charged Density, mEq/mL | |
|---|---|
| Cartilage | 0.098–0.132a |
| 20% ChS Hydrogel | 0.095 ± 0.018 |
| 40% ChS Hydrogel | 0.197 ± 0.0012** |
Intrinsic fixed charge density from full depth healthy 1–2 month old bovine articular cartilage reported by (Wang et. al., 2002);
p<0.01 compared to PEG-ChS (80:20).
Due to differences in macromer molecular weight and degree of functionalization with crosslinkable moieties (i.e., double bonds) for the PEG and ChS macromers, the resulting double bond concentration (i.e., crosslinkable moieties) decreased with increasing chondroitin sulfate macromer (Table 2). The resulting hydrogel properties, the equilibrium mass swelling ratio, q, which is a measure of the amount of water the hydrogel imbibes, and the compressive modulus were measured and are also given in Table 2. An increase in ChS content resulted in significant increases in the equilibrium swelling ratio although the equilibrium water contents were greater than 91% for all gel formulations. The addition of ChS also significantly enhanced the compressive modulus of the hydrogels by ~60%, although higher amounts of ChS did not further influence the modulus.
Table 2.
PEG and PEG-ChS Hydrogel Formulations and Their Resulting Properties
| Hydrogel | PEGDMa (w/w) % | ChS-MAb (w/w) % | [DB](M)c | qd | Ke (kPa) |
|---|---|---|---|---|---|
| PEG-only | 100 | 0 | 0.065 | 9.6 ± 0.3 | 46 ± 5 |
| 20% ChS | 80 | 20 | 0.061 | 11.1 ± 0.3*** | 78 ± 8* |
| 40% ChS | 60 | 40 | 0.058 | 12.9 ± 0.2***, # | 70 ± 3* |
Poly(ethylene glycol) dimethacrylate (PEGDM) macromer concentration prior to polymerization;
Methacrylated chondroitin sulfate (ChS-MA) macromer concentration prior to polymerization;
Total double bond concentration ([DB]) in the macromer solution prior to polymerization based on ChS-MA (~48,700g/mol) (Bryant et. al., 2004b) substituted with 23 methacrylates per ChS molecule as determined by NMR and PEGDM (3000 g/mol);
Mass Swelling Ratio (q);
Tangent Compressive Modulus (K);
p<0.05,
p<0.001 compared to PEG-only gel;
p<0.001 compared to PEG-ChS gels (80:20).
2.2 Pericellular matrix development in PEG and PEG-ChS gels
In an effort to isolate and study the effects of fixed negative charges on chondrocyte response, we sought to identify early cultures times where there would be limited matrix deposited by the chondrocytes. Specifically, matrix deposition was assessed through immunofluorescence for chondroitin-6-sulfate and by Calcein AM, which stains the cytosol of live cells (Fig. 1). Immediately after photoencapsulation and within the first 12 hours of culture, live chondrocytes could be seen within the hydrogel, but with no observable signs of chondroitin sulfate deposition as evident by the lack of positive staining for chondroitin-6-sulfate. However, 24 hours post-encapsulation, a pericellular matrix containing chondroitin-6-sulfate was present surrounding the chondrocytes.
Fig. 1.
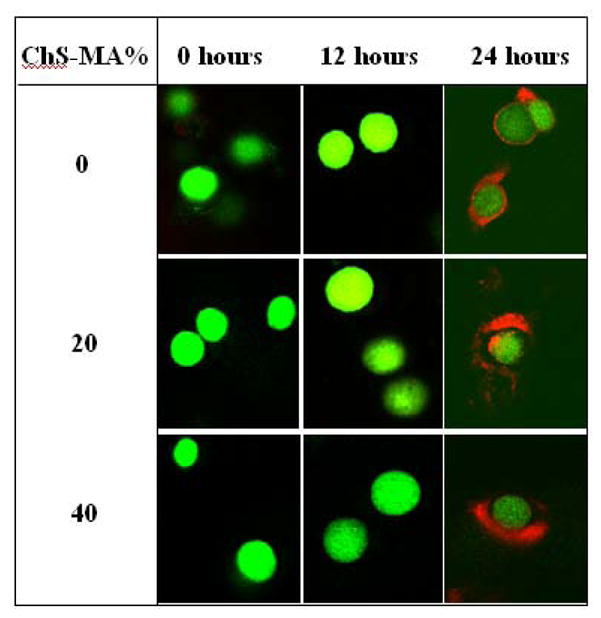
Confocal microscopy images of articular chondrocytes encapsulated in PEG-only and PEG-ChS gels. Live cells fluoresce green. Matrix deposition is stained for chondroitin-6 sulfate (red). No pericellular matrix is observed until 24 hours post-encapsulation. Original magnification is 40×.
Based on these findings, our experimental design is shown in Fig 2. Freshly isolated chondrocytes were encapsulated in PEG hydrogels, which were free-swollen for 6 hours to allow the cells to adjust to their new environment and then cultured for 6 hours under one of three conditions: i) free swelling, ii) uncompressed controls, where the constructs were placed in the bioreactor, but not subject to dynamic mechanical stimulation, or iii) subject to dynamic mechanical stimulation applied in a sinusoidal waveform at 0.3 Hz and 15% amplitude strain. After which time, the constructs were removed from the bioreactor and cultured for an additional 36 hours under free swelling conditions.
Fig. 2. Schematic of experimental design.
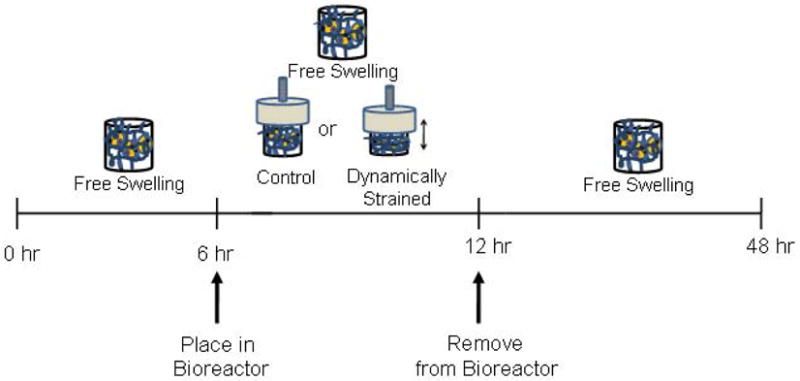
Cell-laden PEG-only and PEG-ChS gels were cultured under free swelling conditions for 6 hours to allow the cells to adjust to their new environment. The constructs were then cultured under free swelling, uncompressed controls or dynamically loaded (0.3Hz and 15% amplitude strain) conditions for 6 hours. The uncompressed control samples were placed under conditions, which mimic the bioreactor, but not subjected to compressive loading. The constructs were removed from the bioreactor and cultured under free swelling conditions for an additional 36 hours.
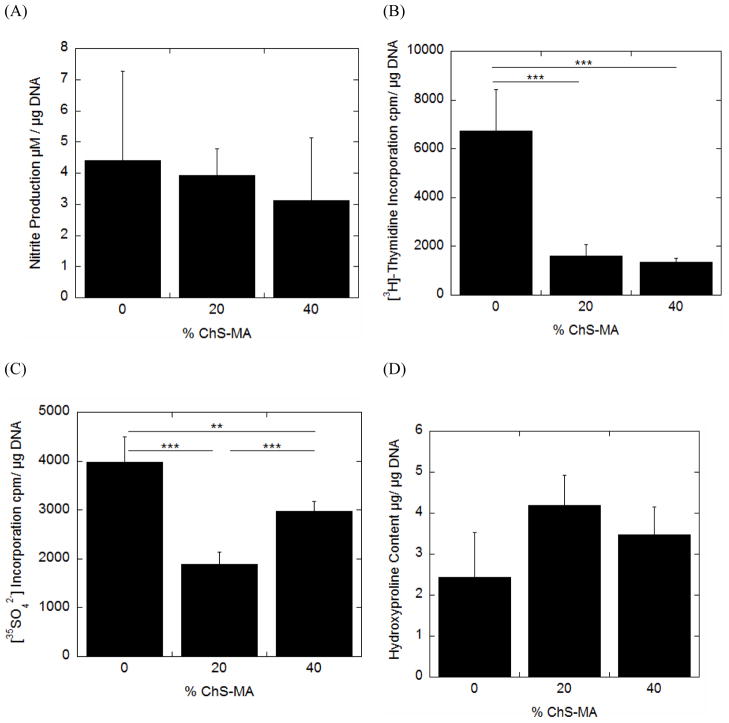
2.3 The effects of ChS incorporation into PEG on chondrocyte bioactivities in the absence of load
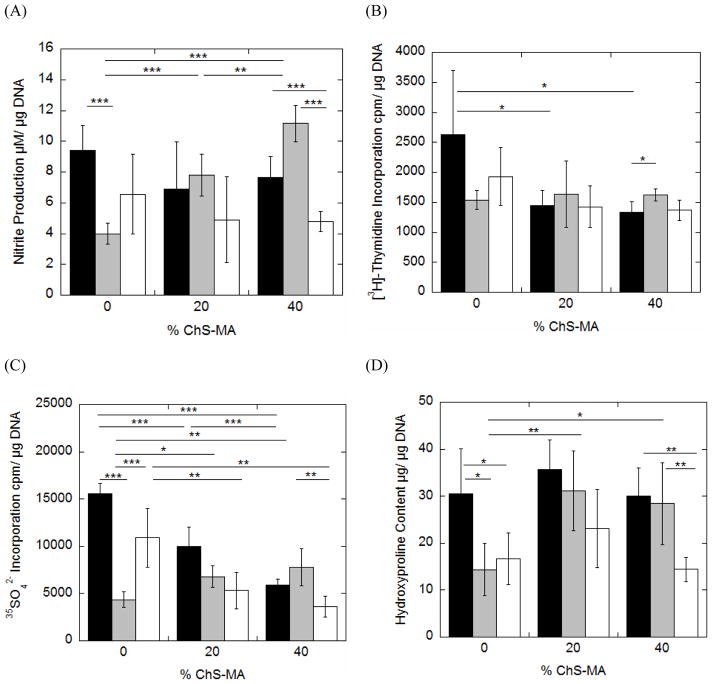
For each hydrogel formulation, chondrocyte bioactivity was assessed through nitric oxide production as measured by the stable end product, nitrite (Fig. 3A), cell proliferation ([3H]-thymidine incorporation) (Fig. 3B) and proteoglycan synthesis (35SO42− incorporation) (Fig. 3C) between 6 and 12 hours of culture (see Fig. 2). Total collagen content (Fig. 3D) measured after 12 hours of culture represents total accumulation over the 12 hour period. Nitrite production was not affected by the incorporation of ChS. Cell proliferation was reduced approximately 4-fold with the incorporation of ChS, but was independent of ChS concentration. Proteoglycan synthesis was reduced by ~50% and 25% for the 20 and 40% ChS hydrogels, respectively. No significant changes in total collagen content were observed among the three gel formulations.
Fig. 3.
The effects of incorporating negatively charged chondroitin sulfate into neutral PEG hydrogels when cultured under free swelling conditions on nitric oxide production as measured by nitrite production (A), cell proliferation (B), proteoglycan synthesis (C) and total collagen content (D) for chondrocytes encapsulated in PEG-only or PEG-ChS gels. Nitrite production, cell proliferation and proteoglycan synthesis were measured for the culture period between 6 and 12 hours. Total collagen content, as measured through hydroxyproline, represents the total accumulation over the first 12 hour culture period. Each cell response is normalized to total DNA content and reported as mean ± standard deviation (n =4–5); *p <0.05, **p<0.01, ***p<0.001.
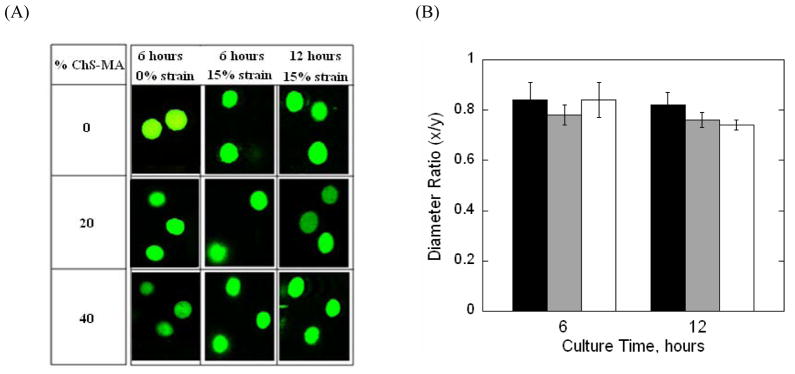
2.4 Chondrocyte Deformation
To assess the level of deformation experienced by the chondrocytes when subjected to dynamic compressive strains, chondrocyte morphology for cells encapsulated in PEG-only or PEG-ChS gels was assessed immediately prior (i.e., at 6 hours of culture) and after (i.e., at 12 hours of culture) being subjected to dynamic mechanical stimulation. Specifically, chondrocyte morphology was assessed using a custom designed cell straining rig associated with a confocal laser scanning microscope. A 15% strain was applied to the hydrogel constructs to mimic the strains in the bioreactor. Representative images of encapsulated cells within the strained hydrogel are shown in Fig. 4A. The application of a compressive strain resulted in a change in cellular morphology from a generally rounded phenotype to an oblate ellipsoid morphology for all three gel formulations at 6 and 12 hours of culture. To quantify cell deformation, a diameter ratio (x/y) was determined by measuring the maximum cell diameters parallel to the direction of the applied strain (x) and the cell diameter perpendicular to the direction of the applied strain (y) (Fig. 4B). Prior and after placing the gels in the bioreactor, diameter ratios under an applied 15% strain were similar across all gel formulations.
Fig. 4.
(A) Representative confocal microscopy images of live chondrocytes (green) encapsulated in PEG-only or PEG-ChS gels and subjected to either not strain or a gross unconfined, 15% static compressive strains. Gels were imaged before (at 6 hours) being placed into the bioreactor and after (at the first 12 hours of culture) being subjected to dynamic loading. Prior to placing in the bioreactor, cellular morphology was generally round, but with the application of a 15% strain, the cells adopted an oblate ellipsoid morphology for all gel formulations before and after being subject to loading; Original magnification is 40×. (B) Chondrocyte deformation was quantitatively assessed under a gross, unconfined, 15% static compressive strain before and after dynamic loading for PEG-only (black bars) and PEG-ChS gels containing 20% ChS-MA (gray bars) and 40% ChS-MA (white bars). Cellular deformation was quantified by a diameter ratio (x/y) defined by the ratio of the cell diameter parallel to the direction of strain (x) and the cell diameter perpendicular to the direction of the applied strain (y) measured at full width half maximum height; **p<0.01.
2.5 The effects of ChS incorporation into PEG on chondrocyte bioactivities in the presence of dynamic load
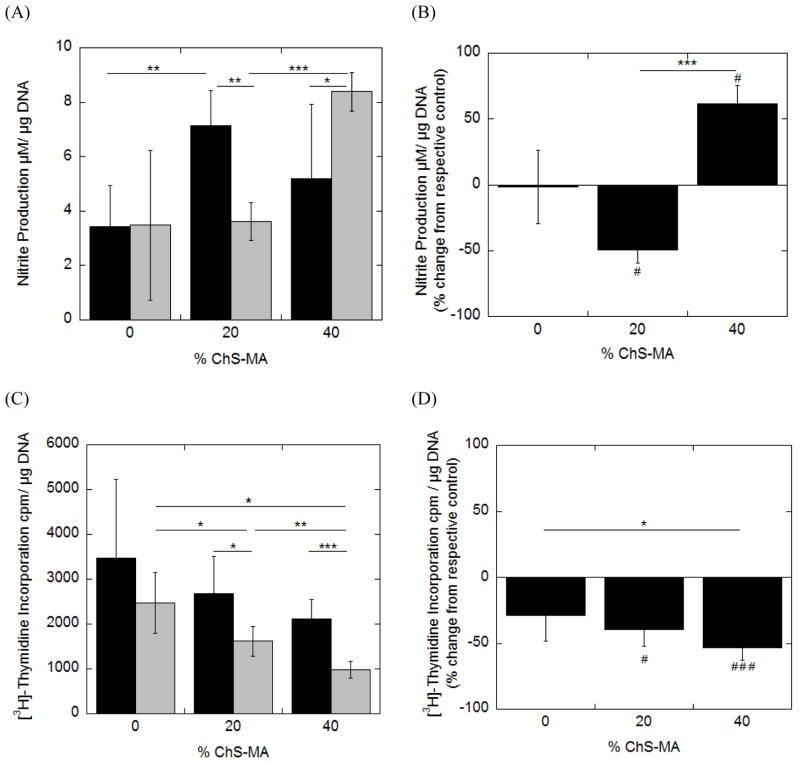
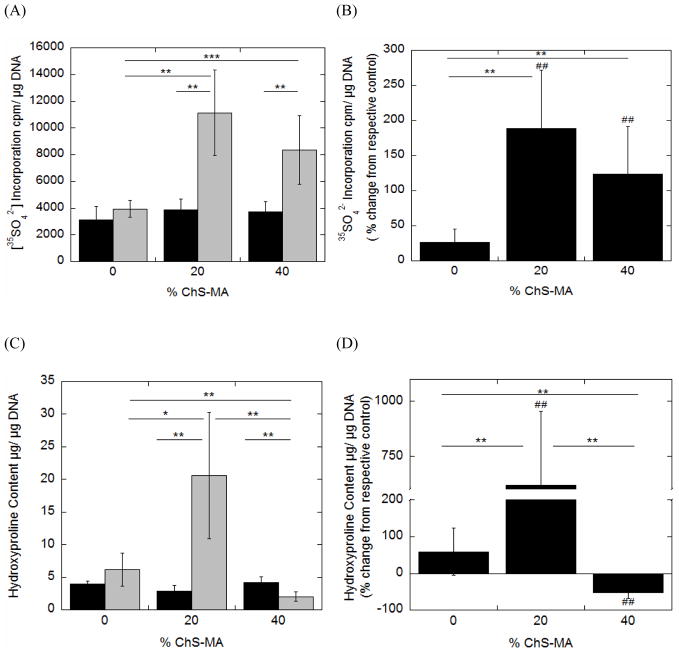
Hydrogels were placed into the bioreactor either as uncompressed controls or dynamically strained specimen (Fig. 2). Nitrite production (Fig. 5A), cell proliferation (Fig. 5C), proteoglycan synthesis (Fig. 6A) and collagen content (Fig. 6C) were measured immediately upon removal from the bioreactor. The mean response from the dynamically loaded samples was normalized to their respective uncompressed controls in an effort to isolate the effects of dynamic loading on cell response (Fig. 5B, 5D, 6B, 6D, respectively).
Fig. 5.
The effects of incorporating negatively charged chondroitin sulfate into PEG hydrogels and applying dynamic mechanical stimulation on nitric oxide production as measured by nitrite production (A,B) and cell proliferation (C,D) for chondrocytes encapsulated in PEG-only or PEG-ChS gels and cultured as uncompressed controls (black bars) or dynamically strained specimen (gray bars). To isolate the effects of dynamic loading, mean responses for the dynamically strained specimen were normalized to their respective uncompressed control counterparts and presented as a percent change from uncompressed control (B,D). Data are given by mean ± standard deviation (n =4–5); *p < 0.05, **p<0.01, ***p<0.001; # p<0.05, ##p<0.01, ###p<0.001. An asterisk(s) above a column in B and D panels denotes significance from uncompressed control.
Fig. 6.
The effects of incorporating negatively charged chondroitin sulfate into PEG hydrogels and applying dynamic mechanical stimulation on proteoglycan synthesis (A,B) and total collagen content (C,D) for chondrocytes encapsulated in PEG-only or PEG-ChS gels and cultured as uncompressed controls (black bars) or dynamically strained specimen (gray bars). Proteoglycan synthesis represents synthesis that occurred during the loading culture period. Total collagen content represents the total collagen produced over the first 12 hour culture period. To isolate the effects of dynamic loading, mean responses for the dynamically strained specimen were normalized to their respective uncompressed control counterparts and presented as a percent change from uncompressed control (B,D). Data are given by mean ± standard deviation (n =4–5); *p < 0.05, **p<0.01, ***p<0.001; # p<0.05, ##p<0.01, ###p<0.001. An asterisk(s) above a column in B and D panels denotes significance from uncompressed control.
Nitrite production was significantly higher in the 20% ChS uncompressed control specimen when compared to the PEG-only uncompressed controls, although no significant differences were found for the 40% ChS uncompressed controls when compared to the other gel formulations. Dynamic loading, however, led to a ~50% higher production of nitrite in the 40% ChS compared to the other gel formulations. When normalized to their respective uncompressed controls, dynamic loading had no effect on nitrite production for the PEG-only gels, but inhibited nitrite production by 50% in the 20% ChS gels and stimulated nitrite production by 62% in the 40% ChS gels.
Cell proliferation was statistically similar for all gel formulations in the uncompressed control specimen. With the application of dynamic loading, cell proliferation decreased significantly with increasing concentrations of ChS in the hydrogel. When normalized to their respective uncompressed controls, loading did not affect cell proliferation in the PEG-only gels, but significantly inhibited cell proliferation in the ChS containing gels. Fixed charged density and mechanical loading were significant factors impacting cell proliferation (Table 3).
Table 3.
ANOVA Two-Factor Fixed Effects Model for Fixed Charged Density and Dynamic Loading
| Factor* | Measure | p-value |
|---|---|---|
| Fixed Charged Density | Nitrite Production | 0.17 |
| Cell Proliferation | 6.9×10−6 | |
| Proteoglycan Synthesis | 0.08 | |
| Collagen Content | 0.02 | |
| Mechanical Load | Nitrite Production | 0.35 |
| Cell Proliferation | 0.01 | |
| Proteoglycan Synthesis | 1.8×10−6 | |
| Collagen Content | 0.01 |
This analysis was performed on the cellular responses during the dynamical loading culture period.
Proteoglycan synthesis was statistically similar for all gel formulations under uncompressed control conditions. However, incorporation of ChS resulted in a ~3–4-fold increase in proteoglycan synthesis when compared to the PEG-only gels under dynamic loading. When normalized to their respective uncompressed controls, loading did not influence proteoglycan synthesis in the PEG-only gels, but resulted in a ~162% and ~123% increase in proteoglycan synthesis for the hydrogels containing 20% and 40% ChS, respectively. Mechanical loading, but not FCD, was a significant factor impacting proteoglycan synthesis (Table 3).
Total collagen content was statistically similar for all gel formulations under uncompressed control conditions. However, the incorporation of 20% ChS into PEG hydrogels led to 4-fold increase in the total collagen content when compared to PEG-only gels under dynamic loading. Higher amounts of ChS (40% ChS) inhibited collagen deposition and was ~52% lower compared to the PEG-only gels. When normalized to their respective uncompressed controls, loading resulted in a 565% increase in collagen deposition within the 20% ChS hydrogels while loading inhibited collagen deposition by 52% in the 40% ChS. Fixed charged density and mechanical loading were significant factors impacting collagen production (Table 3).
2.6 The effects of longer-term culture post-loading on chondrocyte bioactivities
After removing the specimen from the bioreactor, uncompressed control and dynamically loaded specimen were cultured for an additional 36 hours to elucidate whether an initial loading application had longer-term benefits on cellular function. Free swelling specimen were also cultured for an additional 36 hours. Chondrocyte bioactivity was measured by nitrite production (Fig. 7A), cell proliferation (Fig. 7B), and proteoglycan synthesis (Fig. 7C) during the culture time between 12 and 48 hours, while total collagen content (Fig. 7D) represented the total amount accumulated over the entire 48 hour culture period.
Fig. 7.
Evaluation of the longer-term effects of the loading period on nitric oxide production as measured by nitrite (A), cell proliferation (B), proteoglycan synthesis (C) and total collagen content (D) by chondrocytes encapsulated in PEG-only or PEG-ChS gels. After the 6 hour loading period, uncompressed control (gray bars) and dynamically strained specimen (white bars) were removed from the bioreactor and cultured for 36 hours under free swelling conditions. Free swelling conditions (black bars) were also evaluated. Nitrite production, proteoglycan synthesis and cell proliferation were evaluated during the 36 hour free swelling culture period while total collagen content represents the entire 48 hour culture period. Data are given by mean ± standard deviation (n =4–5); *p <0.05, **p<0.01, ***p<0.001.
Nitrite production was statistically similar as a function of hydrogel formulation for the free swelling and the dynamically loaded conditions. However, there was a significant increase in nitrite production with increasing ChS concentrations for the uncompressed control specimen. In the PEG-only gels, nitrite production was significantly lower in the uncompressed control gels compared to the free swelling specimen. No differences in nitrite production were found in the 20% ChS gels for the three different culture conditions. In the 40% ChS gels, nitrite production was lowest in the dynamically loaded specimen.
For cell proliferation, there were generally no significant differences among gel formations and culture conditions with a few notable exceptions. Under the free swelling conditions, incorporation of ChS into the hydrogel significantly inhibited cell proliferation. There was a slight, but significant increase in cell proliferation in the uncompressed control gels for the 40% ChS when compared to the free swelling gels.
For proteoglycan synthesis, there was a significant decrease in proteoglycan synthesis with increasing ChS content in the hydrogels for the free swelling culture conditions and the dynamically loaded conditions. However, the uncompressed control specimen did not show any differences among the three gel formulations. Within the PEG-only gels, proteoglycan synthesis was lowest in the uncompressed control specimen, but was statistically similar for the free swelling and dynamically loaded specimen. There were no differences in proteoglycan synthesis for the 20% ChS gels with culture condition. For the dynamically loaded 40% ChS gels, proteoglycan synthesis was significantly reduced compared to the uncompressed control gels.
For total collagen content, there were no differences in collagen content for the free swelling conditions for the three different formulations and no differences among the loaded specimen. However, collagen contents were significantly higher in the gels containing 20% and 40% ChS when compared to PEG-only gels and when cultured under uncompressed control conditions. In the PEG-only gels, collagen contents were significantly lower in the uncompressed control and dynamically loaded specimen when compared to the free swelling. However, there were no differences in collagen content among culture conditions for the 20% ChS gels. In the 40% ChS gels, collagen content was significantly lower in the dynamically loaded gels when compared to the other culture conditions.
3. Discussion
The chemistry of synthetic hydrogels was manipulated in an effort to introduce fixed negative charges into the 3D environment to probe their role in mediating chondrocyte response to mechanical stimulation. To account for extracellular events that occur independent of a charged matrix during mechanical stimulation, including cell deformation and fluid flow, neutral PEG-only hydrogels were employed. However, incorporation of charge into the hydrogels generally influences the material properties of the hydrogel leading to higher water contents and mechanical properties, which may influence the biomechanical cues. Although there were differences in mechanical properties among the hydrogel formulations, the level of cellular deformation and hence the strain sensed by the cells was similar over the loading period. Fluid flow is also known to influence chondrocyte response, which is dictated in part by the crosslinking of collagen in native cartilage (Korhonen et al., 2006). In crosslinked hydrogels, the degree of swelling provides a measure of the degree of crosslinking (Peppas et al., 2006). Overall the gels exhibited high water contents ranging from 91 to 93% with increasing ChS concentration suggesting that differences in fluid flow is likely minimal among the gel formulations. Differences in gel crosslinking may also impact diffusional properties of large molecules, such as growth factors, and subsequently impact how cells respond to the different 3D culture environments (Peppas et al., 2006). However, we expect this effect to be minimal due to the inherently high swelling ratios of the hydrogels where the mesh sizes are estimated to be between ~125–185 Å for the three hydrogel formulations (Mason et al., 2001). Therefore, neutral PEG hydrogels may serve to remove many of the effects that result due to loading in the absence of charge (e.g., cell deformation, fluid flow), while the PEG-ChS hydrogels allow us to probe the role of FCD in the absence and in the presence of loading. Although, it is important to point out that additional studies are necessary to confirm whether or not these small changes in swelling and mechanical properties have an impact on cellular responses.
Interestingly, the incorporation of chondroitin sulfate had a negative impact on cell proliferation and proteoglycan synthesis. This finding was unexpected based on previous reports describing the positive pharmacological effects of chondroitin sulfate (Bassleer et al., 1998; Chan et al., 2005; Legendre et al., 2008). Under free swelling conditions, the incorporation of charge into the hydrogel network will influence the osmotic environment within the hydrogel leading to higher osmolarities. Chondrocytes are known to be highly sensitive to changes in their osmotic environment (Urban et al., 1993). Here, chondrocytes were encapsulated in culture medium, which was adjusted to match the physiologic osmotic environment of native cartilage (~400 mOsm). Recent studies from our group showed that chondrocyte survival was significantly enhanced during photoencapsulation in PEG hydrogels when a physiological osmolarity medium was employed (Villanueva et al., 2009). However, the osmolarity within the hydrogel environment will likely be above physiological levels as a result of the charged matrix (Mow et al., 1999). Several studies have reported decreases in chondrocyte matrix synthesis when cultured in medium with osmolarities above or below physiological levels (Urban et al., 1993; Negoro et al., 2008). In addition, a hyper-osmotic environment will lead to cell shrinkage (Erickson et al., 2001), which has been shown to be inhibitory for cell proliferation (Lang et al., 2000). Interestingly, the 40% ChS hydrogels resulted in higher proteoglycan synthesis compared to the 20% ChS hydrogels, although still lower than PEG hydrogels. The presence of fixed negative charges may lead to sequestering of positively charged growth factors, which could have a positive effect of cells. The increased ability to sequester growth factors in the higher FCD gels may have been able to partly overcome the adverse effects associated with increased osmolarity. The incorporation of negative charges may also facilitate adsorption of proteins which promote cell adhesion and therefore cell-hydrogel interactions to affect how cells sense and respond to the different 3D culture environments(Thevenot et al., 2008). Additional studies are necessary to elucidate the role of charge in mediating chondrocyte response and more specifically in the inhibition of cell proliferation and proteoglycan synthesis. Interestingly, nitrite and collagen were not affected by the incorporation of charge into the hydrogel network and therefore may be less sensitive to the presence of electrical charge, changes in osmolarity, and/or cell-hydrogel interactions.
Chondrocyte response was markedly different when the charged hydrogel matrices were subjected to dynamic loading. Dynamic loading had no effect on chondrocyte response in the neutral PEG hydrogels, suggesting that cellular strain at the levels reported here and the induction of fluid flow by 0.3 Hz were not sufficient to mediate changes in chondrocyte response to loading, at least at early culture times. These findings are similar to findings previously reported by the authors (Bryant et al., 2004a; Villanueva et al., 2008). The introduction of 20% ChS into the 3D hydrogel environment, however, led to superior chondrocyte response through stimulation of matrix synthesis and inhibition of nitric oxide, a signaling molecule thought to mediate cartilage degradation. On the other hand, dynamic loading of the 40% ChS hydrogels stimulated nitric oxide production and collagen production, but did lead to increased proteoglycan synthesis although not to the same degree as the 20% ChS hydrogels. Numerous studies have reported that physiological levels of mechanical stimulation enhance matrix deposition in cartilage explants, which likely results from a combination of mechano-electrochemical signals (Sah et al., 1989; Kim et al., 1995; Lee et al., 1997; Carver and Heath, 1999; Mauck et al., 2000). In the 20% ChS gels, the FCD was estimated to be near the range reported for bovine cartilage while the FCD of the 40% ChS gels resulted in FCD’s higher than physiological levels for bovine (Wang et al., 2002), although these FCDs have been reported to be physiological in human tissues (Maroudas et al., 1969; Kim et al., 1995; Mow et al., 1999). Our findings suggest that an electrically charged matrix, as presented by these artificial matrices, impacts how chondrocytes respond to mechanical stimulation.
Understanding the mechanisms by which dynamically loading a charged matrix impacts cell response, however, is challenging because the dynamic environment is complex resulting in ion flow, streaming potentials, and dynamic changes in osmolarity (Mow et al., 1999). The magnitude of the streaming potentials generated during loading depends non-monotonically on the fixed charge density of the tissue, where physiologically levels of FCD result in maximum streaming potentials under a specific applied stress (Gu et al., 1993). The effective FCD will also increase during compression of the hydrogel indicating that the maximum FCD within our hydrogels during dynamic loading will be higher than that reported in Table 1. Chondrocytes are known to be sensitive to dynamic changes in osmolarity resulting in changes in cell volume (Erickson et al., 2001), calcium signaling, upregulation of ECM genes (Chao et al., 2006), and matrix synthesis (Negoro et al., 2008). In addition, the presence of electrical charge may influence the type of matrix deposited where any differences in the composition of the pericellular matrix will impact the biochemical and biomechanical cues perceived by the cells (Guilak et al., 2006). Although there was no detectable presence of chondroitin sulfate during the first 12 hours, it is possible that other matrix molecules may have been deposited in the pericellular matrix regions in response to the 3D hydrogel environment and/or loading. Interestingly, collagen type VI, the primary protein present natively in the PCM of cartilage, was detected in the pericellular region of the chondrocytes at all time points suggesting that this protein may be one of the first matrix molecules deposited by the cells and/or not sufficiently removed during digestion (data not shown). Nonetheless, there were no observable differences among the three hydrogel formulations over the course of 12 hours. Although we cannot decouple these different events in our charged hydrogel matrices, our results indicate that physiological levels of fixed negative charges associated with chondroitin sulfate play an important role in mediating mechanotransduction pathways that enhance matrix deposition. These findings suggest that streaming potentials and/or dynamic changes in osmolarity are key extracellular events in this process while fluid flow and cell deformation appear to have less of an effect. Cell-matrix interactions may also be playing an important role.
Within the physiological environment of the joint, cartilage is normally subjected to intermittent dynamic loading patterns. Therefore, we were interested in assessing whether the enhanced matrix deposition observed during the application of loading in the 20% ChS hydrogels was sufficient to maintain continued synthesis of matrix once removed from the bioreactor. Waldman et al. (Waldman et al., 2006) demonstrated that a single application of dynamic loading applied within the first 24 hours of culture resulted in enhanced mechanical properties of the engineered tissue long-term suggesting that early loading can have a positive impact on tissue growth. In this study, the rates of nitric oxide production, cell proliferation, and proteoglycan synthesis (i.e., on a per hour basis) were reduced when compared to the rates observed during the 6–12 hour culture period regardless of gel formulation or prior culture conditions. This finding suggests that initially freshly isolated chondrocytes are highly metabolically active until they have had time to deposit some of their own matrix (Fig. 1). Quinn et al. (Quinn et al., 2002) reported similar findings where matrix synthesis decreased with culture time for chondrocytes encapsulated in 3D agarose constructs. Nonetheless, chondrocyte response post-loading was highly dependent on the prior culture conditions (i.e., free swelling, uncompressed control, or loaded) as well as gel formulation. In the PEG-only gels, prior culture within the bioreactor as uncompressed controls resulted in significantly lower nitric oxide production, proteoglycan synthesis, and total collagen deposition compared to free swelling constructs. Interestingly, this effect was not observed in the ChS containing gels where the uncompressed constructs were statistically similar to the free swelling constructs for each of the cellular responses. In the uncompressed constructs, the weight of the permeable platen inherently imparts a small tare strain on the constructs, which may have impacted the cellular responses. We estimated the tare strain to be ~3% in the PEG-only gels and ~2% in the ChS containing gels based on their compressive moduli. This difference in cellular strain is small and not likely a contributing factor, however, the combined impact of gel chemistry and a small static strain may have led to the differences in observed cellular responses. For the 20% ChS hydrogels during the free swelling, post-loading period, there were no differences in these cellular responses suggesting that the prior loading environment did not have a sustained effect on matrix synthesis. However, the total accumulated proteoglycans will be markedly higher in the loaded 20% ChS hydrogels compared to the other hydrogel formulations. For collagen deposition, a very different response was observed in the 20% ChS gels. At the end of the 48 hour culture period, the total amount of collagen deposited in the hydrogels was similar among all culture conditions. This result indicates that there was a ~8-fold and 10-fold increase in collagen deposition in the free swelling and uncompressed control 20% ChS gels during the 36-hr free-swelling period suggesting that the static environment promoted collagen deposition, but at a slower rate. For the 40% ChS gels, the prior dynamic loading condition resulted in decreased nitrite production, but also led to decreased proteoglycan synthesis and overall lower collagen contents compared to free swelling and/or uncompressed controls further confirming that non-physiological extracellular events resulting from a charged matrix (e.g., streaming potentials and/or dynamic changes in osmolarity) negatively impact cellular response longer-term.
It is important to note that local presentation of the negative charges in our synthetic environment will be largely different from the way negative charges are presented in native tissue. In our hydrogel system, the chondroitin sulfate and PEG macromers are thoroughly mixed prior to polymerization likely resulting in a uniform distribution of chondroitin sulfate chains through the hydrogel network. Contrarily, the negative charges in cartilage are localized within large aggrecan macromolecule forming a bottle-brush structure that consist of many linear chains of chondroitin sulfate and other charged glycosaminoglycans. The molecular distribution of charge within the aggrecan molecules and their distribution within the tissue contribute to the unique biomechanical properties of cartilage (Dean et al., 2006; Dean et al., 2003) impacting the electrical fields generated within the tissue during loading (Hart, 2008). Although the FCD within our hydrogels is estimated to be in the physiological range for the 20% ChS hydrogels, the electro-mechanical signals perceived by the chondrocytes may differ from physiological signals due to the differences in the charge presentation. Nonetheless, these synthetic hydrogel systems offer a controlled 3D environment from which to probe and study the impact of electrical charge on mediating cellular responses.
In summary, this study has attempted to gain insight into the importance of electrical charge in mediating chondrocyte response to mechanical loading by utilizing neutral PEG hydrogels and by systematically incorporating negative charges into the hydrogel via chondroitin sulfate. Overall our findings suggest that for the loading parameters employed here, extracellular events associated with fluid flow and cell deformation, alone, are not sufficient to mediate cellular response to loading within crosslinked hydrogels, while the FCD has a dramatic affect on how chondrocytes respond to mechanical stimulation suggesting that streaming potentials, dynamic changes in osmolarity, and/or ion flow may be important mechano-regulators of chondrocytes. Moreover, the density of fixed negative charges dramatically affected how chondrocytes responded to loading where physiological FCD’s stimulated matrix synthesis and inhibited nitric oxide production while non-physiologically high levels were less beneficial. Additional studies are needed to assess whether a dose-dependent response exists within the physiological ranges of FCD and to better understand the impact of cellular response to a range physiological FCD’s. This study underscores the importance of the mechano-electrochemical environment as presented within the confines of an artificial culture environment, but may provide new insights into chondrocyte mechanotransduction events.
4. Materials and Methods
4. 1 Macromer synthesis
Poly(ethylene glycol) (3000 MW, Fluka, St. Louis, Missouri) was dissolved in methylene chloride and reacted with excess methacrylic anhydride, and triethylamine at room temperature for 96 hours. The reaction by-products were removed by precipitation in cold acetone. The final product, poly(ethylene glycol) dimethacrylate (PEGDM) was further purified and recovered by precipitation 2× into cold ethyl ether. The product was determined to be 88% methacrylated as determined by 1H NMR (Varian VYR-500). Specifically, the area under the curve for the vinyl resonances (δ=5.7ppm and δ=6.1ppm) associated with the methacrylate substitution was compared to that of the methylene protons (δ=4.3ppm) in the PEG backbone.
A photoreactive and crosslinkable chondroitin sulfate macromer was synthesized following protocols adapted from (Bryant et. al., 2004b) and (Smeds and Grinstaff, 2000). In brief, chondroitin sulfate A (ChS, Sigma, St. Louis, Missouri), which contains ~70% chondroitin-4-sulfate and ~30% chondroitin-6-sulfate, was dissolved in deionized water (DI-H2O) at 25% w/v and reacted with methacrylic anhydride at a ratio of 1:8 at 4C° for 24 hours. The pH of the reaction was maintained at a value of 8 by the addition of sodium hydroxide. The resulting product, chondroitin sulfate methacrylate (ChS-MA), was precipitated in cold methanol and dialyzed in DI-H2O for 48 hours and recovered by lyophilization. The reaction allows for multiple substitutions of methacrylates to the free hydroxyl groups present within each repeat unit of ChS (Bryant et. al., 2004b). The degree of methacrylation was determined to be 23% by 1H NMR (Varian VYR-500), which indicates there are 23 methacrylates on average attached to each molecule of ChS-MA. The area under the curve for the vinyl resonances (δ=5.5–6.2ppm) was compared to that for the acetyl groups (δ=1.7–2.0ppm).
4.2 Hydrogel Preparation and Characterization
PEGDM and ChS-MA macromers at varying ratios of PEGDM:ChS-MA (100:0, 80:20, 60;40) were dissolved in PBS to a final macromer concentration of 10% w/v and combined with 0.05% w/v photoinitiator (Irgacure 2959®, Ciba Specialty Company). The macromer solution was thoroughly mixed and then exposed to 365 nm light at ~4 mW·cm−2 for 10 minutes to form solid cylindrical hydrogels (5mm in diameter and height).
The equilibrium mass swelling ratio (q) was calculated by ms/md where ms is the swollen mass and md is the dry polymer mass. In brief, hydrogels without cells were allowed to freely swell in PBS for 24 hours in an incubator at 37°C and 5% CO2 to determine the swollen mass of the gels. The gels were lyophilized for 24 hours to determine the dry polymer mass. The tangent compressive modulus (K) was determined using a mechanical tester (Bose LM1 Test Bench, Eden Prairie, Minnesota) in unconfined compression with non-porous platens and under hydrated conditions. A constant strain rate of 0.02mm/s was applied to the hydrogels and the resulting stress recorded. The tangent modulus was calculated for the linear range of the stress-strain curve. A sample size of 5 was used.
The actual amount of ChS incorporated into the hydrogel was determined after free swelling the gels for 24 hours to allow for any unreacted macromers to diffuse out of the gel and by using the dimethylmethylene blue dye method (Farndale et. al., 1982). The actual amount of ChS incorporated into the hydrogels for the 20% ChS and 40% ChS macromer formulations was 154 and 290 mg/g gel wet weight, respectively. The fixed charge density was determined based on the amount of ChS incorporated into the hydrogel where there are two moles of equivalent charge per repeat unit within ChS and the average molecular weight of ChS is ~48,700 g/mol (Bryant et. al., 2004b).
4.3 Chondrocyte Isolation
Articular cartilage from the metacarpalphalangeal joints of six 1–2 year old bovines (Arapahoe Foods, Lafayette, Colorado) was removed under sterile conditions within several hours of slaughter and washed in PBS containing 2.6 mg·mL−1 of potassium chloride and 5.2 mg·mL−1 of sodium chloride to attain isosmotic conditions (i.e., 400 mOsm) (Urban et al., 1993). In addition, all solutions used for the isolation of chondrocytes were adjusted to a physiological osmolarity of 400 mOsm. The cartilage slices were combined and rewashed in PBS supplemented with 1% penicillin streptomycin (PBS-P/S, Invitrogen, Carlsbad, California), diced finely and digested in a solution of 0.2% collagenase type II (Worthington Biochemical Corp, Lakewood, New Jersey) containing Dulbecco’s Minimal Essential Medium (DMEM, Invitrogen, Carlsbad, California) and 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, California) for 17 hours at 37°C. The isolated chondrocytes were centrifuged 3 times at 1200 rpm for 10 minutes. The cell pellet was combined and resuspended in 30 mL of 400 mOsm PBS-P/S. Cell viability was determined by tyrpan-blue exclusion and was determined to be greater than 98% prior to encapsulation.
4.4 Chondrocyte Encapsulation
Isolated chondrocytes were photoencapsulated in the PEG-only and PEG-ChS hydrogels (~5mm in diameter and height) at a concentration of 4×106 cells·mL−1 macromer solution; a cell concentration commonly used in agarose constructs to study chondrocyte mechanotransduction (Chowdhury et al., 2006; Chowdhury et al., 2001). This cell concentration equates to approximately 50% of the estimated cell concentration in bovine cartilage explants (~600,000 cells/g cartilage, unpublished data). A low cell concentration was employed to minimize contributions from paracrine signaling within the gel. The constructs were allowed to freely swell for 6 hours in individual wells of a standard 24-well tissue culture plate containing 1.5mL per well of chondrocyte medium (DMEM containing 10mM HEPES, 0.1M non-Essential Amino Acids, 0.4 mM L-proline, 50 mg·L−1 of L-ascorbic acid, 1X P/S 10% FBS, 0.5 μg·mL−1 of fungizone; Invitrogen, Carlsbad, California) supplemented with 2.6 mg·mL−1 potassium chloride, and 5.2 mg·mL−1 sodium chloride.
4.5 PCM visualization
Free swelling constructs were assessed immediately after photoencapsulation and after 12 and 24 hours post-encapsulation (n=3 per timepoint). The PEG-only and PEG-ChS gels were cut in half along the horizontal axis. In situ immunofluorescent staining was assessed for each of the constructs by placing the gels in PBS supplemented with 0.5 units/mL chondroitinase ABC (Sigma, St. Louis, Missouri) and 1% bovine serum albumin (BSA; Sigma, St. Louis, Missouri). Samples were treated for 1 hour with anti-chondroitin-6-sulfate (clone MK-302, Chemicon, Billerica, Massachusetts) in DMEM + 20% FBS (1:50) and rinsed twice with Earle’s Balanced Salt Solution (EBSS, Gibco, Carlsbad, California). The gels were then incubated for 1 hour in DMEM+20% FBS with goat anti-mouse IgG labeled with 1:20 Alexa Fluor 546 (Invitrogen, Calrsbad, California). Live cells were counterstained with 2μM calcein-AM (Invitrogen, Calrsbad, California) and a total of 3 regions of interest were imaged using confocal laser microscopy with a 40× objective (Zeiss LSM 510, Thornwood, New York). The antibody for chondroin-6-sulfate, which recognizes chondroitin-6-sulfate stubs, preferentially in clusters within high density cartilage proteoglycans, did not stain the ChS incorporated into hydrogels.
4.6 Mechanical loading
The experimental setup is depicted in Fig. 2. After freely swelling for 6 hours, the gels were placed in a new 24-well tissue plate and into a custom mechanical loading apparatus (Villanueva et. al., 2008) containing 24 permeable platens of Porex® high density polyethylene (HDPE; 40–70μm) and a 2mm thick porous base of the same material. Each construct was cultured in 1.5 mL of chondrocyte medium supplemented with 1 μCi·mL−1 of [3H] thymidine and 10 μCi·mL−1 of [35SO42−] (Perkin Elmer, Shelton, Connecticut). The samples were subjected to continuous, unconfined dynamic compression at 0.3Hz and 15% amplitude strain under sterile conditions for 6 hours. In parallel, a custom-built control box was used to place an additional and equal number of gels per condition using the same type of permeable platens and base and chondrocyte medium as described above. These samples, defined as the uncompressed control, were used to mimic the environment within the bioreactor. To calculate the offset strains imparted by the pins onto the gels, we considered the initial height of the gels, the weight of the pins, and the gel compressive modulus. The calculated offset strains imparted on the gels by the pins were 2.7, 1.8, and 1.7% for the PEG-only, PEG-ChS with 20% ChS and PEG-ChS with 40%ChS gels, respectively. In parallel, additional samples were cultured under free swelling conditions in individual wells of a 24-well tissue culture plate.
4.7 Chondrocyte Deformation
Chondrocyte deformation was assessed after 6 hours of free swelling culture and after 12 hours of culture as free swelling, uncompressed control or dynamically loaded specimen. The hydrogel constructs were treated with 2 μM calcein-AM and 2 μM ethidum homodimer (LIVE/DEAD ® Assay, Invitrogen, Carlsbad, California) and incubated at 5% CO2 and 37°C for 20–30 minutes. The gels were placed in a custom-built cell straining device similar to that described by Knight et al. (Knight et al., 1996). The cell strainer sits on the stage of an inverted laser scanning confocal microscope (Zeiss LSM 510, Thornwood, New York) and the constructs were subjected to a 15% gross compressive strain to mimic the strains of the bioreactor. Using a 40× oil immersion objective, chondrocytes within the hydrogels were imaged at their maximum width. A total of three different regions within the hydrogel were imaged and 3 gels were analyzed per condition for an n of ~60 cells. Cell morphology was determined by a cell diameter ratio (x/y) where x is the cell diameter parallel to the applied strain and y is the cell diameter perpendicular to the applied strain.
4.8 Biochemical Assays
At specified time points, the culture medium was removed from each well and stored at −20°C until further analysis. Hydrogels (n=5) were also removed, homogenized, and placed in an enzymatic digestion of papain (125 mg/mL of papain (Worthington Biochemical Corp, Lakewood, New Jersey), 10 mM of L-cysteine-HCl (Sigma, St. Louis, Missouri), 100 mM of phosphate (Sigma, St. Louis, Missouri) and 10 mM of ethylenediaminetetraacetic acid (EDTA) (Biorad, Hercules, California)) at a pH of 6.3 for 16 hours at 60°C. For the samples that were cultured for an additional 36 hours, the medium was also removed for further analysis and the corresponding gels were enzymatically digested in papain.
Total DNA content was quantified in the papain digests using the Hoeschst 33258 (Polysciences, Warrington, Pennsylvania) fluorescence assay. In the papain digests, the amount of [35SO42−] incorporation into the newly synthesized proteoglycans was measured using the Alcian blue precipitation method (Masuda et al., 1994). [3H]-Thymidine incorporation into newly synthesized DNA was measured in the papain digests by precipitation into 10%w/v trichloroacetic acid. Nitrite, a stable end product of nitric oxide, was measured in the medium using the Griess Reagent Kit (Promega, Madison, Wisconsin). The data were normalized to total DNA content.
4.9 Statistical Analysis
Data are reported as mean values ± standard deviation. Single factor ANOVA was performed for each cell response and a confidence level of 0.95 was considered significant. An ANOVA two factor fixed effects model was used to measure the effects of fixed charged density and mechanical loading on nitrite production, cell proliferation, and proteoglycan synthesis and collagen content during the loading period (Table 3). A confidence level of 0.95 was considered statistically significant.
Acknowledgments
This work was supported by a research grant from the NIH (K22 DE016608), a NASA Harriett Jenkins Predoctoral Fellowship and a Department of Education’s Graduate Assistantship in Areas of National Need Fellowship to IV and an Undergraduate Research Opportunity Grant to SKG. Confocal microscopy was performed at the Nanomaterials Characterization Facility at the University of Colorado.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akanji OO, Lee DA, Bader DL. The effects of direct current stimulation on isolated chondrocytes seeded in 3D agarose constructs. Biorheology. 2008;45:229–243. [PubMed] [Google Scholar]
- Bassleer C, Rovati L, Franchimont P. Stimulation of proteoglycan production by glucosamine sulfate in chondrocytes isolated from human osteoarthritic articular cartilage in vitro. Osteoarthritis Cartilage. 1998;6:427–434. doi: 10.1053/joca.1998.0146. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocyte photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Crosslinking density influences chondrocyte metabolism in dynamically loaded photocrosslinked poly(ethylene glycol) hydrogels. Ann Biomed Eng. 2004a;32:407–417. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- Bryant SJ, Davis-Arehart KA, Luo N, Shoemaker RK, Arthur JA, Anseth KS. Synthesis and characterization of photopolymerized multifunctional hydrogels: water-soluble poly(vinyl alcohol) and chondroitin sulfate macromers for chondrocyte encapsulation. Macromolecules. 2004b;37:6726–6733. [Google Scholar]
- Carver SE, Heath CA. Increasing extracellular matrix production in regenerating cartilage with intermittent physiological pressure. Biotech Bioeng. 1999;62:166–174. [PubMed] [Google Scholar]
- Chan PS, Caron JP, Rosa GJM. Glucosamine and chondroitin sulfate regulate gene expression and synthesis of nitric oxide and prostaglandin E2 in articular cartilage explants. Osteoarthritis Cartilage. 2005;13:387–394. doi: 10.1016/j.joca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Chao PHG, West AC, Hung CT. Chondrocyte intracellular calcium, cytoskeletal organization, and gene expression responses to dynamic osmotic loading. Am J Physiol-Cell Physiol. 2006;291:C718–C725. doi: 10.1152/ajpcell.00127.2005. [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Bader DL, Lee DA. Dynamic compression inhibits the synthesis of nitric oxide and PGE(2) by IL-1 beta-stimulated chondrocytes cultured in agarose constructs. Biochemical and Biophysical Research Communications. 2001;285(5):1168–1174. doi: 10.1006/bbrc.2001.5311. [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Bader DL, Shelton JC, Lee DA. Temporal regulation of chondrocyte metabolism in agarose constructs subjected to dynamic compression. Arch Biochem Biophys. 2003;417:105–111. doi: 10.1016/s0003-9861(03)00340-0. [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Appleby RN, Salter DM, Bader DA, Lee DA. Integrin-mediated mechanotransduction in IL-1 beta stimulated chondrocytes. Biomechanics And Modeling In Mechanobiology. 2006;5(2–3):192–201. doi: 10.1007/s10237-006-0032-3. [DOI] [PubMed] [Google Scholar]
- Dean D, Seog J, Ortiz C, Grodzinsky AJ. Molecular-level theoretical model for electrostatic interactions within polyelectrolyte brushes: Applications to charged glycosaminoglycans. Langmuir. 2003;19(13):5526–5539. [Google Scholar]
- Dean D, Han L, Grodzinsky AJ, Ortiz C. Compressive nanomechanics of opposing aggrecan macromolecules. Journal Of Biomechanics. 2006;39(14):2555–2565. doi: 10.1016/j.jbiomech.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Erickson GR, Alexopoulos LG, Guilak F. Hyper-osmotic stress induces volume change and calcium transients in chondrocytes by transmembrane, phospholipid, and G-protein pathways. Journal of Biomechanics. 2001;34(12):1527–1535. doi: 10.1016/s0021-9290(01)00156-7. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric micro- assay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Frank EH, Grodzinsky AJ. Cartilage electromechanics I. Electrokinetic transduction and the effects of electrolyte pH and ionic strength. J Biomech. 1987;20:615– 627. doi: 10.1016/0021-9290(87)90282-x. [DOI] [PubMed] [Google Scholar]
- Gu WY, Lai WM, Mow VC. Transport of fluid and ions through a porous- permeable charged hydrated tissue, and streaming potential data on normal bovine articular cartilage. J Biomech. 1993;26:709–23. doi: 10.1016/0021-9290(93)90034-c. [DOI] [PubMed] [Google Scholar]
- Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA. The pericellular matrix as a transducer of biomechanical and biochemical signals in articular cartilage. Annals of the New York Academy of Sciences. 2006;1068:498–512. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: a confocal microscopy study. J Orthop Res. 1995;13:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- Hansen U, Schunke M, Domm C, Ioannidis N, Hassenpflug J, Gehrke T, Kurz B. Combination of reduced oxygen tension and intermittent hydrostatic pressure: a useful tool in articular cartilage tissue engineering. J Biomech. 2001;34:941– 949. doi: 10.1016/s0021-9290(01)00050-1. [DOI] [PubMed] [Google Scholar]
- Hart FX. The mechanical transduction of physiological strength electric fields. Bioelectromagnetics. 2008;29(6):447–455. doi: 10.1002/bem.20411. [DOI] [PubMed] [Google Scholar]
- Hascall VC, Hascall GK. Cell biology of extracellular matrix. Plenum Press; New York City, NY: 1981. pp. 39–63. [Google Scholar]
- Hwang NS, Varghese S, Lee HJ, Theprungsirikul P, Canver A, Sharma B, Elisseeff J. Response of zonal chondrocytes to extracellular matrix-hydrogels. FEBS LETTERS. 2007;581:4172–4178. doi: 10.1016/j.febslet.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YJ, Bonassar LJ, Grodzinsky AJ. The role of streaming potentials, fluid flow and pressure in the stimulation of chondrocyte biosynthesis during dynamic compression. J Biomech. 1995;28:1055–1066. doi: 10.1016/0021-9290(94)00159-2. [DOI] [PubMed] [Google Scholar]
- Knight MM, Lee DA, Bader DL. Distribution of chondrocyte deformation in compressed agarose gel using confocal microscopy. J Cell Eng. 1996;1:97–102. [Google Scholar]
- Korhonen RK, Julkunen P, Rieppo J, Lappalainen R, Konttinen YT, Jurvelin JS. Collagen network of articular cartilage modulates fluid flow and mechanical stresses in chondrocyte. Biomech Model Mechanobiol. 2006;5:150. doi: 10.1007/s10237-006-0021-6. [DOI] [PubMed] [Google Scholar]
- Lang F, Ritter M, Gamper N, Huber S, Fillon S, Tanneur V, Lepple-Wienhues A, Szabo I, Gulbins E. Cell volume in the regulation of cell proliferation and apoptotic cell death. Cellular Physiology and Biochemistry. 2000;10(5–6):417–428. doi: 10.1159/000016367. [DOI] [PubMed] [Google Scholar]
- Lee DA, Bader DL. Compressive strains at physiological frequencies influence the metabolism of chondrocytes seeded in agarose. J Orthop Res. 1997;15:181–188. doi: 10.1002/jor.1100150205. [DOI] [PubMed] [Google Scholar]
- Légaré A, Garon M, Guardo R, Savard P, Poole AR, Buschmann MD. Detection and analysis of cartilage degeneration by spatially resolved streaming potentials. J Orthop Res. 2002;20:819–826. doi: 10.1016/S0736-0266(02)00002-5. [DOI] [PubMed] [Google Scholar]
- Legendre F, Baugé C, Roche R, Saurel AS, Pujol JP. Chondroitin sulfate modulation of matrix and inflammatory gene expression in IL-1β stimulated chondrocytes-study in hypoxic alginate bead cultures. Osteoarthritis Cartilage. 2008;16:105–114. doi: 10.1016/j.joca.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Li Q, Williams CG, Sun DD, Wang J, Leong K, Elisseeff JH. Photocrosslinkable polysaccharides based on chondroitin sulfate. J Biomed Mater Res A. 2004;68:28–33. doi: 10.1002/jbm.a.20007. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969;177:492– 500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- Mason MN, Metters AT, Bowman CN, Anseth KS. Predicting controlled-release behavior of degradable PLA-b-PEG- b-PLA hydrogels. Macromolecules. 2001;34(13):4630–4635. [Google Scholar]
- Masuda K, Shirota H, Thonar EJ. Quantification of 35S-labeled proteoglycans complexed to alcian blue by rapid filtration in multiwall plates. Anal Biochem. 1994;217:167–175. doi: 10.1006/abio.1994.1105. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CCB, Wong DD, Chao PHG, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs EA. Endogenous nitric-oxide physiology, pathology and clinical relevance. Eur J Clin Invest. 1991;21:361–374. doi: 10.1111/j.1365-2362.1991.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Mow VC, Guo XE. Mechano-electrochemical properties of articular cartilage: their inhomogeneities and anisotropies. Ann Rev Biomed Eng. 2002;4:175–209. doi: 10.1146/annurev.bioeng.4.110701.120309. [DOI] [PubMed] [Google Scholar]
- Mow VC, Wang CC, Hung CT. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthritis Cartilage. 1999;7:41–58. doi: 10.1053/joca.1998.0161. [DOI] [PubMed] [Google Scholar]
- Negoro K, Kobayashi S, Takeno K, Uchida K, Baba H. Effect of osmolarity on glycosaminoglycan production and cell metabolism of articular chondrocyte under three-dimensional culture system. Clin Exp Rheumatol. 2008;26:534–541. [PubMed] [Google Scholar]
- Nicodemus GD, Bryant SJ. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng Part B, Reviews. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto S, Takagi M, Wakitani S, Nihira T, Yoshida T. Effect of chondroitin sulfate and hyaluronic acid on gene expression in a three-dimensional culture of chondrocytes. J Biosci Bioeng. 2005;100:123–126. doi: 10.1263/jbb.100.123. [DOI] [PubMed] [Google Scholar]
- Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine: From molecular principles to bionanotechnology. Adv Mater. 2006;18:11–25. [Google Scholar]
- Quenneville E, Binette JS, Garon M, Legare A, Meunier M, Buschmann MD. Fabrication and characterization of nonplanar microelectrode array circuits for use in arthroscopic diagnosis of cartilage diseases. IEEE Trans Biomed Eng. 2004;51:2164–2173. doi: 10.1109/TBME.2004.836522. [DOI] [PubMed] [Google Scholar]
- Quinn TM, Schmid P, Hunziker EB, Grodzinsky AJ. Proteoglycan deposition around chonrdocytes in agarose culture: Construction of a physical and biological interface for mechanotransduction in cartilage. Biorheology. 2002;39:27–37. [PubMed] [Google Scholar]
- Sandell LJ, Aigner T. Articular cartilage and changes in arthritis cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah RL, Kim YJ, Doong JH, Grodzinsky AJ, Plaas AHK, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- Smeds KA, Grinstaff MW. Photocrosslinkable polysaccharides for in situ hydrogel formation. J Biomed Mater Res. 2000;54:115–121. doi: 10.1002/1097-4636(200101)54:1<115::aid-jbm14>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Sun DD, Guo XE, Likhitpanichkul M, Lai WM, Mow VC. The influence of the fixed negative charges on mechanical and electrical behaviors of articular cartilage under unconfined compression. Trans ASME. 2004;126:6–16. doi: 10.1115/1.1644562. [DOI] [PubMed] [Google Scholar]
- Thevenot P, Hu WJ, Tang LP. Surface chemistry influences implant biocompatibility. Curr Top Med Chem. 2008;8(4):270–280. doi: 10.2174/156802608783790901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban JPG, Hall AC, Gehl KA. Regulation of matrix synthesis rates by the ionic and osmotic environment of articular chondrocytes. J Cell Physiol. 1993;154:262–270. doi: 10.1002/jcp.1041540208. [DOI] [PubMed] [Google Scholar]
- Varghese S, Hwang NS, Canver AC, Parnduangji T, Lin DW, Elisseeff J. Chondroitin sulfate based niches for chondrogenic differentiation of mesenchymal stem cells. Matrix Biology. 2008;27:12–21. doi: 10.1016/j.matbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Villanueva I, Hauschulz DS, Mejic D, Bryant SJ. Static and dynamic compressive strains influence nitric oxide production and chondrocyte bioactivity when encapsulated in PEG hydrogels of different crosslinking densities. Osteoarthritis Cartilage. 2008;16:909–918. doi: 10.1016/j.joca.2007.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanueva I, Bishop NL, Bryant SJ. Medium osmolarity and PCM development improves chondrocyte survival when photoencapsulated in PEG hydrogels at low densities. Tissue Eng-Part A. 2009 doi: 10.1089/ten.tea.2009.0001. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman SD, Couto DC, Grynpas MD, Pilliar RM, Kandel RA. A single application of cyclic loading can accelerate matrix deposition and enhance the properties of tissue-engineered cartilage. Osteoarthritis Cartilage. 2006;14:323–330. doi: 10.1016/j.joca.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Wang CCB, Guo XE, Sun D, Mow VC, Ateshian GA, Hung CT. The functional environment of chondrocytes within cartilage subjected to compressive loading: A theoretical and experimental approach. Biorheology. 2002;39:11–25. [PubMed] [Google Scholar]